Inexpensive Cell Migration Inquiry Lab using Zebrafish
Published online:
Abstract
Cell migration is an important and interesting topic that has the potential to engage students in cell biology by its intrinsically active nature. However, most ways to investigate cell migration require expensive equipment not typically available for an entire undergraduate laboratory section. This lesson describes a hands-on laboratory lesson for upper-level undergraduate students that uses Danio rerio (zebrafish) embryos to investigate neutrophil migration with a student-designed hypothesis. The embryos are treated with specific inhibitors to cytoskeletal proteins, depending on the student hypothesis, or treated with a different experimental condition, then anesthetized, wounded with a syringe needle, and incubated to give time for the neutrophils to move to the wound site. The following lab session, the fixed embryos are treated with an inexpensive lipophilic dye that stains neutrophils and the students count the neutrophils to obtain quantitative data about their experimental conditions. Students analyze and describe their hypothesis and results in a report in the style of a scientific paper. Students report that the inquiry lab was fun and challenging and helped them learn more about how cell migration works.
Citation
Cooper, K.M. 2016. Inexpensive Cell Migration Inquiry Lab using Danio rerio. CourseSource. https://doi.org/10.24918/cs.2016.6Society Learning Goals
Cell Biology
- Cytoskeleton Structure and function
- How do the different components of the cytoskeleton support a variety of cell functions, such as cell shape, division, movement, sensing the environment, and cell-cell communication?
Science Process Skills
- Process of Science
- Locate, interpret, and evaluate scientific information and primary literature
- Pose testable questions and hypotheses to address gaps in knowledge
- Plan, evaluate, and implement scientific investigations
- Interpret, evaluate, and draw conclusions from data
- Construct explanations and make evidence-based arguments about the natural world
- Address novel questions through authentic research experiences
- Modeling/ Developing and Using Models
- Build and evaluate models of biological systems
- Quantitative Reasoning/ Using Mathematics and Computational Thinking
- Apply the tools of graphing, statistics, and data science to analyze biological data
- Communication and Collaboration
- Share ideas, data, and findings with others clearly and accurately
Lesson Learning Goals
Students will:- understand the role of the cytoskeleton in the process of cell migration.
- understand the importance of good experimental design and quantitative measurements when testing a hypothesis.
Lesson Learning Objectives
Students will:- formulate a hypothesis and design an experiment with the proper controls.
- describe the steps involved in the zebrafish wounding assay (treating zebrafish embryos with drugs or control substances, wounding the embryo, staining the embryo, and counting neutrophils near the wound).
- summarize results into a figure and write a descriptive figure legend.
- perform appropriate statistical analysis.
- interpret results in a discussion that draws connections between the cytoskeleton and cell migration.
- put data into context by appropriately using information from journal articles in the introduction and discussion of a lab report.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
Active learning has been repeatedly shown to be the most effective way to encourage student learning (1), and in science classes we have built-in active learning opportunities in the laboratory. Cell migration is a perfect topic to actively visualize in a lab session. While the concept of a cell moving from one place to another under its own power is usually an easy idea for the students to grasp, the underlying mechanisms and proteins involved are more abstract. An active, inquiry-driven, hands-on investigation of these ideas is likely to improve student learning about cell migration and the function of proteins like the cytoskeleton.
Neutrophils excel at migration, are the most abundant white blood cell in the human body, and are an important part of the immune system. They can move in a rapid and directional manner toward a wound or an infection, and are then involved in engulfing bacteria, releasing chemicals, and helping wounds heal (2). More recently it was discovered that neutrophils can later move away from the wound or site of infection (retrograde migration) (3), however researchers are still investigating when this process is for the benefit of the organism or if it is contributing to pathology (4). Many of the molecular mechanisms that enable the directional migration of the neutrophil have been elucidated and well-studied, including the role of integrin adhesion molecules (5), G protein-coupled receptors (6), signaling molecules like PI3-Kinase (6), and cytoskeletal proteins. It is known specifically that actin is required for movement (7) but microtubules are more important for proper directional migration (8, 9).
Zebrafish (Danio rerio) are an important model organism to investigate neutrophil migration because the zebrafish embryos are optically transparent for several days after fertilization and cell movements can be observed with light microscopy. The fin is the thinnest part of the fish; therefore researchers often create a small hole or tear in the fin of the zebrafish embryo with a sharp needle to entice the neutrophils to the area for either observation using videomicroscopy or to fix and stain them to quantify the total number of neutrophils responding to the wound. Retrograde migration was first observed in zebrafish using these techniques and confocal imaging (3). Zebrafish fin wounding continues to be an important tool for learning more about the general mechanisms of neutrophil migration and also in translational research in search of drugs to improve diseases with inflammation pathology (10).
While many of the current studies use fluorescent tags or transgenic fish expressing fluorescent protein in their neutrophils and confocal videomicroscopy, neutrophils can also be stained with a simple lipophilic dye called Sudan Black B and then visualized using only a basic stereomicroscope. Sudan Black B seems to have first been used for neutrophil staining in 1947 (11), but continues to be used in research labs for easy quantification of migration (12). It is much more efficient to count stained neutrophils in hundreds of wounded fish embryo fins than to do separate two to six hour movies for every embryo. Because the stain is less expensive than fluorescent-tagged antibodies and only requires a stereomicroscope for visualization, this staining method offers a way to move a real zebrafish neutrophil research technique to the teaching laboratory for undergraduate students.
This inquiry lab suggests investigating the role of cytoskeleton proteins in neutrophil migration as a starting point, and the basic techniques could be expanded to ask other novel questions about the mechanisms of neutrophil migration using other inhibitors, as this is still an area of active research, and new discoveries are continuously being made in regards to signaling molecules (13). There are also possible questions related to the larger topic of the immune system. Although these questions could be more difficult to study in the undergraduate lab, interesting class discussion could include the role of zebrafish in research studies determining the contributions of neutrophils in the microenvironment of a tumor (14), or how neutrophils could play a role in autoimmune diseases (typically thought to only involve the other "adaptive" parts of the immune system) (15). Therefore, this lab can introduce students to the mechanisms of how cells are able to move while also potentially introducing aspects of immunology.
In this lab, students investigate how the elements of the cytoskeleton affect neutrophil migration in different ways. Because actin is required for movement (7) and microtubules are more important for proper directional migration (8,9) students can visualize these differences in the role of cytoskeletal proteins for themselves. This lab can also be expanded to allow the students to ask additional questions and do novel research about cell migration or the function of the immune system.
One problem with most cell migration research is that it often involves expensive equipment (microscopes with cameras capable of time-lapse microscopy) that most colleges and universities cannot provide to each student in a laboratory section. Additionally, experiments on neutrophils usually require isolation of human neutrophils, which is a time-consuming process and may require Biosafety Level 2 protocols, thus requiring more safety precautions. This inquiry lab gets around both of these problems by using the zebrafish embryo as a model system. When a small wound is made in the tail fin of the anesthetized 3-day-old embryo, neutrophils from the blood stream migrate into the fin and to the wound site (see supporting file S1: Cell migration in zebrafish movie).
Zebrafish are becoming a more popular model system for research and for teaching and therefore many lab exercises and inquiry labs have been published, though most involve genetics or development lab exercises, like one that involves studying when motor behavior develops in the zebrafish (16) and many are available online at the Zebrafish in the Classroom website (http://www.zfic.org/classroom%20experiments/index.html). A few involve traditional cell biology topics, such as Wnt signaling (17). However, I could not find any published lab related to cell migration or the migration of neutrophils in the zebrafish. Therefore, this inquiry lab was developed to give students hands-on experience of the function of cytoskeletal proteins in cell migration, an engaging and exciting lab involving living organisms, and practice collecting, analyzing, and incorporating quantitative data into a lab report in the style of a journal article.
This inquiry lab is designed for an upper-level undergraduate cellular and molecular biology lab with students who are majoring in biology or a related field. The entire procedure takes two three-hour laboratory sessions to investigate cytoskeletal function questions; additional sessions would be needed for additional student-directed research.
The students found this inquiry lab to be very interesting, enjoyable but challenging, and memorable. The students work on dexterity and hand-eye coordination by looking through a microscope while wounding the embryos, and the visual nature of the data they collect is a nice change from other more abstract cellular and molecular biology labs, such as looking at bands on gels. Additionally, by considering each fish as its own "experiment," the students have replicates of their quantitative data to do statistical analysis, as well as to create graphs and figures with their photographs of the fish. The lesson could be strictly used to investigate the cytoskeleton's role in cell migration or could be more open-ended, where students are allowed to test various hypotheses related to cell migration or the function of the immune system (alcohol treatment, temperature, etc.). Zebrafish are a fairly easy organism to keep and breed, though embryos can also be ordered from the Zebrafish International Research Center (ZIRC https://zebrafish.org/) instead. The lab exercise is great way to make the study of cell biology and the cytoskeleton more visual for students and to give them practice asking questions, designing an experiment, and analyzing data.
INTENDED AUDIENCE
This inquiry lab is designed for an upper-level undergraduate cellular and molecular biology lab with students who are majoring in biology or a related field, and has been tested at a small liberal arts college during multiple semesters, each with two lab sections of between 15-18 students each.
REQUIRED LEARNING TIME
The laboratory requires two sessions to complete, at least one day and up to one week apart. It works best with two three-hour sessions, though could be modified to fit two two-hour sessions by shortening the incubation time or having the instructor or teaching assistant finish one step after the first laboratory session has ended.
PRE-REQUISITE STUDENT KNOWLEDGE
Students will receive general information on the functions of specific cytoskeletal proteins (actin and microtubules) prior to the lab. Students should be able to do calculations related to making dilutions and other basic laboratory calculations, and they should be familiar with use of a stereomicroscope or receive instruction on basic use.
PRE-REQUISITE TEACHER KNOWLEDGE
The instructor should be familiar with the role of the cytoskeleton in the process of cell migration, using stereomicroscopes, and directing students in their use. Additionally, they either need to have zebrafish and the knowledge of how to set up pairs to obtain embryos or have another source for the embryos.
SCIENTIFIC TEACHING THEMES
ACTIVE LEARNING
The student must do some investigations into the published literature to develop their own hypothesis to test in the inquiry lab, which requires active synthesis of information and creativity in developing their new question. Additionally, students will manipulate the zebrafish throughout the experiment and count the number of cells that moved. The student is physically performing all aspects of the lab and therefore learning the concepts is directly related to the hands-on work.
ASSESSMENT
Progress toward the learning outcomes were measured primarily with a lab report in the form of a scientific journal article worth approximately 5-10% of their final course grade. Previous inquiry labs in the course have focused on writing various components of a scientific journal article. This report is the first time the students pull everything together to write all the sections about one inquiry lab. Additional assessment is with an essay question related to cytoskeletal function on the exam. To evaluate the effectiveness of the lab, students were surveyed several months after the class ended to see how well they remembered the lab and how well they thought it helped them learn. (The Loras College Institutional Review Board (IRB) approved the survey.)
INCLUSIVE TEACHING
Students work in the same group of two or three for all lab activities during the semester, which aids student comfort with group members and development of teamwork skills. All group members research the cytoskeleton and combine knowledge to develop their hypothesis. All group members are involved in the lab activities; however, there can be some division of labor such that students can choose certain tasks based on their strengths. The hands-on nature of the lab activities engages a variety of learners. Some find wounding the embryos to be their strength, while others excel at determining drug concentrations and making needed dilutions for treatments. The major assessment is an individual written report in the style of a scientific paper which allows students with testing anxiety to maximize their performance.
LESSON PLAN
PRE-LABORATORY PREPARATION
INTRODUCING THE TOPIC TO THE STUDENTS
The lesson requires introduction ahead of time, plus two lab session (see Table 1: Inexpensive Cell Migration Teaching Timeline). In a class period or a lab section about a week before starting the lab, give the students a short introduction to zebrafish as a model system, cell migration, and introduce the idea of different cytoskeletal proteins being involved in the process. This presentation will take around 15 minutes. (see Supporting File S2: Pre-lab Presentation Slides) The students then work with their lab partner outside of class to determine the hypothesis they want to investigate in their inquiry lab and read over the details of the protocol to prepare for lab (see Supporting File S3: Student Handout).
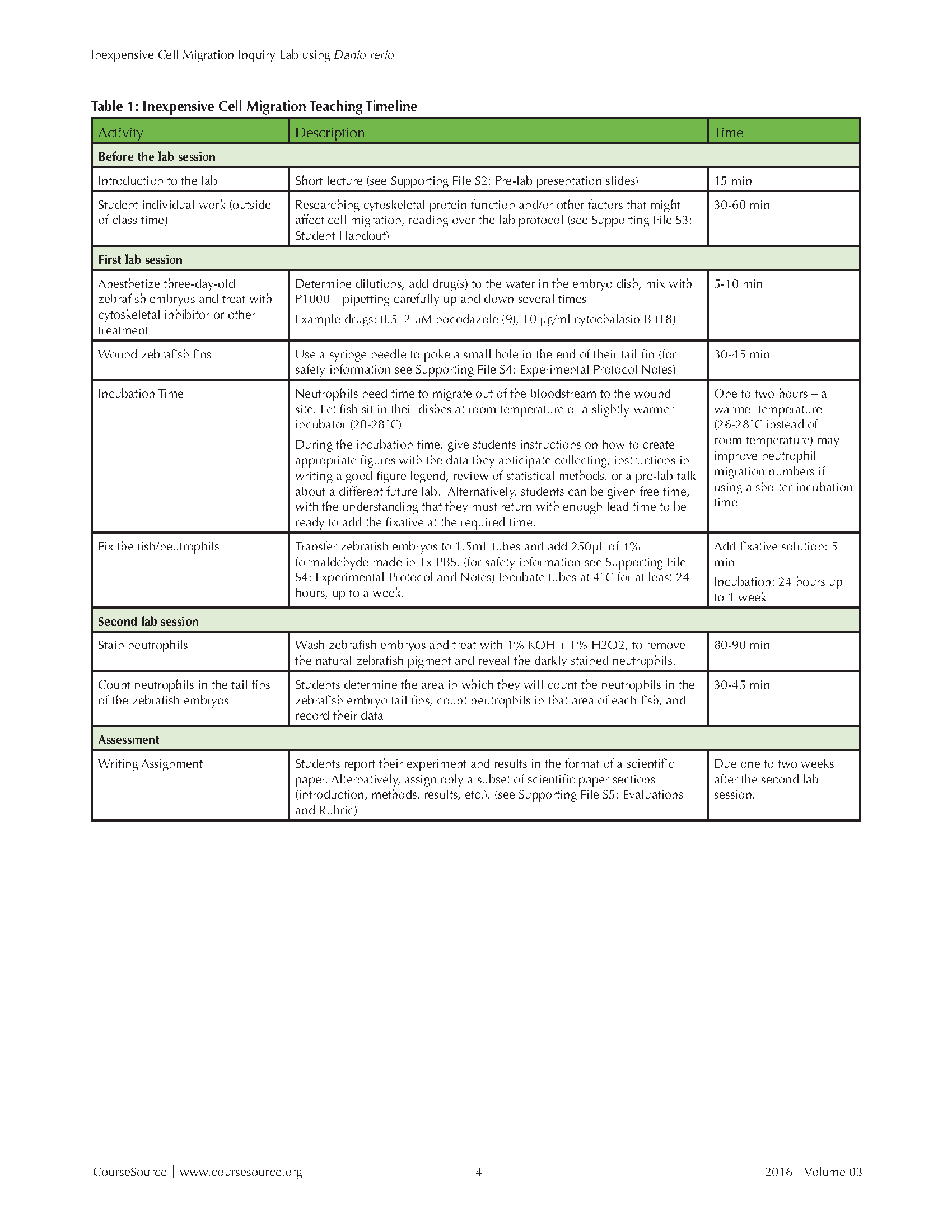
Table 1. Inexpensive Cell Migration - Teaching Timeline
OBTAINING ZEBRAFISH EMBRYOS
Institutional Animal Care and Use Committees (IACUC) oversee animal research on most university and college campuses. This committee should be consulted to see what steps and protocols are necessary to undertake this inquiry lab with students. Institutions without federal research money may not be required to have an IACUC committee or protocol but are still encouraged to follow best practices for care and use of zebrafish and their embryos. Example IACUC-approved zebrafish care and use protocols can be found on the Zebrafish International Resource Center's website: (https://zebrafish.org/documents/protocols.php)
Obtaining embryos could be the most challenging part of the laboratory for most instructors if they do not already have a zebrafish facility of their own. It is probably not practical to obtain and keep adult zebrafish to breed for embryos just for this lab exercise. It is outside the scope of this protocol to explain the proper care needed for them to breed reliably. General information on zebrafish care and breeding can be found on the zebrafish model organism website (zfin.org) including specific protocols at https://zfin.org/zf_info/zfbook/chapt2/2.6.html. If the instructor does not have his/her own facility, I would recommend asking a colleague in the area with a zebrafish facility for embryos first. Alternatively, they can be ordered from the Zebrafish International Resource Center (ZIRC) (https://zebrafish.org/documents/fees.php) or from somewhere like Carolina Biological, although I have not used embryos from either source specifically for this inquiry lab.
One potential problem with ordering zebrafish embryos from ZIRC and Carolina Biological is that there is less control over when the zebrafish are bred and therefore how old the embryos will be on your lab day. The embryos are ideally three days-post-fertilization for this laboratory experiment. At around four to five days old the swim bladder inflates in the embryos and then it becomes much more challenging for the students to wound the fins. Two-day-old embryos typically have a less-robust neutrophil response, and they are often still in their chorions (outer membrane surrounding the embryo) which must be removed with pronase treatment or manually (https://www.youtube.com/watch?v=3LbYTEu1Fo8), a process that students are apt to do clumsily, ruining many of the embryos. One possible method to deal with embryos that arrive too many days before the lab is to change the temperature at which they are kept. Embryos are typically raised at 28.5°C, but keeping them at a slightly lower temperature (~22-25°C) can slow development. However, embryo survivability is typically lower at these lower temperatures. Zebrafish embryos cannot eat before their swim bladder inflates and slowly begin to learn how to eat in the next few days following that, so no food is necessary for embryos being raised for this experiment.
When determining how many embryos to obtain, consider that each student group will need four to five zebrafish embryos for each of their treatment conditions. Additionally, factor in that many embryos naturally die in the process of development. Ask your supplier for an estimate of this loss before calculating how many embryos you will need to order. Other tips and suggestions, as well as a list of the reagents needed is available (see Supporting File S4: Materials List and Instructor Hints).
FIRST LAB SESSION
In the first lab session the students must be encouraged to quickly determine what cytoskeletal inhibitor they are going to test and determine the appropriate amount to add to the embryo water. The final concentrations of the drugs can either be given to the students [examples: 0.5-2 µM nocodazole (9), 10 µg/ml cytochalasin B (18)] or the students could be required to come to lab with the proper drug concentration determined from the literature. Students tend to have difficulty calculating the amount of concentrated drug to add to the embryo water to get the appropriate final concentration, and they spend too much time on this step. Encourage them to move quickly, offering to check their math if they are struggling. If the recommended final concentrations of inhibitors are given to the students ahead of time they can do the math for making dilutions on their own time and come in prepared to simply do the pipetting. Most inhibitors of cytoskeletal proteins are toxins and students should be instructed as to their safe use.
Students typically find watching the embryos very interesting and exciting, seeing their hearts beating and the blood flowing brings to life much of the science they've already learned over the years. Wounding the embryos with a small syringe needle can be a bit challenging. Students need to be instructed on needle safety before using the syringe needles. There should be approved needle/sharps collection containers for the used needles. Other chemical and general safety instruction should be given before the laboratory if there is not general instruction given to all science majors in a separate class. In order to wound the fish, using a stereomicroscope with a clear glass base and a mirror to tilt the light to get a very clear view of the fin is the best way to see the area to be wounded. It is helpful to draw a fish fin on the board and show them with the chalk/marker where to poke through with the needle (usually poking all the way down to the plastic petri dish) and how to then pull outward to the end of the fin to create the wound. Student groups sometimes ask for a demonstration from the instructor with their own fish at their station. Other groups want the instructor to come over and check their first fish to make sure it was done correctly. The students quickly seem to get comfortable with the wounding process, though individual student hand-eye dexterity and skill does vary.
After wounding, the embryos are incubated to allow the neutrophils time to migrate to the wound. The incubation time (which can be at room temperature or in a slightly warmer incubator up to 29°C) can be used for many possible purposes. Part of the time should be taken to remind the students what they need to do with the embryos when the incubation is over. After a quick observation to determine if the hearts are still beating in the embryos (and therefore that the fish survived the treatment), the zebrafish must be transferred to 1.5 mL tubes, the liquid removed, and formaldehyde added. The formaldehyde should be added in a fume hood, and students should be given specific safety information about this chemical before using. After that short reminder of the future work and safety, the remaining time could be used as a lab report writing workshop, a PowerPoint preview for the next lab in the semester, discussion of class material, an exam review session, or free time for the students. At the end of the incubation time, upper-level students with some lab experience typically have no problems doing the last step of transferring the zebrafish to the formaldehyde even without direct supervision, as long as they have had the process explained ahead of time to them and had the opportunity to ask questions. The flexibility of this step is useful if the lab time is scheduled for less than three hours or if a group had significant problems with the wounding step and took much longer than most groups.
SECOND LAB SESSION
After one to seven days of incubation in formaldehyde at 4°C, the neutrophils in the zebrafish embryos are stained with Sudan Black. (The zebrafish embryos can also be fixed in formaldehyde for one hour at room temperature instead and then the rest of the procedure carried out immediately. However, this timing does not lend itself well to most lab session lengths.) The trickiest part of the staining procedure is that the Sudan Black stain is dark, making the embryos impossible to see in the tube. The students sometimes accidentally remove them while removing the stain and washing. The loss of embryos can be minimized by informing them that it is okay to leave a little of the Sudan Black or washing solution on the embryos at each step, to keep the tip away from the bottom of the tube, and to remember how much space the zebrafish embryos take up at the bottom of the tube.
After the staining is complete, the neutrophils are easiest to count with a white background under the embryos. If the stereomicroscope only has clear glass base, a piece of plain white paper can be put underneath the plastic petri dish and the light adjusted to come from the top, if possible. If the lab is equipped with a stereomicroscope with an attached camera/screen it is useful to show the class a finished, stained zebrafish and point out a dot that is actually a neutrophil. However, still photos (Figure 1) can also be projected on a PowerPoint slide to assist with their identification skills.
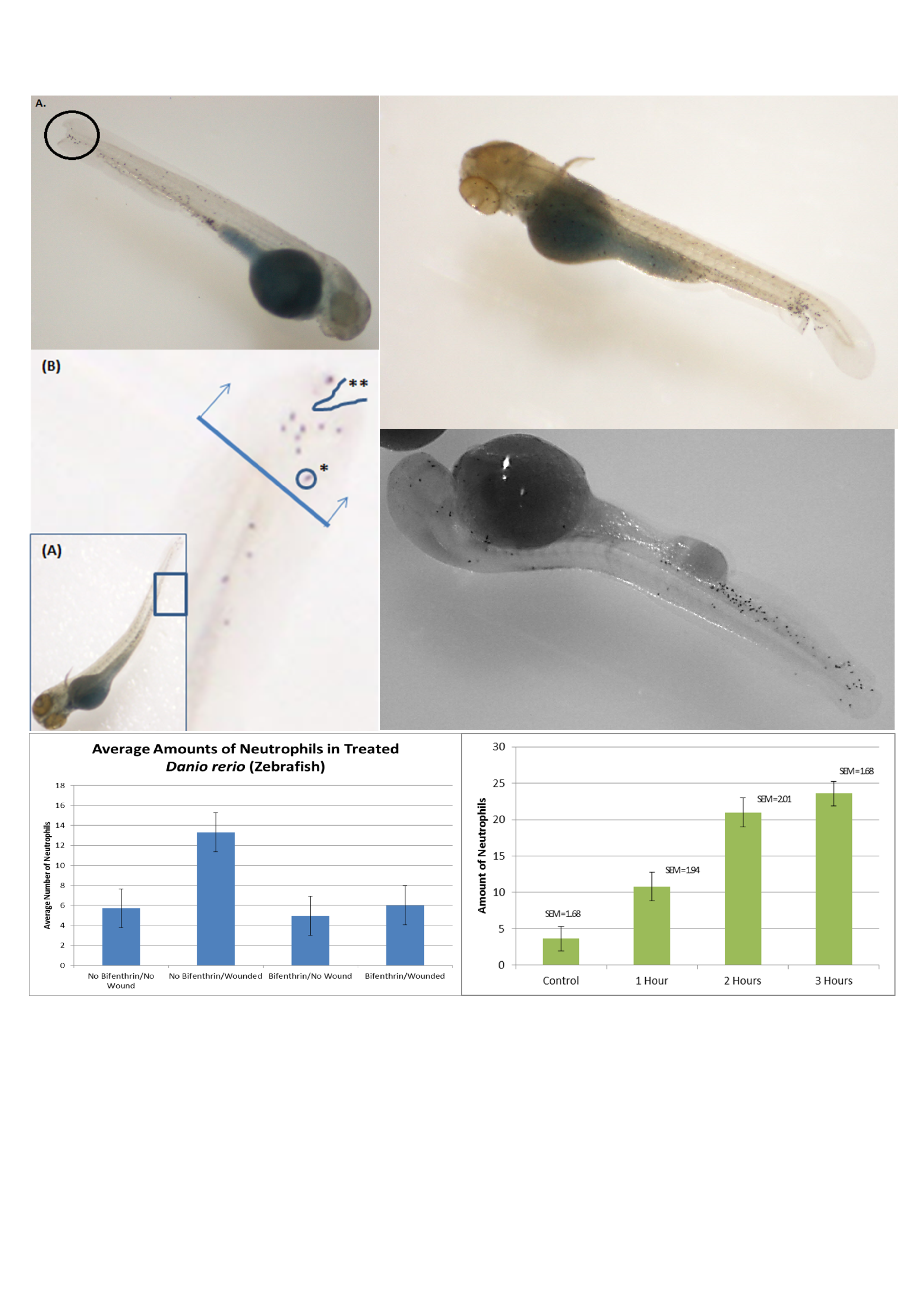
Figure 1. Student-created figures of their experimental data. Students took photos of a zebrafish embryo to create a figure for their lab report showing typical neutrophil response to a wound and pointing out the area near the wound where the neutrophils were counted. Quantitative data of number of neutrophils migrating in each zebrafish was also collected and made into a graph; in this example the variables were completely student-generated, toxin treatment or the time needed for neutrophils to respond, rather than being specifically directed to examine the cytoskeleton function.
The students catch on very quickly, but they are a little hesitant at first as to what is a neutrophil because of how small the cells appear even at the highest setting on a typical stereomicroscope. It might be possible to visualize the embryos on a compound microscope to see the cells at higher magnification, but due to the embryos being in liquid it would probably need to be an inverted microscope. This has not been tested. Students also typically need some advice to define the "area around the wound." I encourage them to define this however they feel is appropriate, but to remember that every embryo they evaluate - including ones that were not wounded - needs to have that same size and location of area considered for counting. Typically, non-wounded zebrafish do not have any neutrophils in the tail fin area, unless there has been accidental damage of the fish. The tiny bit of detergent added to the buffer is very important during this last counting step to facilitate moving the embryos around in the dish to obtain a good view of the fin for counting. The neutrophils should be counted directly after the staining process is complete. I have not tested counting the cells a day or two later, but do know that waiting a full week to count the neutrophils does not work; the stain faded and the cells could not be counted.
ASSESSMENT
The data from this lab are quantitative (each fish embryo has a number of neutrophils that migrated to the area of the wound) and by considering each zebrafish embryo a replicate statistical analysis can be done by the students (ANOVA or t-test, depending on the number of groups). The fact that each fish is not exactly its own completely independent experiment should be pointed out to the students. As there is not enough time in the semester to fully repeat the lab several times for statistical analysis of the data, this assumption can give students practice with statistics on data they have collected in a cell biology lab.
The assessment for the lab is typically a full laboratory write-up in the style of a scientific paper with an introduction, methods, results (including figures with legends), and discussion. Additionally, an essay question on the exam assesses their general knowledge of the function of the cytoskeletal proteins. Students were asked to complete a survey about their opinions six months after the course ended. (For details on all assessment, see Supporting File S5: Evaluations and Rubric) However, these assessments can be modified to fit the specific learning goals, the focus of the lab course, or the specific writing skills being taught during the course unit in which this inquiry lab is performed.
TEACHING DISCUSSION
The goal of this laboratory experiment was to give students an insight into the molecular mechanisms of cell migration with a hands-on, visual lab. It also offers an opportunity for the students to work on developing hypotheses and their experimental design skills. The scope of the experimental design possibilities can be either limited to questions involving how the components of the cytoskeleton affect cell migration in the zebrafish or be broadened to allow the students freedom to read the literature and design any experiment to investigate a factor that might affect cell migration. This flexibility means the lab can be adjusted to fit the learning goals of the class. I observed that the students were able to write accurately about cell migration in their laboratory papers, suggesting that the lab helped them understand the process.
To investigate student perception of their own learning, students were asked to complete a survey six months after the class ended. When given a list of all the inquiry labs done that semester and no indication of which lab I was especially interested in learning about, 9 of the 10 students responding to the survey picked the zebrafish cell migration lab as the one they remembered the best (approximately half the class responded to the survey, though some students had graduated and weren't able to be contacted). Additionally, 8 of 10 students picked it out of the list as their favorite lab. Later on a different page of the electronic "SurveyMonkey" survey (www.surveymonkey.com) (see Supporting File S6: Student Opinion Survey), 9 of 10 students said the zebrafish cell migration lab was either "very useful" or "extremely useful" for learning the concept of cell migration. Students were also asked to select multiple words from a list if they agreed that they pertained to the zebrafish cell migration lab, and "memorable," "challenging," and "fun" were some of the chosen words (Figure 2).
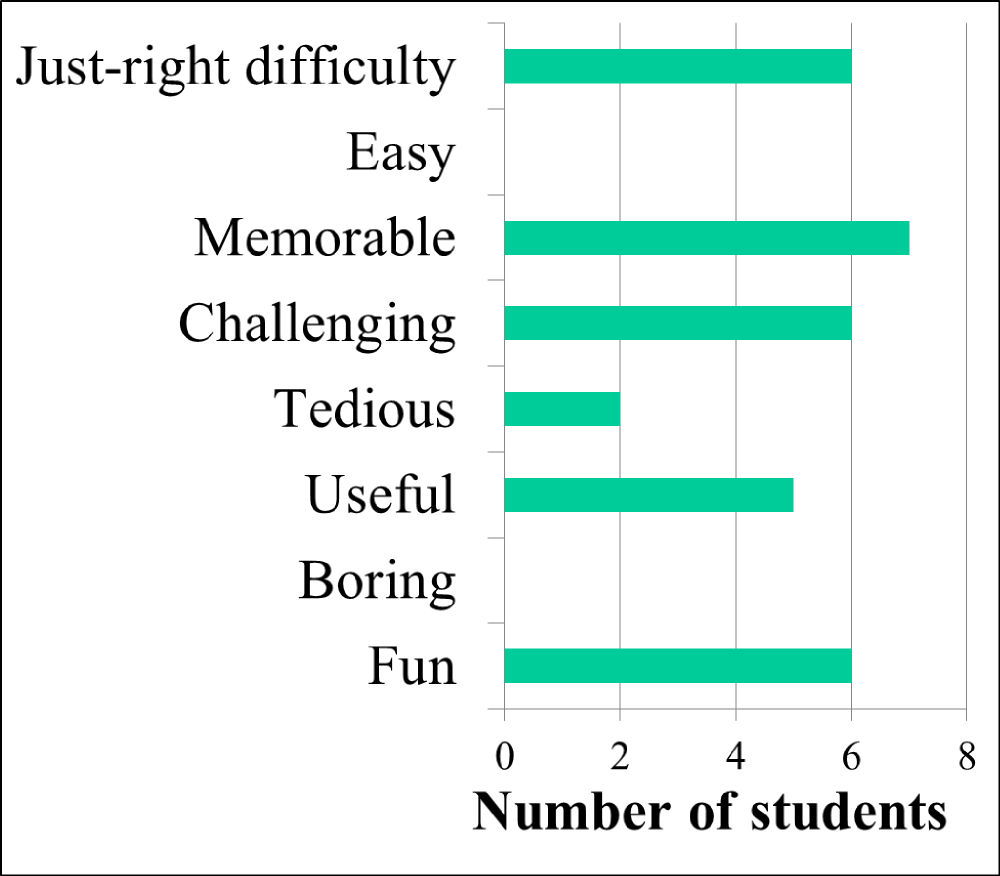
Figure 2. Students find the lab memorable - not easy or boring. Students were asked if various words described the zebrafish cell migration lab they had performed about six months prior. Ten students responded and were allowed to choose as many of the words as they thought applied to the lab.
While the experiment was originally designed for two lab sessions that each meet for 3 hours at a time, it could also be adapted to fit into two lab sessions that each meet for 2 hours by shortening incubation times, asking students to come back to transfer embryos to formaldehyde, or having the instructor or a teaching assistant do the last step later after the lab session. Broadening the focus from cytoskeletal proteins to other aspects that might affect cell migration or the function of the immune system allows the lab to possibly be adapted to fit other types of classes, not just a cell biology class. An introductory biology lab could adapt the lab to have the primary focus put on allowing the students to develop their own hypotheses, without being quite as concerned as to the mechanistic connections between the treatment and cell migration. When given more flexibility, I have seen students very excited about trying to figure out if vitamin C, alcohol, or high temperatures (mimicking fever) can affect the immune system because of the possible connections to their own lives. Alternatively, a class could dive even deeper into the mechanisms of signaling and migration because of the availability of inhibitors for cellular signaling molecules.
I typically do this lab with two lab sections of 15-18 students each. Scaling up to more students in a lab section would be possible; the hardest part would be that the instructor often needs to instruct individual groups on the fin wounding. Having a teaching assistant to assist with that instruction would be useful if there were more students in a lab section. A greater number of lab sections would also be possible, with the real limiting factor being the number of zebrafish embryos your zebrafish facility is able to produce. Also note that because the age of the embryos is important, if the lab sections meet on different days of the week, separate batches of embryos will likely be needed for each lab section.
This basic inquiry lab offers students a chance to use a model organism of growing importance in cell biology to study an active topic - cells moving and what proteins are necessary for that process - in an inexpensive way. The data that are obtained (the graph showing averages +/- standard error of the mean for the treatments, plus an additional figure showing a picture of an example zebrafish with neutrophils at the wound site, if technology permits) allow students practice with statistical analysis of data. With small alterations the protocol can be adapted for other types of classes and become either a more open-ended exploration of the immune system or a more focused investigation into the mechanisms of cell migration. Students thought the lab was helpful in their learning and memorable.
SUPPORTING MATERIALS
- S1: Inexpensive Cell Migration: Movie of neutrophils migrating in fish fin (FishWound.avi)
- S2: Inexpensive Cell Migration: Pre-lab presentation slides (Pre-lab presentation slides.pptx)
- S3: Inexpensive Cell Migration: Student handout (Cell Migration in Zebrafish handout.docx)
- S4: Inexpensive Cell Migration: Experimental Protocol Notes (Experimental Protocol Notes.docx)
- S5: Inexpensive Cell Migration: Evaluations and Rubric (Evaluations and Rubric.docx)
- S6: Inexpensive Cell Migration: Student Opinion Survey (Student Opinion Survey.docx)
ACKNOWLEDGMENTS
I would like to thank Anna Huttenlocher for getting me excited about zebrafish and providing the lab and equipment where the movie of the neutrophil migration in the zebrafish fin was taken. I would also like to thank Benjamin J. Perrin for allowing me to share his movie of the neutrophil migration in the zebrafish fin and for thoughtful discussions about cell biology lab ideas. And finally, I would like to thank my colleagues at Loras College, all the students who did this lab exercise over the years, and those who helped me take care of the zebrafish.
References
- Handelsman J, Miller S, Pfund C. 2007. Scientific Teaching. New York, NY:W.H. Freeman.
- Kolaczkowska E, Kubes P. 2013. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13: 159-175.
- Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. 2006. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol 80: 1281-1288.
- Nourshargh S, Renshaw SA, Imhof BA. 2016. Reverse Migration of Neutrophils: Where, When, How, and Why? Trends Immunol 37: 273-286.
- Langereis JD. 2013. Neutrophil integrin affinity regulation in adhesion, migration, and bacterial clearance. Cell Adh Migr 7: 476-481.
- Mocsai A, Walzog B, Lowell CA. 2015. Intracellular signaling during neutrophil recruitment. Cardiovasc Res 107: 373-385.
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. 2003. Cell Migration: integrating signals from front to back. Science 302: 1704-1709.
- Niggli V. 2003. Microtubule-disruption-induced and chemotactic-peptide-induced migration of human neutrophils: implications for differential sets of signaling pathways. J Cell Sci 116: 813-822.
- Yoo SK, Lam PY, Eichelberg MR, Zasadil L, Bement WM, Huttenlocher A. 2012. The role of microtubules in neutrophil polarity and migration in live zebrafish. J Cell Sci 125: 5702-5710.
- Loynes CA, Martin JS, Robertson A, Trushell DM, Ingham PW, Whyte MKB, Renshaw SA. 2010. Pivotal Advance: Pharmacological manipulation of inflammation resolution during spontaneously resolving tissue neutrophilia in the zebrafish. J Leukoc Biol 87: 203-212.
- Sheean HL, Storey GW. 1947. An improved method of staining leucocyte granules with Sudan Black B. J Pathol Bacteriol 59:336-342.
- Lam P, Yoo SK, Green JM, Huttenlocher A. 2012. The SH2-domain-containing inositol 5-phosphatase (SHIP) limits the motility of neutrophils and their recruitment to wounds in zebrafish. J Cell Sci 125: 4973-4978.
- Xu X, Jin T. 2015. The novel functions of the PLC/PKC/PKD signaling axis in G Protein-Coupled Receptor-mediated chemotaxis of neutrophils. J Immunol Res 2015:817604.
- Powell DR, Huttenlocher A. 2016. Neutrophils in the tumor microenvironment. Trends Immunol 37: 41-52.
- Shelef MA, Tauzin S, Huttenlocher A. 2013. Neutrophil migration: moving from zebrafish models to human autoimmunity. Immunol Rev 256: 269-281.
- McKeown KA, Downes GB, Hutson LD. 2009. Modular laboratory exercises to analyze the development of zebrafish motor behavior. Zebrafish 6: 179-185.
- Ross AW, Bonner J. 2012. Activation of Wnt signaling using Lithium Chloride: Inquiry-based undergraduate laboratory exercises. Zebrafish 9: 220-225.
- Cheng JC, Miller AL, Webb SE. 2004. Organization and function of microfilaments during late epiboly in zebrafish embryos. Dev. Dyn. 231:313-323.
- Brand M, Granato M, N?sslein-Volhard C. 2002. Keeping and raising zebrafish, p.22. In N?sslein-Volhard C and Dahm R (eds.), Zebrafish. Oxford University Press, New York.
- Welf ES, Haugh JM. 2011. Signaling pathways that control cell migration: models and analysis. Wiley Interdiscip Rev Syst Biol Med 3:231-240.
Article Files
Login to access supporting documents
Inexpensive Cell Migration Inquiry Lab using Zebrafish(PDF | 705 KB)
S1- Inexpensive Cell Migration- Movie of neutrophils migrating in fish fin.avi(AVI | 1011 KB)
S2- Inexpensive Cell Migration- Pre-lab presentation slides.pptx(PPTX | 585 KB)
S3- Inexpensive Cell Migration- Student handout.docx(DOCX | 22 KB)
S4- Inexpensive Cell Migration- Experimental Protocol Notes.docx(DOCX | 20 KB)
S5- Evaluations and Rubric.docx(DOCX | 25 KB)
S6- Student Opinion Survey.docx(DOCX | 105 KB)
- License terms

Comments
Comments
There are no comments on this resource.