A new approach to course-based research using a hermit crab-hydrozoan symbiosis
Published online:
Abstract
There are few feasible models for marine-focused inquiry laboratory activities, a notable shortcoming for instructors seeking to engage their students in meaningful, course-based research experiences (CUREs). We describe a multi-week CURE that investigates the symbiosis between hermit crabs and the hydrozoan Hydractinia spp. Although much is known about hermit crab biology, ecology, and behavior, little is known about Hydractinia, and less is known about the relationship between the two symbionts. Given their small size, low cost, and relative ease of maintenance, colonized hermit crabs may be useful subjects for student-driven research projects. We discuss our experiences with this system and offer adopters a suite of resources for in-lab implementation.
Citation
Galush, T., Mazur, C., and Cotner, S. 2017. A new approach to course-based research using a hermit crab-hydrozoan symbiosis. CourseSource. https://doi.org/10.24918/cs.2017.2Society Learning Goals
Science Process Skills
- Process of Science
- Locate, interpret, and evaluate scientific information and primary literature
- Pose testable questions and hypotheses to address gaps in knowledge
- Plan, evaluate, and implement scientific investigations
- Interpret, evaluate, and draw conclusions from data
- Construct explanations and make evidence-based arguments about the natural world
- Address novel questions through authentic research experiences
- Modeling/ Developing and Using Models
- Recognize the important roles that scientific models, of many different types (conceptual, mathematical, physical, etc.), play in predicting and communicating biological phenomena
- Make inferences and solve problems using models and simulations
- Build and evaluate models of biological systems
- Quantitative Reasoning/ Using Mathematics and Computational Thinking
- Apply the tools of graphing, statistics, and data science to analyze biological data
- Communication and Collaboration
- Share ideas, data, and findings with others clearly and accurately
Lesson Learning Goals
Students will:- develop their own meaningful scientific research project, from defining a question, through communicating results in light of a stated hypothesis.
- understand the complexity of real-world symbioses, along with the difficulties associated with assigning fixed designators to fluid relationships (i.e., a "commensalism" can morph into "mutualism" or "parasitism" under certain environmental conditions).
- understand how scientific findings are communicated, from small-group presentations through peer-reviewed literature.
Lesson Learning Objectives
Students will be able to:- define different types of symbiotic interactions, with specific examples.
- summarize and critically evaluate contemporary primary literature relevant to ecological symbioses, in particular that between hermit crabs and Hydractinia spp.
- articulate a question, based on observations of a natural phenomenon (in this example, the hermit crab-Hydractinia interaction).
- articulate a testable hypothesis, based on their own observations and read of the literature.
- design appropriate experimental or observational studies to address their hypotheses.
- collect and interpret data in light of their hypotheses.
- problem-solve and troubleshoot issues that arise during their experiment.
- communicate scientific results, both orally and in written form.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
COURSE-BASED UNDERGRADUATE RESEARCH EXPERIENCES
The Vision and Change in Undergraduate Biology Education report articulates several priorities for educating future scientists, among them the inclusion of authentic research experiences into biology education (1). This recommendation rests on the findings of numerous investigators, including evidence that authentic research experiences contribute to increased student engagement (2), understanding of scientific processes (3), long-term learning (4), and interest in science as a career (5-7). In response to these data, inquiry-based pedagogy, as defined by the National Research Council (NRC; (8)), has been widely implemented in undergraduate collegiate education. Course-based undergraduate research experiences (CUREs) are one manifestation of inquiry-based pedagogy. CURES directly engage all students enrolled in a science course in the process of discovery by integrating lecture topics with real-time research to effectively meet the objectives outlined by the NSF, AAAS, and NRC (1,8).
Excellent models for CURES exist (see (9), and references therein), including suggestions for student-driven research projects in cell biology (10), molecular biology (11), microbial ecology (12), and experimental evolution (13). In 2012, Singer et. al developed an inquiry-based module (i.e. CURE) that allows students to investigate biologically relevant questions regarding the genomics of the marine cnidarian Aiptasia pallida (14,15). Studies using A. pallida have become increasingly common. The study system was awarded the prize in Inquiry-Based Instruction by the AAAS in 2012 in recognition of the module's ability to increase science literacy by converging practical research skills with knowledge of biological processes (16). Apart from genomic research using A. pallida, we are not aware of other CUREs incorporating marine biology. This shortcoming is notable, given student interest in marine biology and the wealth of critical, emerging issues that intersect with marine systems (e.g., climate change, sustainable fisheries, bioprospecting).
We describe a multi-week laboratory research project that engages students in meaningful research and is novel and engaging. Specifically, students discuss contemporary research literature on the relationship between hermit crabs and the cnidarian hydroid Hydractinia spp., then develop and test hypotheses related to this symbiosis and/or other aspects of cnidarian and crustacean biology.
THE SYMBIOTIC PARTNERS
An understudied symbiosis readily observed in coastal waters of the North Atlantic is that of hermit crabs of the family Paguridae and hydroid cnidarians in the genus Hydractinia. Hermit crabs are marine crustaceans that inhabit, compete for, and rely on gastropod shells for protection and growth (17). Hydractinia are marine Cnidarians that colonize gastropod shells (and therefore, hermit crab shells). This colonization of hermit crab shells leads to understudied interspecies interactions.
Hydractinia, including Hydractinia echinata and Hydractinia symbiolongicarpus, are colonial marine hydrozoans found in the shallow ocean waters of the North Atlantic (18). Hydractinia predominantly colonize hermit crab shells of the family Paguridae, but have infrequently been observed colonizing bivalve shells, stones, and docks. Hydractinia are broadcast spawners with daily gamete release that is regulated by photoperiod (19). Embryogenesis leads to a free-swimming planula larva. Using unknown chemical cues from a small subset of marine bacteria that colonize the shells of gastropods, the planula larvae settle and undergo metamorphosis via stem-cell differentiation to become mature colonies (cover image and Figure 1; (18,20)). Mature Hydractinia colonies exhibit polyp polymorphism. All polyps in a single colony arise via asexual reproduction (e.g. budding) and individual, genetically identical polyps differentiate into gastrozooid (feeding), dactlyozooid and tentaculozooid (defense), and gonozooid (reproductive) polyps (18).
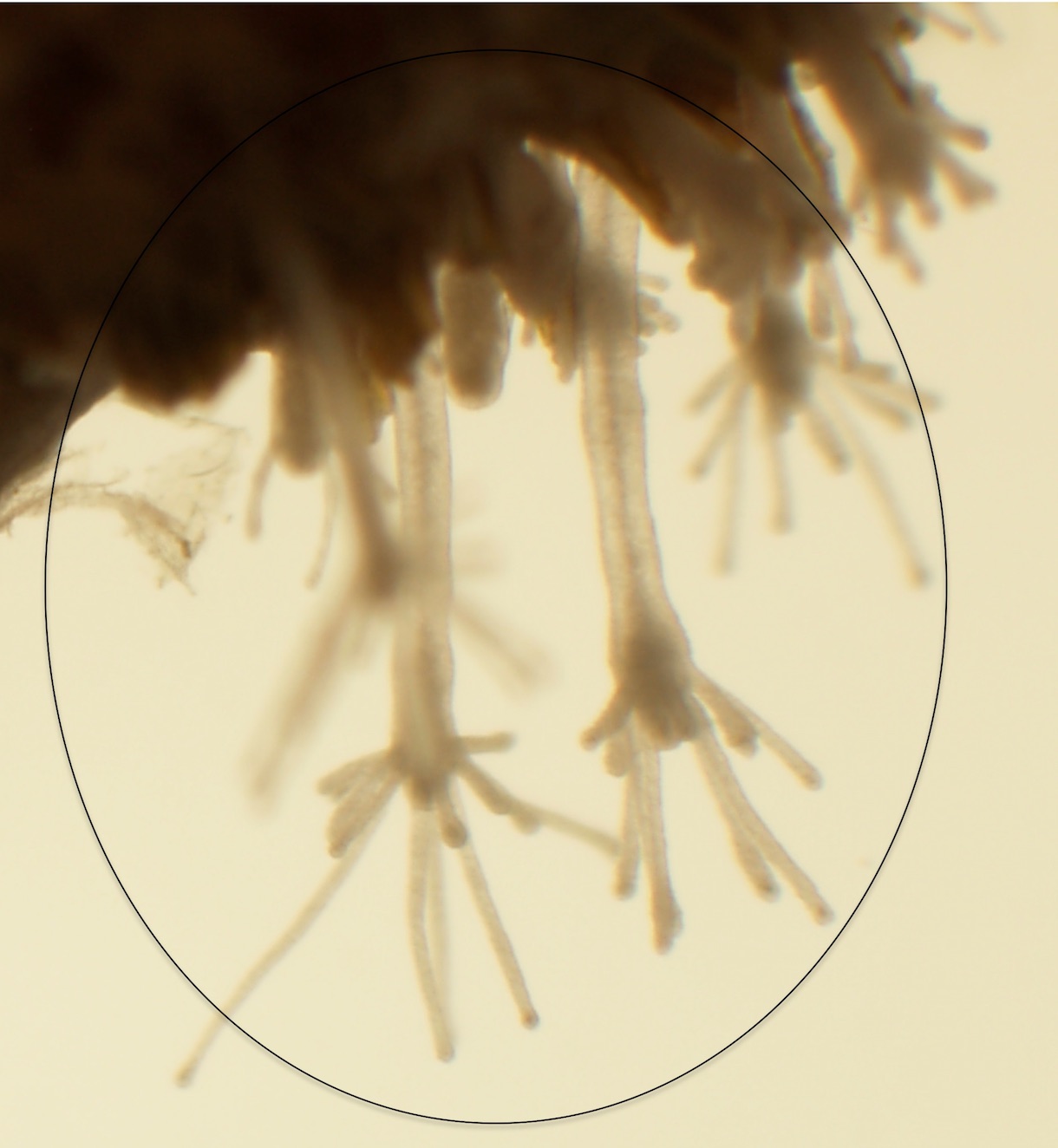
Figure 1. Hydrozoan polyps on a hermit-crab shell (photo by Tiffany Galush)
Numerous aspects of the Hydractinia life cycle make it practical and provocative for student-led research, including the ability to: control reproduction with light exposure, induce metamorphosis with common laboratory chemicals (e.g., cesium chloride), propagate the organism on multiple surfaces including petri dishes and glass slides, and observe the entire life cycle in two to three months (18). These features make Hydractinia an interesting model organism to serve the diverse interests of emerging scientists in the teaching laboratory.
Hydractinia has been established as a model organism to explore allorecognition (21), genetics (22), developmental biology (23), and cnidarian ecology (24). Such studies focus solely on analysis of Hydractinia, but our focus is the understudied Hydractinia - hermit crab symbiosis. The life cycle of Hydractinia depends on marine bacteria, notably Pseudoalteromonas espejiana, that colonize the shells occupied by Paguroid hermit crabs (20). Once colonized, the occupied hermit crab shells become the locus for recruitment, development, and reproduction of Hydractinia, initiating a complex symbiosis between crab and cnidarian. At high population densities of Hydractinia, up to 42% of hermit crab shells can be the home to multiple, genetically distinct colonies (21), leading to both inter- and intraspecific interactions. These intricate interactions complicate the characterization of the hermit crab-Hydractinia symbiosis.
The benefits that Hydractinia receive from their symbiont are well established, including mobility, access to food scraps, and congregation of conspecifics to facilitate reproduction (25); however, contradicting conclusions have been drawn regarding the advantages that the Hydractinia colony might provide the hermit crab. For example, Brooks and Mariscal found that hermit crabs were protected by Hydractinia from predation by stone crabs, but experiments performed by Bach et al. found that colonized hermit crabs were not protected from stone crab predation (26,27). Similarly, Buckley and Ebersole (28) determined that colonization does not protect hermit crabs from blue crab predation. Aside from the possible reduction of predation, Hydractinia also protect hermit crabs from harmful endosymbionts (28). Costs incurred by colonized hermit crabs may include decreased fitness in the form of reduced clutch size (29). The limited number of experiments that examine the associated costs/benefits of this symbiosis, the conflicting conclusions from published experiments, and the stochastic nature of marine systems offer a foundation for students to extend scientific knowledge by (a) engaging in scientific discourse and (b) testing their own hypotheses.
INTENDED AUDIENCE
This CURE, referred to hereafter as the Hydractina-Pagurus CURE (HP-CURE), was designed for undergraduate students with an interest in marine biology; the only prerequisite was a college-level biology course with laboratory experience. The average class size across the three semesters in which we implemented the CURE was 20 students; these students came from a range of majors, with an emphasis on those studying biology, physiology, and animal sciences.
REQUIRED LEARNING TIME
The HP-CURE extends through a full fifteen-week semester, as part of a Marine Animal Diversity Laboratory (BIOL 2007) at the University of Minnesota. This course meets for three hours a week, with students spending ~1.5 hours a week on projects. Depending on project demands, students may need to perform research outside of the scheduled class period. On average, projects can be completed with 23 hours in the lab and 5-10 hours outside the lab.
PRE-REQUISITE STUDENT KNOWLEDGE
Our prerequisite is an introductory biology course with an accompanying lab. Concepts and skills with which students should be familiar include taxonomy, types of symbioses (e.g. mutualism, commensalism, and parasitism), reading scientific literature, and writing lab reports. The laboratory instructor provides introductory materials to familiarize students with Hydractinia and to start the process of scientific inquiry.
SCIENTIFIC TEACHING THEMES
ACTIVE LEARNING
Students are active participants in all aspects of their research projects. Students set up and maintain their own aquaria, read and discuss assigned research literature, supplement assigned literature with their own literature searches, work collaboratively in small groups to design multi-week experiments prompted by their own interests, collect and analyze data, and present their results to their classmates.
ASSESSMENT
Formative assessments include: three jigsaw assignments in which students read and discuss assigned scientific articles; scaffolded reporting in which students submit their lab reports in stages (project ideas, introduction, methods, results), and as initial drafts for review; and weekly check-ins with the laboratory instructor. Summative assessments include the final written laboratory report and the final in-class presentation. Group members also evaluated contributions of their group members using a short "group-member evaluation form" distributed online during the last week of class.
INCLUSIVE TEACHING
Beyond the simple prerequisite of an introductory-biology course, any student can participate in the activities we describe. We structure our student groups around common interests and offer several opportunities for individuals to "play to their strengths" during the collaboration. In the final group-member evaluation, individuals are encouraged to consider all the ways that each member contributed to the effort, from finding key articles, to imagining new ways of designing experiments, to developing a particularly engaging final presentation.
LESSON PLAN AND GRADING
GRADING
This project constitutes 18% of the total course grade. The draft of the paper is 5%, the final paper is 7.5% and the project presentation is 2% of the grade. Jigsaw activities are worth 3.5% of the final grade. Other aspects of the course grade include exams (48%) and various assignments (34%), including many dissections, unrelated to the project.
WEEKS 1-3: AN INTRODUCTION TO HYDRACTINIA
Students met once a week with the instructor in a 3-hour class period, with ~1.5 hours devoted to project work. During the first 3 weeks of the course, students assembled one small saltwater aquaria (10 gallons) per group and read instructor-selected scientific literature about the experimental system in a "jigsaw" format.
AQUARIUM SET-UP AND INITIAL MAINTENANCE (SEE SUPPORTING FILE 1 FOR DETAILS)
Student groups established saltwater environments within the 10 gallon aquariums. During the first week of class, students assembled the aquaria, including establishing a heater, filter, and lighting system. Students were given a substrate of sand; students then add live rock and artificial saltwater to the aquarium. Each student group generally required only one aquarium. During the second and third week of the semester, students recorded baseline data on water-quality parameters (using test kits that measure ammonia, nitrate and nitrite, phosphate, and pH), salinity in terms of specific gravity (using a hydrometer or refractometer), and temperature. Students continued to measure and record water parameters throughout the semester.
LITERATURE DISCUSSION
During the first week of the semester, students were required to come to lab prepared to discuss the article "The hydroid Hydractinia: a versatile, informative cnidarian representative" by Uri Frank, Thomas Leitz and Werner A. Muller (18). Students completed a 5-point Pre-Reading Guide that leads them through the main concepts of the article (see Supporting File S2). Students met in randomly assigned reading groups of four during class to discuss the article. Following the group discussions, the instructor led the lab in a general discussion of the main concepts. Then, students discussed their research interests and formed research teams of two to four students, based on common research interests. For a class of 20 students, we typically had five to six research teams. The research teams discussed potential project ideas. Formal research questions are submitted during the third week of class. The research teams remained together for the entire semester.
The second and third weeks of lab continue the literature exploration, but in a "jigsaw" format. A week before the planned discussion, we randomly assigned students to read one of four papers. As literature regarding Hydractinia is limited, this project integrates a set of eight scientific articles to explore the biology and life history of Hydractinia (see Supporting File S3). We devoted time during class for small and large group discussion of the articles, to clarify questions students may have. Students are required to complete a Scientific Paper Reading Guide for their assigned article (see Supporting File S4), use it during group discussions, and submit it for up to five points toward the final project grade (each five-point assignment is worth 1.167% of the final grade). Initially, students who have read the same paper met for ~15 minutes to deconstruct their article. This discussion assured that students who have read an assigned article agree on the authors' hypothesis, methods, results, and conclusions. In addition, students had the opportunity to resolve questions that arose while they were reading the article with both their peers and the instructor, who circulated among the groups and discussed each article with the students, ensuring that all questions and queries about the article had been addressed. This student-student and student-instructor interaction ensured that any misconceptions or misunderstandings were clarified.
Next, students assorted into "mixed" groups, with each group having at least one individual prepared to discuss each of the four articles. Over the next 20 minutes, each student in the group shared information about his or her article with others in the group. This jigsaw method allowed students to discuss questions, methods, and conclusions from four different articles while only requiring detailed preparation of one paper.
By the third week of class, students had explored nine different articles focused on Hydractinia and the Hydractinia-hermit crab symbiosis. At this point, students worked with their research team members to submit two formal project ideas, for which they could earn up to five points towards the final grade. Students generally submitted project ideas in the form of a question. Although many students opted to explore the Hydractinia-hermit crab symbiosis, the instructors were open to other research project ideas involving Hydractinia. Instructors reviewed the proposed project ideas and gave feedback to the students during the following lab, by holding private discussions with each group. Approved projects typically were low-cost, required minimal purchase of supplies, could be completed within the timeline of the class, and were creative. Research teams then chose between their approved project ideas, selecting a final question to explore. The teams submitted a "materials needed" list to the instructors. Materials needed can vary from non-toxic paint to live (invertebrate) predators. We generally limited specialized project supplies to $35-40 per group; however, since most supplies needed for the research were already available in the laboratory, the actual cost per project was much lower. Once materials had been gathered or purchased (usually by the fourth or fifth week of class), students began their projects.
WEEKS 4-12: PROJECT WORK TIME
In the next eight to nine weeks, students spent one to two hours per week working on their projects in class and were encouraged to spend time outside of class working on their projects as needed. The laboratory was accessible to students on weekdays during regular business hours (8AM-5PM) and students were encouraged to make special arrangements with instructors and other laboratory faculty to access the laboratory outside of these hours if essential for the project. Students generally worked unsupervised in the laboratory but had access to nearby laboratory staff. Each student project was different, but ultimately focused on answering a question related to Hydractinia, hermit crabs, or, ideally, the symbiosis between the two. Student groups submitted one final, collaborative paper and prepared an in-class presentation to be delivered during the final weeks of class. The rubric for the paper and presentation is available in Supporting File S5.
Initially, each research team received five Hydractinia-colonized gastropod shells inhabited by hermit crabs during the fourth week of class. We ordered the colonized shells from Gulf Specimen Marine Laboratories (Panacea, FL) two to three weeks in advance and had them delivered on the day of the Week 4 lab meeting. Students used a drip protocol to acclimate their crabs to the aquaria during project work time (Supporting File S1). Student experiments began the week following acclimation, to allow organisms to recover from the transportation and acclimate to their new conditions.
We also ordered additional Hydractinia colonies (approximately 10), which were acclimated to an additional 30-gallon aquarium used as a reservoir. This reservoir was maintained similarly to student run aquaria by laboratory staff year-round. The organisms in the reservoir can provide additional experimental organisms as the need arises, or replace organism losses.
In week five, students began their experiment. For the duration of the experiment, student groups measured water-quality parameters weekly. All organisms were fed three times weekly. Feeding schedules can be designed to better suit the instructor's schedule, but we used a Monday-Wednesday-Thursday schedule. Because lab met on Thursdays, students were responsible for the Thursday feeding and laboratory staff completed the other two feedings. Hermit crabs received Hikari Crab Cuisine Rapidly Sinking Sticks for Bottom Feeders & Crustaceans (roughly 2-3 pellets per crab, placed in a shallow petri dish within the aquarium) and Hydractinia received either live brine shrimp, cultivated in house using the San Francisco Bay hatchery system, or a mix of Reef Nutrition brand oyster eggs and rotifers. Students aimed for ammonia, nitrate, nitrite, and phosphates to be 0 ppm, pH to be between 7.8-8.4, and specific gravity to be 1.018 ppm-1.025 ppm. Along with weekly water-quality testing, students also performed weekly water changes. Students generally removed 15% of the water in the aquarium and added an equivalent amount of cured saltwater, depending on the outcome of the water-quality tests (i.e., high nitrates, nitrites, and/or phosphates warrant a larger water change). If student aquaria experienced high ammonia levels, a water change was not performed; instead, the ammonia-binding agent API Ammo Lock Ammonia Remover Aquarium Water Conditioner was added to the water per the directions on the bottle (see Supporting File S1). Laboratory staff assured that water levels are kept consistent and topped off aquaria with RO water or saltwater throughout the week to account for evaporation.
To help students stay on track with writing their final papers, groups periodically submitted draft versions of the key parts of their work including (1) the project ideas, (2) introduction, (3) methods, and (4) results and discussion. Each draft submission contributes 1.25% of the final grade in the course.
WEEKS 13-15: PROJECT PRESENTATION AND PAPER SUBMISSIONS
Student projects concluded during the 13th week of the semester. Although students could collect data through the 13th week of the semester, some student projects naturally ended before this time. At the culmination of their project, each student group acclimated (via the drip method) their crabs and colonies into the 30-gallon reservoir aquarium and cleaned out their aquaria for students to use in the upcoming semester. Crabs that survived to the next semester are used in new student projects.
Student group presentations took place during Weeks 14 and 15, which provided sufficient time for five to six presentations. Each presentation was ~15 minutes long, with four presentations given each week. Students commonly used PowerPoint slides or Prezi to describe their project.
During Week 14, each student group collaborated to peer review the research paper of one or two other teams. Students used the project rubric (Supporting File S5) to guide their peer review. Final papers, which the student teams edited in response to the peer reviews, were due on the day of the final exam.
During week 15, students completed a team-member evaluation that contributed to class participation points (Supporting File S6). Students rated themselves and their group members based on the peer review form using a Likert-scale response system ranging from 1 (e.g. extremely unsatisfied) to 5 (extremely satisfied). Individual scores of all questions were averaged and any points lost during peer evaluation were subtracted from the student's final participation score. To earn all team participation points, students had to have an average peer rating of 4 or higher. As encouragement for students to complete the short peer evaluation, any student who did not complete the peer evaluation lost 5 points (out of a total of 30 participation points) from their course grade. The complete project timeline is described in Supporting File S3.
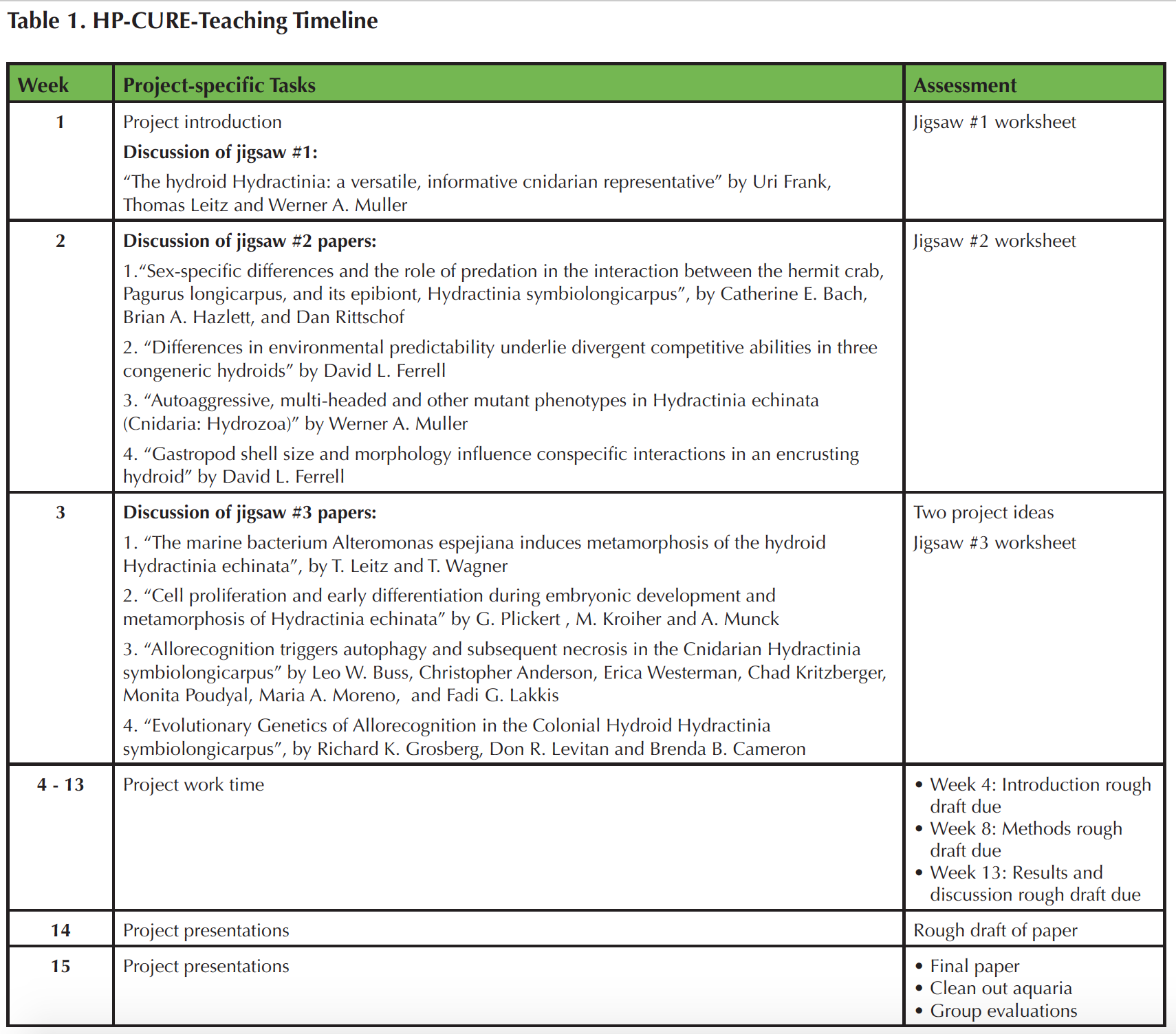
Table 1. HP-CURE-Teaching Timeline.
TEACHING DISCUSSION
STUDENT PROJECTS
Unlike many CUREs, the HP-CURE projects were driven by the students themselves, rather than a faculty research interest. Students created the questions/hypotheses, developed the methods, performed the experiments, troubleshot and problem-solved, and analyzed their own results. Examples of student projects are provided below:
Project 1: Do Hydractinia prefer to grow on occupied or unoccupied shells?
- Hypothesis: Hydractinia will grow better on occupied shells
- Methods: 4 Hydractinia-colonized shells (2 occupied by a hermit crab and 2 unoccupied) were placed in an aquarium. We ordered occupied and unoccupied shells from the supplier to meet the research team's needs. Hydractinia colony growth and expansion was observed over several weeks.
- Results: Hydractinia preferred to grow on occupied shells. Hydractinia on unoccupied shells succumbed to algal over-growth.
Project 2: Nudibranch preference for Hydractinia on hermit crab-occupied versus unoccupied shells.
- Hypothesis: Nudibranchs will prefer to prey on Hydractinia growing on unoccupied shells
- Methods: Various unspecified nudibranchs obtained from Gulf Specimen were isolated into mesh coffee filters suspended in marine aquaria. Colonized shells (unoccupied or occupied by a hermit crab) were placed inside of the coffee filter with the nudibranch. A GoPro was used to film Nudibranch predation patterns.
- Results: Nudibranchs do not have a preference for the crab-occupancy status of hermit crab shells colonized by Hydractinia, as they were observed to eat Hydractinia under both conditions.
Project 3: Predation by calico crabs on hermit crabs in Hydractinia-colonized and non-colonized shells occupied by hermit crabs.
- Hypothesis: Calico crabs will prefer to prey on hermit crabs with non-colonized shells
- Methods: Hermit crabs with bare shells and with Hydractinia-colonized shells were placed in aquaria with starved calico crabs. Calico crab predation patterns were observed.
- Results: Hydractinia does not deter calico crab predation on hermit crabs
LESSON EFFECTIVENESS
Based on feedback from student surveys and an end-of-term focus group, students who participated in the research projects were satisfied with the experience. Many reported feeling that they have been allowed to explore an authentic marine experience and appreciate that the activity wasn't guided by a textbook or lab manual. When many students first step into the lab, they have little experience in designing hypotheses and experiments. By the last day of class, students reported that they feel they have the skills to think critically and constructively about their own experimental results, as well as to effectively examine other student projects in a scientific and evidence-based manner. This claim is supported by student behavior during project presentations, when peers question the experimental methods and findings of other groups.
Students quickly became familiar with the Hydractinia-hermit crab symbiosis and can explain different types of symbiotic interactions. Through our weekly interactions with students, we saw that they use this expertise as they:
- articulated a testable hypothesis based on their own observations and their interpretations of the literature
- designed appropriate experimental or observational studies to address their hypotheses
- modified their experiments; thought critically about the data they are collecting; troubleshot when problems arise; collaborated with other student groups and the instructors
- communicated their findings orally and in writing.
At the time of their final submissions, the group's research paper (and presentations) were often above the rubric standards, indicating that students gained the specific science-process skills listed as "Learning Objectives."
STUDENT REACTIONS
Students reported that they "enjoy the idea of a semester long project" in a biology class. Most students (73%, n= 80) felt that a semester-long project is beneficial for a biology laboratory course because it introduces practical research skills that are unusual in most lower-level courses. Students stated that the projects "helped them learn how to run [their] own experiment, make knowledgeable conclusions from [their] results, and make compromises to methods when problems arose." Students also felt that it was satisfying to problem-solve when setbacks arise and they enjoyed sharing their data with their peers. Students appreciated the jigsaw activities, but they didn't love them: some students reported that having jigsaws for the first three weeks of the course is unnecessary for the development of project ideas. They also appreciated the opportunity to submit a group evaluation for each member of their group.
In contrast to their support of a semester-long project, student reactions to working with Hydractinia were mixed. The majority of students (73%; n=80) enjoyed working with an organism that is understudied and they liked the opportunity to collect and analyze their own data. Students also reported that they enjoyed working with symbionts and liked to think critically about what is going on between the two symbionts in this system. However, students found that at times the crabs were more interesting than the Hydractinia, possibly because detailed observation of Hydractinia requires a dissecting microscope. Student opinions of Hydractinia varied from "I love Hydractinia" to "the crabs were more interesting than the Hydractinia." Other students reported that "Hydractinia are hard to work with because of their size."
The biggest complaint students have about the HP-CURE is the timeline: only 40% (n= 80) of the students were satisfied with the timeline of the project. Many students suggested condensing the jigsaws into one or two weeks, allowing for more project time. Students said that submitting their project ideas on the third week of class did not give them enough time to collect data. However, students appreciated having a timeline for project drafts. They liked that each draft is "low stakes" and that the updates kept them on track with writing the paper throughout the semester. Students suggested assembling their aquaria with acclimated specimens at least two weeks prior to the first class. Overall, students endorsed "starting project work time earlier in the semester" so that they have more time to collect data, analyze results, and deal with problems that arise.
SUGGESTIONS FOR IMPROVEMENT
The student-driven HP-CURE in the Marine Animal Diversity course at the University of Minnesota interests students and provides them with opportunities to develop as scientists. These projects have become one of the highlights of the course. However, some aspects of the project could be modified to enhance the overall student experience. First, setting up the aquaria one month prior to the first class meeting would be ideal. For the first two weeks, each aquarium should hold saltwater with live rock and live sand as a substrate. A filter (described in Supporting File S1) should be placed in the aquarium and the water should be filtered and subjected to a 12-hour on/12-hour off light cycle. The last two weeks should involve the introduction and acclimation of the symbionts to the larger reservoir aquarium, so that they are ready for immediate use.
The timeline of the project could be modified as well. We are confident that the jigsaw activities are crucial to project development. Even though some students feel that three weeks of jigsaws are too many, it doesn't seem feasible to have students discuss all papers in fewer than three weeks. Therefore, we recommend continuing to carry out the jigsaw activities over a period of three weeks, but adding in a small amount of laboratory time during each class period. Perhaps students could perform aquarium maintenance and water quality testing during this time.
Finally, and critically, we have not yet been able to breed and maintain colonies of Hydractinia in the lab. As a result, we order colonized hermit crabs each semester for the student projects. Colonized crabs are not expensive, but the cost (at ~$14 per colony from Gulf Specimen suppliers) is not negligible, and our hope is to eventually breed our own colonies.
In conclusion, we continue to improve the HP-CURE experience by modifying our project timeline and supporting materials. We welcome collaborators interested in sharing ideas for curricular improvements, hydroid husbandry, and course-based undergraduate research projects using the Hydractinia-hermit crab symbiosis, or other marine organisms.
SUPPORTING MATERIALS
- S1. HP-CURE-Aquarium design and animal husbandry
- S2. HP-CURE-Pre-jigsaw reading guide
- S3. HP-CURE-HP-CURE Timeline
- S4. HP-CURE-Scientific paper reading guide
- S5. HP-CURE-Grading rubric for final paper and presentation
- S6. HP-CURE-Group-member evaluation form
ACKNOWLEDGMENTS
We're indebted to many colleagues near and far, but especially Adam Engelhardt, John Paul Kettinger, Missy Rudeen, Sandy Mand, and Alex Cramer.
References
- Brewer CA, Smith D. 2011. Vision and change in undergraduate biology education: a call to action. Am Assoc Adv Sci Wash DC.
- Hunter A-B, Laursen SL, Seymour E. 2007. Becoming a scientist: The role of undergraduate research in students' cognitive, personal, and professional development. Sci Educ 91:36-74.
- Seymour E, Hunter A-B, Laursen SL, DeAntoni T. 2004. Establishing the benefits of research experiences for undergraduates in the sciences: First findings from a three-year study. Sci Educ 88:493-534.
- Laursen S, Hunter A-B, Seymour E, Thiry H, Melton G. 2010. Undergraduate Research in the Sciences: Engaging Students in Real Science. John Wiley & Sons.
- Eagan MK, Hurtado S, Chang MJ, Garcia GA, Herrera FA, Garibay JC. 2013. Making a Difference in Science Education The Impact of Undergraduate Research Programs. Am Educ Res J 50:683-713.
- Lopatto D. 2007. Undergraduate Research Experiences Support Science Career Decisions and Active Learning. CBE Life Sci Educ 6:297-306.
- Russell SH, Hancock MP, McCullough J. 2007. Benefits of Undergraduate Research Experiences. Science 316:548-549.
- National Research Counsel. 2000. Inquiry and the National Science Education Standards: A Guide for Teaching and Learning. National Academies Press, Washington, D.C.
- Adams DJ. 2009. Current trends in laboratory class teaching in university bioscience programmes. Biosci Educ.
- Hurd DD. 2008. A microcosm of the biomedical research experience for upper-level undergraduates. CBE Life Sci Educ 7:210-219.
- Harrison M, Dunbar D, Ratmansky L, Boyd K, Lopatto D. 2011. Classroom-Based Science Research at the Introductory Level: Changes in Career Choices and Attitude. CBE-Life Sci Educ 10:279-286.
- Kloser MJ, Brownell SE, Chiariello NR, Fukami T. 2011. Integrating Teaching and Research in Undergraduate Biology Laboratory Education. PLoS Biol 9:e1001174.
- Ratcliff W, Raney A, Westreich S, Cotner S. BioOne Online Journals - A Novel Laboratory Activity for Teaching about the Evolution of Multicellularity.
- Banta LM, Crespi EJ, Nehm RH, Schwarz JA, Singer S, Manduca CA, Bush EC, Collins E, Constance CM, Dean D, Esteban D, Fox S, McDaris J, Paul CA, Quinan G, Raley-Susman KM, Smith ML, Wallace CS, Withers GS, Caporale L. 2012. Integrating Genomics Research throughout the Undergraduate Curriculum: A Collection of Inquiry-Based Genomics Lab Modules. CBE-Life Sci Educ 11:203-208.
- Singer SR, Schwarz JA, Manduca CA, Fox SP, Iverson ER, Taylor BJ, Cannon SB, May GD, Maki SL, Farmer AD, Doyle JJ. 2013. Keeping an Eye on Biology. Science 339:408-409.
- American Association for the Advancement of Science. 2013. Science Magazine Prize Goes To Teaching Tool for Undergraduate Genomics Course. AAAS - Worlds Larg Gen Sci Soc.
- Hazlett BA. 1996. Reproductive behavior of the hermit crab Clibanarius vittatus (Bosc, 1802). Bull Mar Sci 58:668-674.
- Frank U, Leitz T, M?ller WA. 2001. The hydroid Hydractinia: a versatile, informative cnidarian representative. BioEssays 23:963-971.
- Ballard WW. 1942. The Mechanism for Synchronous Spawning in Hydractinia and Pennaria. Biol Bull 82:329-339.
- Muller WA. 1973. Induction of metamorphosis by bacteria and ions in the planulae of Hydractinia echinata; an approach to the mode of action.
- Rosengarten RD, Nicotra ML. 2011. Model Systems of Invertebrate Allorecognition. Curr Biol 21:R82-R92.
- Mokady O, Buss LW. 1996. Transmission Genetics of Allorecognition in Hydractinia symbiolmgicarpus (Cnidaria: Hydrozoa). Genetics 143:823-827.
- M W, R U, M K, S B. 1996. Metamorphosis and pattern formation in Hydractinia echinata, a colonial hydroid. Int J Dev Biol 40:313-322.
- Katsukura Y, Ando H, David CN, Grimmelikhuijzen CJP, Sugiyama T. 2004. Control of planula migration by LWamide and RFamide neuropeptides in Hydractinia echinata. J Exp Biol 207:1803-1810.
- Dowds BM, Elwood RW. 1983. Shell wars: assessment strategies and the timing of decisions in hermit crab shell fights. Behaviour 1-24.
- Bach CE, Hazlett BA, Rittschof D. 2006. Sex-specific differences and the role of predation in the interaction between the hermit crab, Pagurus longicarpus, and its epibiont, Hydractinia symbiolongicarpus. J Exp Mar Biol Ecol 333:181-189.
- Brooks WR, Mariscal RN. 1985. Protection of the hermit crab Pagurus pollicaris say from predators by hydroid-colonized shells. J Exp Mar Biol Ecol 87:111-118.
- Buckley WJ, Ebersole JP. 1994. Symbiotic organisms increase the vulnerability of a hermit crab to predation. J Exp Mar Biol Ecol 182:49-64.
- Damiani CC. 2003. Reproductive costs of the symbiotic hydroid Hydractinia symbiolongicarpus (Buss and Yund) to its host hermit crab Pagurus longicarpus (Say). J Exp Mar Biol Ecol 288:203-222.
Article Files
Login to access supporting documents
A new approach to course-based research using a hermit crab-hydrozoan symbiosis(PDF | 325 KB)
S1. HP-CURE-Aquarium Set Up and Animal Husbandry.docx(DOCX | 21 KB)
S2. HP-CURE-Pre-Jigsaw Reading Guide.docx(DOCX | 63 KB)
S3. HP-CURE-Teaching Timeline.docx(DOCX | 91 KB)
S4. HP-CURE-Scientific Paper Reading Guide.docx(DOCX | 63 KB)
S5. HP-CURE-Grading Rubric for Final Paper and Presentation.doc(DOC | 250 KB)
S6. HP-CURE-Group-Member Evaluation Form.docx(DOCX | 86 KB)
- License terms

Comments
Comments
There are no comments on this resource.