Dynamic Daphnia: An inquiry-based research experience in ecology that teaches the scientific process to first-year biologists
Published online:
Abstract
This authentic research experience lesson teaches the core concept of systems and the competencies of quantitative reasoning, communication, and the ability to apply science. The research is student driven, the results are unknown, and the students engage in an iterative process to gather data, collaborating with classmates. It is designed for first-year biology majors, in a class size of 15-30 students who can work in groups of three. Students will learn to properly design an experiment, work as teams, analyze data, evaluate conclusions, and communicate findings to others. Additionally, this lesson also incorporates self-reflection and peer assessment when students produce a poster as a summative assessment. Over a five–week period, students will explore how an abiotic factor affects growth, reproduction, and survival of Daphnia. Students are asked to compare their results to published literature. By the end, students should have a better understanding of science as an ongoing process where results are being updated and furthering the state of knowledge.
Citation
Gleichsner, A.M., Butler, S.R., and Searle, C.L. 2019. Dynamic Daphnia: An inquiry-based research experience in ecology that teaches the scientific process to first-year biologists. CourseSource. https://doi.org/10.24918/cs.2019.2Lesson Learning Goals
The ultimate goal of this lesson is to teach students how to think and act like scientists. This goal includes that students will develop the skills necessary to design and complete simple experiments. They will also learn how to consult the primary literature and to make hypotheses and predictions. Students will develop basic statistical skills that will help them interpret and visually display simple data sets and results. To conduct their experiments, students will learn how to use basic laboratory equipment, such as microscopes and pipettes, which will serve as a basis for other research or laboratory courses.Lesson Learning Objectives
Students will be able to:- Construct written predictions about 1 factor experiments.
- Interpret simple (2 variables) figures.
- Construct simple (2 variables) figures from data.
- Design simple 1 factor experiments with appropriate controls.
- Demonstrate proper use of standard laboratory items, including a two-stop pipette, stereomicroscope, and laboratory notebook.
- Calculate means and standard deviations.
- Given some scaffolding (instructions), select the correct statistical test for a data set, be able to run a t-test, ANOVA, chi-squared test, and linear regression in Microsoft Excel, and be able to correctly interpret their results.
- Construct and present a scientific poster.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
Engaging in research as an undergraduate has been linked to increases in overall student success (3) including problem solving abilities, independence, communication skills, and understanding of the scientific process (collecting, analyzing, and interpreting data) (4). They have also been linked to higher confidence and enthusiasm towards research (5), higher student GPA (6), increased graduate school enrollment (7, 8), and retention (9, 10).
There are generally four different contexts in which undergraduates can engage in research (2) including traditional laboratory classes, research internships, inquiry-based labs, and Course-based Undergraduate Research Experiences (CUREs). Traditional laboratory classes typically have one instructor for many students, the instructor generally provides students with very guided laboratory exercises, the outcome of the experiments is known, data generated are usually very expectable, and the conclusions are limited to the scope of the course. Additionally, in traditional laboratory classes, students generally collaborate among themselves and not with anyone outside of the course. In research internships, an instructor generally works one on one with a student, the direction of the research is driven by a mix of the instructor and the student, the outcome of the experiments is unknown, and the relevance of the research is broader than the internship itself (i.e. is novel). Moreover, in research internships, the student generally collaborates with a research group, usually working on a subset of a larger project. The third type, inquiry-based labs, have generally one instructor with many students, the direction of the research is primarily guided by the students, the results may be known or unknown, the relevance of the research is typically limited to within the course, and collaboration occurs among the students in the course. The last context in which undergraduates can engage in research is through a CURE course. In a CURE, there is generally one instructor and teaching assistant(s) for many students, the direction of the project is guided by the students and the instructor, the outcome of the experiments is unknown, and the relevance of the research extends beyond the course (i.e. is novel). In CUREs, collaboration generally occurs among students in the course as well as with TA’s and the instructor, and can extend beyond the course resulting in paper acknowledgements that include students, or authorship on scientific papers. Both CURE and inquiry-based courses allow a larger number of students to benefit from a novel research experience compared to participating in individual research internships under faculty mentors in research labs (11).
Here, we present an inquiry-based lesson in which student groups design, conduct, analyze, and present research in the field of ecology using the model freshwater organism Daphnia. Student research direction is primarily guided by the students, but also advised by the instructional team to guide student groups towards novel research questions. Students consult the scientific literature to design research experiments that may ultimately provide preliminary results for future work outside of the class. While all projects designed in this lesson have results that are not entirely known, all students are not expected to design and conduct truly novel research that produces results that are relevant beyond the scope of the course. In this aspect, this lesson falls under the criteria of an inquiry-based research experience rather than a CURE. The primary goal of this lesson is not to produce publishable results, but rather to provide students with experience with the entire scientific process, during which students gain the skills necessary to think and act like a scientist, preparing them for future science careers.
This lesson is aligned with the recent education reforms focused on quantitative reasoning and communication (1), and is designed to set up first-year undergraduate science majors for success in several ways. First, like most first year courses, we aim to help students learn and retain fundamental biological knowledge that they need to build on in future classes. To do so, this course uses a cooperative learning approach (12). Though cooperative learning can be hard to define, here we use Dillenbourg’s definition of cooperative learning, which is when two or more people attempt to learn something together (13). Cooperative learning helps students retain the material better than if it were presented in a standard lecture setting (14). Second, this course aims to create a nurturing environment for first year students to help retain them in the sciences and finish their degree (15). One important factor in providing a nurturing environment is having small class sizes that allow students to be more engaged (16), but also increases the potential for students to have more positive interactions with faculty. The use of teaching assistants has also been linked to degree retention and positive experiences in post-secondary education classrooms (17), and can be helpful in creating a sense of community and increasing interactions between students, graduate students, and faculty (18). Third, this lesson aims to increase inclusivity and diversity in the sciences. Lessons incorporating inquiry-based research experiences not only allow educators to involve more students in research compared to a standard laboratory PI-student setting (19) but also result in more diverse students (socioeconomic and ethnic minorities) being involved in hands on research experience (20). Fourth, we introduce statistical tests to help students understand variation and how data is collected. Statistical skills are important to undergraduates in STEM fields (1, 21, 22), and lack of this foundational knowledge can be a hindrance to even experts in the field (23, 24). Teaching students about variation and statistics can help them understand how science works and its limitations. Finally, this class helps first year students begin to develop scientific communication skills. Scientific communication skills rarely develop spontaneously, but once learned can be applied to higher level thinking (25).
To accomplish these goals, we use Daphnia as a model organism system to provide students in this course with novel research experience. Invertebrates are commonly used in undergraduate classes because they are easy and inexpensive to acquire and maintain, typically have life cycles and life history that are hospitable to laboratory environments, and have measurable traits that are conducive to scientific inquiry (26). In addition to the ease of their use, the use of live animals as part of authentic research experiences has been shown to increase understanding of scientific concepts (27). Drosophila fruit flies and Caenorhabditis elegans nematodes are perhaps the most commonly used invertebrates in undergraduate classrooms, as they are molecularly well described and therefore ideal for teaching genetics (28-31), and physiology (32). Examples of other invertebrate model systems that have been used in undergraduate classrooms include Sacchromyces cerevisiae yeast to investigate cell biology (33), hermit crabs and their symbionts to study ecology (34), Planaria to examine toxicology (35), Paramecium to explore sensory signaling (36-38), and blackworms to examine physiology (39, 40).
Daphnia have historically been used as a model system for toxicology, as they are sensitive to water contamination and exhibit rapid physiological responses when exposed to toxins (41, 42). Daphnia are also easy to observe and measure. Their bodies are clear, allowing for visual observation of internal organs and measurement of physiological characteristics such as heart rate and feeding rate. Life history characteristics, including growth, mortality, and reproduction are also easily determined. As with other model systems (39, 40), labs examining Daphnia response to novel stimuli such as Nicotine or Caffeine have been developed (43), but their use as an educational system remains undeveloped beyond this limited scope. Here, we present this organism as an ideal candidate to explore a broad range of ecological questions with undergraduate students. In addition, we expand upon single lab formats that capture rapid responses in invertebrates to create an inquiry-based research experience lesson in which students use this model system to design and implement their own experiments over several weeks, learning the scientific process and ecological principles in the process of scientific discovery.
Authentic research experiences in many disciples have been developed (see 11 and 44 for a review of some of these), including modules or courses in genetics/bioinformatics (45, 46), neuroanatomy (47), physiology (48), and ecology (49-51, 34). Courses that implement authentic research experiences into their curricula strive to meet similar learning outcomes to those described here, primarily an increase in the ability of students to think and act like scientists in terms of experimental design, data analysis, and presentation. Courses that focus on ecological principles often use plants as study organisms (49, 51) or require that classes go into the field to collect data, which is not always possible. These courses are also seldom published in a format that is conducive to the implementation of the course by other instructors. In addition to this, there is a lack of representation of both marine and freshwater authentic research experiences in ecology (although see 34 for another non-field based ecology course using hermit crabs). Aquatic ecology is an engaging topic to undergraduates as it is a field impacted by large scale climate change as well as issues such as pollution and environmental disturbance. Here, we present the details for an inquiry-based authentic research experience course using a charismatic freshwater organism in which students can engage in an inquiry-based research experience over a five-week period. Students in this course gain knowledge in ecology as well as the skills needed to be a scientist, by designing, implementing, and analyzing their own research projects. Students in this course have completed projects that become the preliminary research that they use as a basis for further research with course instructors. We provide materials for any instructor wishing to adopt the Daphnia system into their courses (see Supporting Files).
Intended Audience
The intended audience for this lesson is first-year undergraduate biology majors.
Required Learning Time
This lesson is designed for five different two-three hour class periods in a wet lab setting. Class periods should be separated by 3-7 days to allow time for the experiments to run.
Pre-requisite Student Knowledge
Students should be able to read and conduct basic arithmetic. No specific background knowledge is required for this lesson.
Pre-requisite Teacher Knowledge
Teachers should be familiar with basic stoichiometry, microscope use, and aseptic technique (Supporting File S1. Dynamic Daphnia: Lab Manual), as well as the basic terminology associated with ecology and Daphnia biology (Supporting File S2. Dynamic Daphnia: Glossary). Teachers should have access to a large amount of glassware to allow for the culturing of Daphnia as well as student projects (20 – 30 containers per group as a minimum). Teachers should also familiarize themselves with the lifecycles of Daphnia (Supporting File S1. Dynamic Daphnia: Lab Manual and S3. Dynamic Daphnia: Daphnia care).
SCIENTIFIC TEACHING THEMES
Active Learning
Almost all of the material in this lesson is designed from a student-centered perspective to active learning, which has been shown to increase student performance in STEM courses (52). Many of these activities are done in the form of Think-Pair-Share (53) or other collaborative learning (12) to facilitate student engagement as opposed to passive lecturing.
Assessment
Throughout the lesson, there are many opportunities for formative assessments, which include constructing hypotheses and making graphs. Pre-lab quizzes (see Supporting File S4. Dynamic Daphnia: Pre-lab quizzes) provide a formative assessment of student reading comprehension of the topics in each lab (Supporting File S1. Dynamic Daphnia: Lab manual) prior to its completion, as well as an added incentive for students to be prepared for the activities of the day. There are also questions included throughout the lab manual (Supporting File S1. Dynamic Daphnia: Lab manual) that can be used to determine student understanding of the material presented. The summative assessment at the end is making a poster, which can be presented to other classmates, faculty, or the public. To demonstrate the effectiveness of teaching, instructors can use these graphs and writing samples as artifacts for demonstrating that students have met the learning objectives. If instructors are interested in measurement and evaluation of these assessments, we suggest the following resources (54, 55). Additionally, we have included an alignment table (Supporting File S10. Dynamic Daphnia: Alignment Table) so that instructors can easily see how the assessments match up with the learning objectives.
Inclusive Teaching
In this lesson, students can work in small groups (3-4 students). This arrangement can be particularly helpful when students are working on their summative assessment poster, an inclusive learning activity that is included in the lab manual (Supporting File S1. Dynamic Daphnia: Lab manual). To further ensure inclusivity, we have students rotate between groups roles, with groups deciding which role each member will fill. Instructors make a point to ask for student feedback throughout the lesson and to interact individually with each student to ensure that each student feels valued and understands that their contribution is important. Additionally, authentic research experiences in classrooms have been shown to be more inclusive than regular, individually selected research positions, allowing diverse students to gain research experience (20), making this lesson extremely inclusive.
LESSON PLAN
In our lesson, we have an undergraduate teaching intern, a graduate teaching assistant, and a professor in the classroom. Initially, students seem to be much more likely to approach the undergraduate, followed by the graduate student, and then the professor. However, throughout the lesson students become more comfortable with approaching the professor. This format is helpful to provide a nurturing environment for the students, in which they have multiple people to answer their questions and assist them through the scientific process.
This lesson plan includes five weeks (if labs meet once per week) of laboratory exercises during which students learn basic laboratory skills, including micropipetting, microscope use, and data collection while simultaneously providing an authentic research experience in ecology. Students learn the entire scientific process ranging from experimental design to statistical analysis and presentation of results, with an emphasis on developing communication skills. To do this, we use the model organism Daphnia, a genus of aquatic zooplankton, which is inexpensive and easy to maintain. Several species of Daphnia can be ordered online from merchants such as Carolina, Ward’s Science, MBL Aquaculture, and various other places. Many different species are available for use.
Throughout the laboratory exercises outlined below students work in groups of three or four. Groups can be assigned based on instructor preference. Students are expected to read the lab information before class, and have low stakes assignments, in the form of a pre-lab quiz (see Supporting File S4. Dynamic Daphnia: Pre-lab Quizzes), due at the beginning of each lab to increase their incentive to do this. If an instructor desires, they can incorporate this type of assessment on the information either online or at the beginning of class. At the beginning of each class we give a brief presentation that provides an overview and purpose of the activity, where students can locate the materials that they need, and an opportunity to ask any questions before they begin. Below we outline the lesson plans for each laboratory activity in detail.
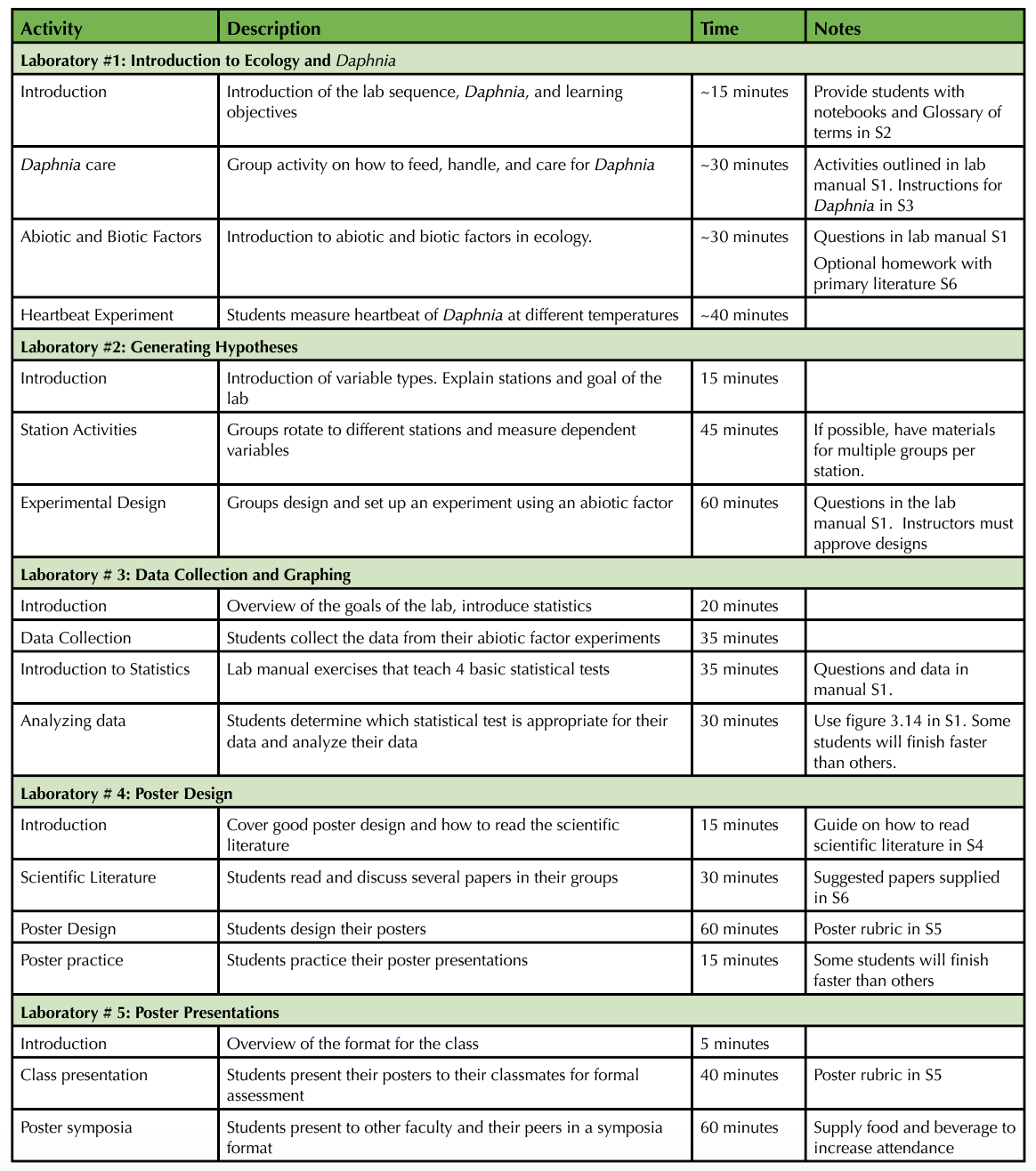
Table 1. Teaching Timeline
Laboratory exercise #1: Introduction to Ecology and Daphnia
Pre-lab preparation
For this lab, students need:
- a composition notebook (1 per student),
- Daphnia in beakers (2 beakers per group),
- empty beakers (2 per group),
- algae food solution (enough to feed all of the beakers),
- cetyl alcohol,
- well water (i.e., a non-chlorinated water source: tap water can be allowed to stand for a few days to eliminate chlorine, or spring water can be used as a quick alternative),
- compound or dissecting microscopes,
- concave slides or watch glasses (depending on the type of microscope used),
- Pasteur pipets,
- open-mouthed glass tubes and bulbs for moving Daphnia, and
- buckets of ice water in which to place beakers of Daphnia (1 per group).
You should also create an excel spread sheet in which the class data can be entered (see Supporting File S5. Dynamic Daphnia: Excel Template). You can choose to have the students use this pooled class data sheet to analyze and interpret the data (calculate means and sketch a graph) or they can use their own individual group data that they will record in their notebooks. The class data for this experiment is used in lab #4 to teach the students how to run a t-test. We recommend pooling the students’ data into one set for the class so that students can have a large enough sample size to run the statistical tests.
Lab Overview
The overarching goal for the first lab is to introduce students to basic ecological principles, the scientific method, and the Daphnia model system that they will be using over the next 4 weeks. The lab combines these three components by having the students come up with a hypothesis and set up an experiment that incorporates the principles of abiotic and biotic factors using Daphnia.
Since this is the first lesson of the series we also introduce the idea of an authentic research experience, and how the ultimate goal of the lesson culminates in each group designing and completing a novel research project. This design helps students to become aware that they need to learn carefully, as the skills they learn in the next few weeks are needed to accomplish their research projects.
We also introduce the concept of a laboratory notebook in which students are expected to keep careful records of their work. This notebook is used as an assessment tool throughout the lesson, as the graphs and writing that student put in their notebooks can serve as artifacts to demonstrate that they have met the learning objectives. We provide students with simple composition notebooks for this use. Notebooks are collected and graded after lab 3 and 5. Instructors can choose the level of detail at which they will assess these notebooks, but having this kind of assessment encourages our students to get into the habit of using their notebooks. As a fun activity, students are allowed to take home their notebooks to decorate the cover after the first lab period.
Lab #1 introduces students to working with Daphnia while also covering the topics of abiotic and biotic factors (Supporting File S1. Dynamic Daphnia: Lab Manual, pages 1-17). Daphnia are easy to maintain and will reproduce asexually (clonally) as long as they are kept in good conditions. An overview of Daphnia anatomy and reproduction is included in the lab manual (See Supporting File S1. Dynamic Daphnia: Lab Manual), and students should gain a general understanding of what it means to be cyclically parthenogenetic, and why Daphnia make a good model organism for ecology. Some students may be confused by the terminology used with the Daphnia system, particularly with its reproductive biology. Instructors may need to clarify that asexual ‘eggs’ are diploid entities in this system (rather than the typical haploid eggs students may have learned about in prior classes), and that females produce haploid eggs for sexual reproduction only when environmental conditions are poor and they have produced some male offspring.
At the start of this class we briefly go over the information about Daphnia with our class and explain why we are using them. We explain that the purpose of today’s lab is to get practice with Daphnia and to begin learning skills that they will use later for their own projects. Since this is the first class we also hand out and explain lab notebook use, and discuss the overview of the course and its emphasis on learning how to do science (culminating in their own projects and presentations). We also assign students to groups and explain the activities for the day before allowing the groups to work through the exercises in the lab manual (Supporting File S1. Dynamic Daphnia: Lab Manual, pages 1-17). First, students practice moving and feeding Daphnia and looking at them under a stereomicroscope. Students have difficulty manipulating Daphnia at first, both in terms of finding the Daphnia in the beakers and catching them, and losing individuals as part of this exercise. Instructors should prepare for questions about micropipette or microscope use and can refer students to the information in their lab manual (S1. Dynamic Daphnia: Lab Manual, pages 8-9, 12-14). Our students often struggle to find and focus on their samples at first.
Additionally, this lab introduces students to reading a scientific paper. We select a handful of simple scientific papers that both introduce students to reading primary literature and provide a foundation to make hypotheses (see Supporting File S6. Dynamic Daphnia: Sample Literature). Students even at the graduate level have difficulty comprehending primary literature (56), and incorporating primary literature into the curriculum has been shown to positively affect student’s ability to design experiments (57). We provide supplemental material (see Supporting File S7. Dynamic Daphnia: How to read scientific paper) on how to read a paper that can be given to the students. Additionally, we provide a group of papers with simple to read figures that students can practice on and use to compare their results to (see Supporting File S6. Dynamic Daphnia: Sample Literature). For additional support on how to read a scientific paper, we also suggest using the C.R.E.A.T.E. method (Consider, Read, Elucidate the hypothesis, Analyze and interpret data, Think of the next Experiment) that was developed by Hoskins et al. (58).
Once students practice moving and observing Daphnia they learn about abiotic and biotic factors in ecology. Students work in groups to discuss how they think certain factors would influence Daphnia biology. At the end of the lab they perform a small experiment testing the impacts of temperature (an abiotic factor) on Daphnia heart rate (physiology). Students should find that the higher the temperature is, the faster the Daphnia’s heart rate will be (ice water treated Daphnia should have a significantly slower heart rate than those at room temperature). This result occurs because higher temperatures increase metabolic processes, increasing the amount of oxygen needed by the Daphnia’s cells, and thus increasing heart rate. It helps to remind students to record the methods as well as the data collection of their experiment in their notebooks while they complete this experiment.
Students should not use a coverslip and must be careful not to crush Daphnia if using a compound microscope for this exercise. Students should sketch the data in a bar chart in their notebooks. Bar graphs are a good way to represent data that have a categorical variable (temperature: room temperature, cold) and a numerical measurement (beats/minute). To graph the data they calculate the average (mean) beats/minute for each treatment. Instructors can have students pool their data into a class data sheet that can then be used to illustrate the impacts of sample size on outcomes, and these data can also be used in laboratory 4 as part of learning statistics.
Laboratory exercise # 2: Generating hypotheses
Pre-lab Preparation
For this lab you first need some generic examples of experimental designs for class discussion (see below for an example). For the laboratory exercise you need to set up different stations at which groups rotate to learn different data they can collect from Daphnia (Supporting File S1, Dynamic Daphnia Lab Manual, pages 18-27).
Station 1 (length) requires stereomicroscopes, fine scaled rulers, watch glasses, open mouth pipets and bulbs, and beakers of Daphnia (containing many individuals).
Station 2 (fecundity) needs many beakers each containing a single adult female Daphnia and her most recent clutch of offspring. If your Daphnia cultures are reared under good conditions, (ie: not over-crowded and with the proper amount of food, see the lab manual; Supporting File S3. Dynamic Daphnia: Daphnia Care), then all of the individuals in your containers should be female. Closer examination using a stereomicroscope reveals whether the individuals are carrying offspring. Instructors should select mature female individuals that have eggs to ensure that they will have offspring for this lab. These beakers need to be set up by the instructor prior to the lab (at least four days prior) to ensure that each adult has a clutch of offspring. You also need stereomicroscopes, watch glasses, and pipets with bulbs.
Station 3 (mortality) needs a set of beakers, some of which should contain a single Daphnia individual, and some that are empty (to represent dead individuals).
Station 4 (male versus female) needs some beakers containing and labeled as female Daphnia and some containing and labeled male Daphnia. To obtain male Daphnia instructors must stress a beaker of Daphnia prior to the lab (via overcrowding, change in photoperiod, and/or food deprivation) and isolate males from the beaker. Overcrowding in Daphnia is dependent on the species being used, as different species vary in size (59). Daphnia magna, a large and easily obtainable species from commercial sources, can be stressed by placing more than 24 adult individuals into 100 mL of water together. We recommend that instructors try to stress their animals at least three weeks prior to this lab to ensure that they give themselves ample time to acquire males. Stress can be detected by the formation of ephippia in females and also signifies that males are present. Male Daphnia are smaller than females and can be identified by the presence of claspers (Figure 1.2 in Supporting File S1: Dynamic Daphnia: Lab Manual). This station also needs stereomicroscopes, watch glasses, and pipets with bulbs.
Station 5 (type of reproduction) needs Daphnia that have ephippia (evidence of sexual reproduction) and those that have eggs/embryos (evidence of asexual reproduction) as well as stereomicroscopes, watchglasses, and glass pipets and bulbs for moving Daphnia. The preparation outlined for station 4 will not only produce males but will also produce females harboring ephippia which can be used for this station. Asexually reproducing females can be collected from unstressed containers of Daphnia. As in station 2, instructors can examine Daphnia using a stereomicroscope to determine that individuals selected are mature and harboring eggs. Since males and ephippia are produced more readily in some species of Daphnia than others, you may or may not be able to easily acquire individuals for stations 4 and 5. However, if you find that it is difficult to induce males or ephippia in female Daphnia, then it is unlikely that your students will see them in later experiments, and stations 4 and 5 can be omitted.
Students also set up experiments using an abiotic factor of their choice. Instructors should be prepared with materials that allow for the manipulation of a variety of abiotic factors, as well as having sufficient numbers of Daphnia and beakers for each group’s experiment. Proper control of Daphnia age for these experiments requires that mature Daphnia (the largest individuals) be placed into containers without young or immature Daphnia 1-2 days prior to the lab. These Daphnia will produce new offspring of known ages (1-2 days old) which should then be used in the experiments that the students set up in this lab. Because Daphnia do not always reliably reproduce, we found that setting up more Daphnia ‘moms’ at this stage was a necessary precaution to avoid not having enough same age Daphnia for the experiments. The number of ‘moms’ required will depend on the number of groups in the class. A conservative estimate is that each adult female Daphnia will have 1 offspring every 2 days. We recommend that each group have a minimum of 20 offspring to use.
Lab Overview
This lab builds upon the previous lab by incorporating key components of the scientific process (hypotheses and predictions) and introduces types of data, while drawing from the concepts of abiotic and biotic factors which were introduced in lab 1. We begin this lab with an introduction of the scientific method and experimental design. Students should have already read the material prior to this class, so we try to make the presentation active by asking students questions (i.e. What is a hypothesis and a prediction? What is the difference between an independent and a dependent variable? Can you give us examples of categorical and numerical data?).
A common source of confusion in science lies in the specific way that a hypothesis and a prediction are defined. We emphasize the difference in this lesson and describe a hypothesis as a statement that explains WHY we expect to see our prediction. A hypothesis should be a scientifically informed piece of information that could explain why something happens. Hypothesis and prediction statements are often written as “If”, “then” statements, where the hypothesis is the ‘if’ and the prediction is the ‘then.’ For example: If higher quality food allows Daphnia to have increased growth (hypothesis), then Daphnia with high quality food will have increased body lengths compared to those with low quality food (prediction).
We also find that it is helpful to present students with an example experiment from which they have to generate a hypothesis and prediction, identify the independent and dependent variables, and label the data types. To encourage participation, instructors should give students a few minutes and work out the example within their groups, before going through it as a class. An easy example that instructors could use would be: A group of scientists wanted to examine the impacts of mercury levels on Daphnia reproduction in a lake. To do this they manipulated mercury levels across 4 groups (None, Low, Medium, and High) and recorded the number of offspring that Daphnia had over the course of 2 weeks. For this example, a hypothesis and prediction could be “If mercury is bad for the health of a Daphnia (hypothesis), then higher levels of mercury will lead to lower reproduction (prediction). The dependent variable (response variable that is measured) is Daphnia reproduction (number of offspring) and the independent variable (variable that is manipulated) is mercury level. In this example the independent variable is binned into 4 treatment groups (none, low, medium, and high) and is therefore categorical. As an aside: you could also point out that the ‘none’ group is the control in this experiment and highlight the importance of controls. The dependent variable is count data, and is therefore numerical.
Next, we tell the students that they will learn how to measure different dependent variables in Daphnia that they can then use as part of their experiments later. To do this we set up 5 stations around the lab, each with an activity that focuses on one of the variables (length, fecundity, mortality, sex, type of reproduction). Each station requires its own set of materials and preparation (outlined above).
The final activity in the lab involves each group choosing an abiotic factor and designing an experiment testing the impact of that factor on survival. The purpose of this is to give students experience with experimental design, without requiring that they design an entire experiment at this stage. This section introduces them to experimental controls, replication, and pseudoreplication. Students decide on an abiotic factor that they want to manipulate and ask the instructor whether it is feasible. Alternatively, you could give them a list of possible abiotic factors from which they could choose. Examples could include: salinity, pH (---HCl, generic acid?), temperature (if heat blocks are available), a ‘pollutant’ (very diluted dish soap, bleach, pesticide, etc.), lighting, substrate (different types of soil or gravel added to the bottom of beakers – you will likely want to sterilize them first), water level, or any other non-living factor available). Students should be encouraged to design meaningful experiments (that could uncover new scientific knowledge). To aid in this endeavor, instructors can help students search for existing scientific literature once they have a set of candidate abiotic factors, to explore what has been discovered already. Students can become discouraged if they find papers that are close to their idea, but should be encouraged to think about ways that they can pursue the idea while adding novelty (for example, testing the idea with a different species of Daphnia to compare results, looking at an individual or population level that is different than what has been done). As different species or community levels often respond differently to stimuli, even this small change in design allows students to contribute knowledge to the existing literature and participate in novel research. Students that design projects that are similar to existing studies should then incorporate these studies into their final presentation and compare their findings to those in the literature that had similar experimental designs.
Once students have chosen an abiotic factor, we have them work together in groups to formulate a hypothesis and prediction, identify the independent and dependent variables, sketch a prediction graph for their results, and ultimately set up their experiments. This time is a good opportunity to talk about the need for including control groups and having a sufficient sample size (e.g., a single Daphnia or beaker per treatment is not enough). Students record all of this information in their notebooks with enough detail to repeat the experiment from their notes.
We remind students throughout this process that their grade does not depend on significant results, to discourage students from falling into the mindset that statistical significance is better in science. Every class has projects that do and do not have statistically significant results and it is important that students do not feel that they failed if they do not fall into the former category. However, it is helpful to try to guide students towards experimental designs that are more likely to yield a result of some kind by avoiding situations in which an experiment completely fails, preventing any data from being collected. If students do not have data to interpret, it can be difficult to learn from the failure.
A complete failure situation usually occurs when students design an experiment in which they use lethal doses of their abiotic factor (too much of a pollutant, conditions such as temperatures that are outside of the range of survival, etc.), killing all individuals in a given treatment. To avoid this situation, we help the students search the scientific literature to see if there are any recommended limits for their factor of choice. If there is no guidance in the literature about the dose or limitations for an abiotic factor we recommend that students design experiments with multiple levels or tiers of their factor to increase the likelihood that some of the levels are hospitable (i.e.: None, Low, Medium, High). This approach has worked well for experiments that use salt and temperature in the past, and also teaches students an important aspect about experimental design.
Another potential issue occurs when students do not have enough replicates in their experiment to run statistical analyses. We avoid this pitfall by reviewing each group’s design before they set up their experiment, to ensure that they have more than one container of Daphnia per treatment. Ideally, we want students to have 10-20 individual containers per treatment, to ensure that accidental losses that decrease sample size (i.e., spilled beakers, misplaced individuals) did not prevent data analysis.
Laboratory exercise # 3: Data Collection and Graphing
Pre-lab preparation
Each group needs the animals and beakers from the experiment that they set up the previous week, as well as pipettes and containers to manipulate Daphnia and aid in data collection. Students also need access to computers to conduct statistical analyses and to graph their data. If computers cannot be provided to them, students should be told to bring personal computers prior to this lab, and need one computer per group.
Instructors should familiarize themselves with Excel or the statistical software of their choosing as students typically require guidance when using these programs for the first time. As an option, instructors can have students practice running a t-test on the heart beat rate data from lab one.
Instructors should also familiarize themselves with each group’s project and the correct statistical analysis that each group should ultimately use. Students often struggle with applying statistics to experimental designs and will likely need help deciding on how to best analyze their data.
Lab Overview
In this lab students collect and analyze the data from their abiotic experiments. To do this, students need to learn about statistics. This lab introduces several basic statistical analyses that students can use. We include a template data sheet for the t-test that instructors can use to compile class data (Supporting File S5, Dynamic Daphnia Excel Template). Specifically, this lab introduces the student’s t-test, one and two-way ANOVAs, the Chi-squared goodness of fit test, and linear regression. All of these tests are parametric, meaning that they assume that the data fit a normal distribution. For the level of this lesson we do not go in to detail about non-parametric tests, but instead use this as an opportunity to expose students to statistics and to help them learn how to identify the type of statistical test that they need based on their experimental design. It is possible that students in your class will encounter the need for a more advanced statistical test, so instructors should be aware that they may need to explain that the handful of tests introduced here are only a basic introduction to statistics.
Instructors should start the class with a general discussion about statistics and why we use them. They should instruct students to work in their groups to collect the data from their experiments and enter their data into their notebooks and Excel (instructors can emphasize the importance of having multiple copies of data). Instructors need to briefly explain the format that students should use to enter their data into Excel so that it can be analyzed by the software program. This formatting is often a source of confusion for students, as they do not realize that most statistical programs require columns for independent variables and one for each replicate of their dependent variable. For example, if an experiment is investigating the impact of different levels of salinity (High, Medium, Low) on Daphnia reproduction, the data needs to be entered into excel with a ‘Salinity treatment’ column containing entries of ‘High,’ ‘Medium,’ and ‘Low’ and a ‘Reproduction’ column with the number of offspring per experimental unit. Students should be reminded to record everything in their notebooks and to answer the questions in the lab manual (S1. Dynamic Daphnia: Lab Manual, pages 28-31). It is also helpful to have students take pictures of their experiments that they can use when they make their scientific posters in lab #5.
Once student groups finish collecting and entering their data, they work through the lab manual exercises for each statistical test (S1. Dynamic Daphnia: Lab Manual, pages 31-44). The students run the tests in Excel or, alternatively, instructors may have access to a different statistical program (SPSS, Minitab, R, SAS, etc.) and can modify this lab to the program of their choice. It should be noted that Excel does not currently have the capabilities to run pairwise comparisons for ANOVAs. Instructors should either not require the addition of these comparisons or use an alternate program to perform them if they are necessary.
Once each group works through the different statistical tests they use figure 3.6 in the lab manual (Supporting File S1. Dynamic Daphnia: Lab Manual, page 42) to decide which statistical test is most appropriate for their experiment. They then use their chosen test to analyze their data and interpret their results. We find that some groups work slower than others during this lab. Any that do not complete their analysis are told that they can finish it in the next lab period. Any who finish early are told to start graphing and interpreting the results from their statistical test (e.g. Were their results significant? Did they support their hypothesis?).
Laboratory exercise # 4: Poster Design
Pre-lab preparation
This lab requires that students have access to computers (at least one per group) so that they can graph and analyze their data, as well as construct a poster in Power Point. In addition to the poster, students need 3-4 relevant papers from the scientific literature. We include some selected papers that we think are easy to understand (Supporting File S6. Dynamic Daphnia: Sample Literature). Students use these papers, or the references from these papers, to find other papers. We provide students with a supplemental material for students about how to read a scientific paper (Supporting File S7. Dynamic Daphnia: How to Read Scientific Papers) and suggest the C.R.E.A.T.E methods as well for additional support (58). If the students do not have experience finding or reading the scientific literature they may struggle to find and access papers, and the instructor should be prepared to help them with this process. It may be helpful to prepare a guide to a single database of choice, such as PubMed, to help students find applicable literature.
We provide template posters in the supplemental materials for this lesson that students can use to make their posters if you choose (Supporting File S8a. Dynamic Daphnia: Poster Template 1 and S8b. Dynamic Daphnia: Poster Template 2).
Lab Overview
The goal of this lab is to have students use the results from their group experiments to develop a scientific poster that they will present in the next class. We begin the class by giving an overview of the lab. We emphasize the importance of presenting research to others, and tell students that their goals for the day are to put their results in context with the rest of the scientific literature, learn how to make a scientific poster, and practice presenting science to others. They also learn about the details of the poster presentation, which will happen in the next lab. We provide students with the grading rubric that we use to grade their posters (Supporting file S9. Dynamic Daphnia: Poster Rubric). This information is outlined in paragraph form in the lab manual (Supporting File S1. Dynamic Daphnia: Lab Manual, pages 45- 49).
One important element to presenting science is having an understanding of how the data being presented fit in the broader context of scientific knowledge. For students to present their data effectively they need to read the scientific literature to determine what other experiments have been done that relate to their work. The first thing that students do in this lab is complete a group activity in which the students read several papers and determine how their results relate to the findings of others. Groups are instructed to find, or are given, papers that are connected in some way to their study. This connection does not have to be about Daphnia or about their specific treatment, but should help students either interpret their results or frame their results in a broader context. Groups then answer the questions in the lab manual (Supporting File S1. Dynamic Daphnia: Lab Manual) and incorporate that literature into the presentation and interpretation of their results.
For the remainder of the lab, groups work together to create their posters. Students can be provided with a poster template to help with formatting (Supporting Files S8a. Dynamic Daphnia: Poster template 1 and S8b. Dynamic Daphnia: Poster template 2). This template is particularly useful if you plan to have the posters printed for display as the file is formatted to be a standard poster size of 3 x 4 feet. Instructors can have students search for scientific poster examples online and find ones that they do and do not like, and identify what characteristics are associated with their opinions. This exercise can lead to a discussion about good and bad poster design. For example, limited text and more images/figures on a poster are generally best.
Throughout the class, while students are designing their posters, the instructor should check in on each group to monitor their progress and answer any questions. By the end of the class students should have a draft of their poster and practice their presentations. Instructors will need to encourage students to practice the presentation aloud to one another. Students should be reminded that they will present their posters in the next class, and may wish to meet outside of class to practice. In addition to this, there is an elevator speech activity in the lab (Supporting File S1. Dynamic Daphnia: Lab Manual, page 49 -51) that can be assigned as homework or can be incorporated into the lab period. This activity helps students summarize their findings in a concise and meaningful way.
Laboratory exercise # 5: Poster Presentations
Pre-lab Preparation
If students are presenting their posters in a symposium format, then instructors will need to figure out the best way to print and display their posters prior to lab, based on their institutions resources and IT support. For example, at our institution it is easiest to print posters with dimensions of 3 ft x 4 ft, since our existing poster display boards are built for 3 ft high x 4 ft wide, so those are the dimensions that we use. They may wish to invite colleagues to attend the event, and could make fliers to advertise. Snacks and refreshments are a good way to entice higher attendance.
Lab Overview
In this lab, students present their posters. For this lesson, we print posters and set up a within-department symposium in a main lobby area, in which faculty, staff, and other students are invited to view the posters and talk to the groups. Fliers advertising the event are posted throughout the building and emailed to the department, and refreshments are served during the poster session. Students are excited to see their professors from other classes attending the poster session. To ensure that each group can be graded, students present their posters in the laboratory classroom prior to the symposia and are graded by both the instructor and their peers using the supplied rubric (S9. Dynamic Daphnia: Poster Rubric). In addition, the instructor visits each group during the symposia to ask additional questions.
TEACHING DISCUSSION
Student Projects
Student groups design their own projects in this course, allowing for diverse interests to be pursued. We assess the viability of each project idea prior to its implementation (i.e. Can we obtain the materials needed quickly or do we have them on hand? Is the idea novel?) and try to accommodate student interests whenever possible. Below are four example projects that have been completed as part of this lesson. Projects 3 and 4 were completed as part of a course that extended the timeline to allow for longer data collection.
Project 1: Does salt pollution negatively impact Daphnia dentifera populations?
Hypothesis and prediction: If higher salt concentrations negatively impact Daphnia dentifera, then individuals in high salt environments will have higher mortality and lower reproduction, leading to declines in population size.
Methods: Students set up 3 treatments including control (no salt), low (environmental levels), and high (2x), with 10 large beakers per treatment, each containing 10 individuals.
Conclusion: Salt results in lower population densities as compared to control treatments. High salt treatments experience the highest level of mortality.
Project 2: Does microplastic contamination alter Daphnia survival?
Hypothesis and prediction: If microplastic contaminants are detrimental to the health of Daphnia, then Daphnia exposed to contaminants will have lower survival than those without exposure.
Methods: Students selected one microplastic leachate, Bisphenol A (BPA) to test. BPA was ordered from Sigma and diluted with instructor assistance. Students chose 3 levels of exposure for their treatments: Control (no BPA), Low (current environmental levels), and High (2x environmental levels). Students set up 10 beakers, each containing 1 individual per treatment. Students recorded mortality in their treatments in the following lab.
Conclusion: BPA impacts survival at high, but not low, concentrations. Students stated that they would have preferred having a longer amount of time for data collection so they could see if long-term exposure at low levels has a negative impact or not.
Project 3: How does leaf tannin concentration influence D. dentifera reproduction and survival?
Hypothesis and prediction: If Daphnia are negatively impacted by leaf tannins, then high tannin concentrations will result in lower survival and reproduction.
Methods: Students set up 4 treatments including a control (no tannins), low, medium, and high tannin concentration, with 10 beakers containing 1 individual each per treatment. Students recorded reproduction (babies produced) and survival for 4 lab periods (2 weeks).
Conclusion: The presence of tannins significantly increases mortality and eliminates reproduction of D. dentifera. Daphnia exposed to tannins had abnormal embryos resulting in no reproduction.
Project 4: Does temperature impact survival in infected Daphnia?
Hypothesis and prediction: If extreme temperatures induce stress in Daphnia, then Daphnia exposed to cold temperatures and infected with Metschnikowia bicuspidata (a fungal parasite) will be more likely to die than infected individuals at normal temperatures.
Methods: Students set up 6 treatments: Control (uninfected, room temperature), uninfected cold, uninfected varying, infected room temperature, infected cold, and infected varying, with 10 beakers per group and 1 individual per beaker. They recorded mortality over 2 lab periods (1 week).
Conclusion: Temperature did not impact whether an infected Daphnia survived or not. Infected Daphnia had significantly higher mortality regardless of temperature.
Learning Outcomes
The primary goal of this lesson is to give students the opportunity to think and act as scientists. This goal is accomplished by having students work in groups to carry out all aspects of the scientific process. Students design and execute an experiment, record, analyze, and interpret their results, and display and present their data to both fellow scientists and the public through poster presentations at a community symposium. Within this goal we have several specific learning objectives that encompass the skills needed to complete the overall goal. Here, we describe how each objective is met.
Construct written predictions about one factor experiments.
Students learn how to create hypotheses and predictions, and then must formulate their own predictions for their experiments, in lab # 2 (Supporting File S1. Dynamic Daphnia: Lab Manual). Each student brainstorms several hypotheses and predictions that they could test and then groups meet to discuss the ideas. These predictions are recorded in their lab notebook using the guidelines provided in the lab manual. Groups present their predictions to their instructors who discuss and evaluate the feasibility and correctness of the predictions proposed. Student ability to describe and defend their prediction was used to indicate that this objective had been met. By the end of the lesson, all students were able to accomplish this task and explain the logic they were using to create their predictions.
Interpret and construct figures with two variables
Students are first introduced to scientific figures in lab #1 (Supporting File S1. Dynamic Daphnia: Lab Manual). They learn how to construct figures in lab #2 and gain experience with figure construction throughout the lesson. Students are given experience interpreting figures in scientific papers as they learn how to read the scientific literature. Their ability to construct and interpret figures is evaluated by their ability to create and interpret figures from their own data that they collect as part of their group experiments. Students construct these figures and explain them as part of their posters that they design and present in labs 4 and 5. Correctness of the presented figures is used to assess whether this objective had been met. Students are individually assessed for this objective as part of a pre-lab quiz (Supporting File S4: Dynamic Daphnia: Pre-lab Quizzes) in which they must interpret a figure. We find that this individual assessment is important to determine individual achievement of this learning outcome. All student groups are able to effectively meet this outcome, but not all individual students are able to do so. We find that ~75% of our students are able to complete this learning outcome by the end of this 5-week lesson.
Design simple one factor experiments with appropriate controls
As part of this lesson, groups of students design and conduct novel research. Students learn about experimental design in lab #2 (Supporting File S1: Dynamic Daphnia: Lab Manual). They fill out the questions in the lab manual to develop their experimental design. Instructors ask students to explain their design and identify the treatments and controls. This activity allows instructors to assess whether students understand the basics of experimental design and whether they achieve this learning objective. Students later present their experiments, allowing for further assessment of their understanding through questioning by the instructor and their peers. All student groups are able to meet this learning outcome, but the level of individual achievement varies. By the end of the five weeks, we find that the majority of students (~90%) are able to design simple 1 factor experiments with appropriate controls, thus meeting the learning objective. Students do vary substantially in how novel or interesting the experiments are that they design, with the majority of students that meet the learning objective (~70%) finding it difficult to design truly novel experiments.
Demonstrate proper use of a two-stop pipette, microscope, and laboratory notebook.
While learning the scientific process students also gain experience using key laboratory techniques and equipment, including notebook and record keeping, micropipetting, and microscope use. These techniques are reinforced repeatedly over multiple labs. Instructors and teaching assistants assess proper pipette and microscope use during the lab while students are using this equipment to ensure that this objective is being met. Laboratory notebooks are assessed after labs 3 and 5 (Supporting File S1: Dynamic Daphnia: Lab Manual) when instructors collect student notebooks to determine whether students are following the guidelines outlined in lab 1, including keeping detailed records of the procedures and data from each lab. While some students (~10%) still require some assistance when using laboratory equipment (confirming proper setting on pipettes, finding specimens with microscopes), the amount of guidance needed by instructors is vastly reduced by the end of the five-week lesson. Laboratory notebook detail and quality improve as notebook checks are conducted. By the end of the lesson all students are demonstrating proper use of a laboratory notebook.
Calculate means and standard deviations
To create figures throughout this lesson students must calculate the mean and standard deviation for their treatments. Students first learn how to do this in lab #1 (Supporting File S1. Dynamic Daphnia: Lab Manual) and then apply this skill to the creation of figures to visualize their own experimental data. Indication that this objective has been met is determined by each student’s ability to create graphs from a set of data in lab #3, where each individual student learns how to run the statistical analyses for different data sets. By the end of the five- week lesson all students demonstrate the ability to calculate these test statistics using Excel.
Select the correct statistical test for a data set, given some scaffolding & Conduct a t-test, ANOVA, chi-squared test, and linear regression in Microsoft Excel, and be able to correctly interpret their results.
Students learn about statistical analyses in lab #3 (Supporting File S1: Dynamic Daphnia: Lab Manual), where they use example data sets and data collected in previous labs to run a t-test, ANOVA, chi-squared test, and linear regression in Microsoft Excel. Figure 3.14 in the lab manual (Supporting File S1: Dynamic Daphnia: Lab Manual) provides instructions for how to select the correct statistical test for the type of data collected. They then apply this knowledge to their own experimental data. This objective is assessed in their poster presentations, as students must demonstrate that they correctly choose the statistical test for their experimental design, are able to conduct the analysis, and are able to interpret and communicate the results of that test. Targeting questioning by the instructor can determine whether this objective has been met for each student. This objective is also partially assessed in the final pre-lab quiz for this lesson (Supporting File S4: Dynamic Daphnia: Pre-lab Quizzes). By the end of the five-week lesson, around 70% of the students are able to meet this learning objective when asked to select a test and interpret its results, without extra guidance from instructors. The remaining 30% are able to accomplish this objective given some instructor direction (targeted questions about what type of data they are working with, what is being compared, etc.).
Construct and present a scientific poster
Students learn how to design and present scientific posters in lab #4 (see Supporting File S1: Dynamic Daphnia: Lab Manual). They use the guidelines presented in this lab to design a poster using the data collected from their group’s novel research project. Students then present this poster to their class as well as to the public or university as part of a symposia. Ability to complete this objective is formally assessed by both the instructor and their peers using a grading rubric (see Supporting File S9: Dynamic Daphnia: Grading Rubric). Given that posters are constructed as a group, we are unable to assess this learning outcome for each individual student. By the end of the five-week lesson, all student groups are able to construct and present a scientific poster to their peers, with all students obtaining passing scores on this assignment.
Lab Learning Objectives
In addition to these lesson specific objectives, each lab (Supporting File S1: Dynamic Daphnia: Lab Manual) includes learning objectives. To assess that each of these objective is met there are a number of assessment artifacts produced from each lab that can be used (Supporting File S10: Dynamic Daphnia: Alignment Table).
Student feedback
Students were asked to provide feedback and overall impressions of the course to help us better understand how well the course was received, and whether students had areas of recommended improvement. Student feedback was not intended to be a rigorous, quantitative measure of lesson effectiveness, allowing for only anecdotal impressions, which we outline here. Overall, students reported an excitement at having taken the course and an appreciation for science and the scientific process. In particular, the ability to have ownership over their own projects made them feel like each lab exercise was worthwhile, and they reported that it made the lab more engaging. While many students dislike working in groups, the majority of our students enjoyed working and developing friendships with the members in their team, even if they did not initially. Students found it exciting that they were conducting experiments with potentially unknown results, which they had not experienced previously in their coursework. In addition to this, students enjoyed learning about statistics in an applied format, rather than by completing calculations on data that was not theirs. Many students expressed the desire to become involved in research as part of their education as a result of taking this course.
It was suggested that the course could have incorporated more specific skills that were used in the following courses in the biology major sequence. In particular, the use of a spectrophotometer was heavily relied upon in the next course, and our students recommended incorporating the use of a spectrophotometer in this course to expose them to it earlier. We recommend that instructors incorporate techniques that best meet the needs of their curricula and the skills that their students require in their subsequent coursework.
Possible alterations
This lesson plan sequence could easily be modified to meet different instructor needs and time frames. For example, the heart beat experiment in lab #1 could be used as a standalone way to have students observe how an abiotic factor can influence organism physiology. Class data from that experiment could then be used to teach students how to run a t-test, as we have suggested in lab #4. Pairing these activities together could fit within a single lab period, and would expose students to ecological concepts and also running statistics. Instructors could also use the concept of the independent project and limit student’s choices of their independent and dependent variables to a set list of factors with a known statistical test outcome. For example – instructors could give them the option of abiotic factors with 3 levels in each factor, and then use the projects to teach them about ANOVAs. This approach would allow student groups to have ownership over a project and learn about experimental design, but save time by not necessitating instruction of multiple statistical tests. Another possible alteration would be to have students give oral presentations instead of a poster symposium at the end of the course. This change would decrease costs by eliminating the need for printing, any refreshments, and any cost associated with hanging posters, etc., while still providing students with the opportunity to present their work to their peers.
Alternatively, this course could be expanded by limiting the scope of the abiotic experiment and then including an additional student-designed final experiment that they ultimately present. To limit the abiotic experiment scope and to use it as a way to teach statistics you could allow students to only choose an abiotic factor, possibly from a list, and control their dependent variable to a single option (a good choice may be mortality or reproduction). They would then run the statistics on this project as part of the statistics lesson in lab #4. Once they have learned all of the concepts of experimental design they could then develop an entirely independent project in their groups, in which they pick their independent and dependent variables and ultimately have to decide on the correct statistics and present their projects in the poster format outlined in labs #5 and #6. This alteration opens up the use of biotic factors such as competitors, diverse communities, or predators in their experiments, and could allow the incorporation of lessons highlighting these ecological principles.
While the course as written is designed for a small class size, components of the course could be applied to larger class sizes to teach targeted learning outcomes, including parts of the scientific process or specific statistical tests. For example, a laboratory activity using Daphnia that is pre-designed to teach students a specific statistical test is much more manageable across larger student numbers than an activity in which students design and test their own hypotheses. The inquiry-based components to this course could be applied to larger numbers of students if they were used in all laboratory sections associated with a high enrollment course. Because each laboratory section would contain a small portion of the large course enrollment, this would allow every student in the class to gain from the same authentic research experience while keeping the number of students per class to a manageable level and maintaining the benefits of a small class size.
General Conclusions
Throughout this lesson plan sequence students acquired basic concepts and laboratory techniques while also learning about hypothesis based questioning, experimental design, and basic statistical tests. Students were able to take ownership over their projects and conduct research on ecology, while learning how to work efficiently and effectively in teams. These students left the course with a better understanding and greater enthusiasm about what science is and how it is conducted, and acquired broader life competencies including resilience, the ability to troubleshoot issues, and increased communication and team building skills that translate inside and outside of the classroom. The artifacts produced by students in the medium of formative (lab notebooks) and summative (posters) assessments can be used by educators as demonstration of effective teaching.
SUPPORTING MATERIALS
- S1. Dynamic Daphnia: Lab Manual - A laboratory manual containing all exercises described in this lesson plan
- S2. Dynamic Daphnia: Glossary - A glossary of terms used in the lab manual.
- S3. Dynamic Daphnia: Daphnia Care - A document with details on how to care for and rear Daphnia
- S4. Dynamic Daphnia: Pre-lab quizzes - A document with questions and answers that can be used for pre-lab quizzes to promote reading prior to the lab period.
- S5. Dynamic Daphnia: Excel Template - An Excel sheet containing a template for class data entry for the temperature heart rate experiment in Lab exercise number 1.
- S6. Dynamic Daphnia: Sample Literature - Sample literature for instructors that they could use in the course
- S7. Dynamic Daphnia: How to read scientific papers - A guide for students on how to read the scientific literature
- S8A. Dynamic Daphnia: Poster template 1 and S8B. Dynamic Daphnia: Poster Template 2- Power Point poster templates, formatted for a 3 x 4 foot poster size, for students to use to design their posters
- S9. Dynamic Daphnia: Poster Rubric - A rubric for grading student poster presentations
- S10. Dynamic Daphnia: Alignment Table - A table aligning each lab's learning objective with its manner of assessment.
ACKNOWLEDGMENTS
We would like to acknowledge Abigail Merrick for helping obtain some of the pictures in the lab manual. We would also like to thank the editors and two anonymous reviewers for their valuable feedback on this manuscript. Also, the authors of Gasper et al. for contributing some of the activities which have been adapted (and cited) from their paper.
References
- AAAS. 2011. Vision and Change: A Call to Action, A Summary of Recommendations; Vision and Change Conference; Washington, DC. www.visionandchange.org
- Auchincloss LC, Laursen SL, Branchaw JL, Eagan K, Graham M, Hanauer DI, Lawrie G, McLinn CM, Paleaz N, Rowland S, Towns M, Trautmann NM, Varma-Nelson P, Weston TJ, Dolan EL. 2014. Assessment of Course-Based Undergraduate Research Experiences: A Meeting Report. CBE- Life Sciences Education 13:29-40
- Nnadozie E, Ishiyama J, Chon J. 2001. Undergraduate research internships and graduate school success Journal of College Student Development 42:145-156
- Lopatto D. 2004. Survey of undergraduate research experiences (SURE): first findings. Cell Biology Education 3:270-277
- Brownell ES, Kloser MJ, Fukami T et al. 2012. Undergraduate biology lab courses: comparing the impact of traditionally based "cookbook" and authentic research-based courses on student lab experiences. Journal of college science teaching 41:36
- Fechheimer M, Webber K, Kleiber PB. 2011. How well do undergraduate research programs promote engagement and success of students? CBE - Life Science Education 10:156-163
- Lopatto D. 2007. Undergraduate research experiences support science career decisions and active learning. CBE- Life Sciences Education 6:297-306.
- Russell SH, Hancock MP, McCullough J. 2007. Benefits of undergraduate research experiences. Science (Washington) 316:548-549
- Nagda BA, Gregerman SR, Jonides J, von Hippel W, Lerner JS. 1998. Undergraduate student-faculty research partnerships affect student retention. The Review of Higher Education 22(1):55-72
- Hathaway RS, Nagda BA, Gregerman SR. 2002. The relationship of undergraduate research participation to graduate and professional education pursuit: An empirical study. Journal of College Student Development 43:614-631
- Wei CA, Woodin T. 2011. Undergraduate Research Experiences in Biology: Alternatives to the Apprenticeship Model. CBE Life Sciences Education 10:123-131
- Lord T. 2009. Cooperative Learning in Using That Really Work To Biology Teaching: Using Constructivist-Based Activities to Challenge Student Teams. The American Biology Teacher 60:8, 580-588.
- Dillenbourg, Pierre. (1999). Chapter 1 (Introduction) What do you mean by 'collaborative learning'?. Collaborative-learning: Cognitive and Computational Approaches. Vol. 1.
- Knight JK, Wood WB. 2005. Teaching More by Lecturing Less. Cell Biol Educ 4:298-310. doi: 10.1187/05-06-0082
- Minchella DJ, Yazvac CW, Fodrea RA, et al. 2012. Biology Resource Seminar : First Aid for the First Year. The American Biology Teacher 64:352-357.
- Gasper BJ, Minchella DJ, Weaver GC, et al. 2012. Adapting to osmotic stress and the process of science. Science 335:1590-1. doi: 10.1126/science.1215582
- O'Neal C, Wright M, Cook C, Perorazio T, Purkiss J. 2007. The Impact of Teaching Assistants on Student Retention in the Sciencies: Lessons for TA Training. Journal of College Science Teaching 36(5), 24
- Dickson, PE, Dragon T, Lee A. 2017. Using Undergraduate Teaching Assistants in Small Classes. Proceedings of the 2017 ACM SIGCSE Technical Symposium on Computer Science Education; 165-170
- Weaver GC, Russell CB, Wink DJ. 2008 Inquiry-based and research-based laboratory pedagogies in undergraduate science. Nat Chem Biol 4:577-580. doi: 10.1038/nchembio1008-577
- Bangera G, Brownell SE. 2014. Course-based undergraduate research experiences can make scientific research more inclusive. CBE Life Sci Educ 13:602-606. doi: 10.1187/cbe.14-06-0099
- Bisgaard S. 1991. Teaching Statistics to Engineers. The American Statistician 45:4 274-283.
- National Research Council (US) Committee on Undergraduate Biology Education to Prepare Research Scientists for the 21st Century. 2003. BIO2010: Transforming undergraduate education for future research biologists. National Academies Press (US).
- Sharpe D. 2013. Why the resistance to statistical innovations? Bridging the communication gap. Psychol Methods 18:572-82. doi: 10.1037/a0034177
- Parker LC, Gleichsner AG, Adedokun OA, et al. 2016. Targeting change: Assessing a faculty learning community focused on increasing statistics content in life science curricula. Biochemistry and Molecular Biology Education 44(6):517-525
- Spektor-Levy O, Eylon B-S, Scherz Z. 2009. Teaching Science Communication Skills in Science Studies: Does It Make A Difference? Int J Sci Math Educ 7:875-903. doi: 10.1007/s10763-009-9150-6
- Wilson-Saunders SE. 2011. Invertebrate models for biomedical research, testing, and education. ILAR Journal 52(2):126-152
- Broder ED, Angeloni LM, Simmons S, Warren S, Knudson KD, Ghalambor CK. 2018. Authentic Science with Live Organisms Can Improve Evolution Education. The American Biology Teacher 80(2);116-123
- Jorgensen EM, Mango SE. 2002. The art and design of genetic screens: Caenorhabditis elegans. National Rev Genet 3:356-369
- Chen J et al. 2005. Discovery-Based Science Education: Functional Genomic Dissection in Drosophila by Undergraduate Researchers. PLOS Biology 3(2):e59
- Jacobs-McDaniels NL, Maine EM, Albertson RC, Wiles JR. 2013. Using model organisms in an undergraduate laboratory to link genotype, phenotype, and the environment. Journal of Biological Education. 47(1):52-59
- Dunne CR, Cillo AR, Glick DR, John K, Johnson C, Kanwal, J, Malik BT, Mammano K, Petrovic S, Pfister W, Rascoe AS, Schrom, D., Shapiro S, Simkins JW, Strauss D, Talai R, Tomtishen III JP, Vargas J, Veloz T, Vogler TO, Clenshaw ME, Gordon-Hamm DT, Lee KL, Marin EC. 2014. Strucred Inquiry-Based Learning: Drosophila GAL4 Enhancer Trap Characterization in an Undergraduate Laboratory Course. PLOS Biology 12(12):e1002030
- Titlow JS, Johnson BR, Pulver SR. 2015. Light Activated Escape Circuits: A behavior and neurophysiology lab module using Drosophila Optogenetics. Journal of Undergraduate Neuroscience Education. 13(3):A166-A173
- Goudsouzian LK, McLaughlin JS, Slee JB. 2017. Using Yeast to Make Scientists: A six-week student-driven research project for the Cell Biology laboratory. CourseSource doi:00.0000/journal.cs.000000
- Galush T, Mazur C, Cotner S. 2017. A new approach to course-based research using a hermit crab-hydrozoan symbiosis. CourseSource http://doi.org/10.24918/cs.2017.2
- Pag?n OR, Coudron T, Kaneria T. 2009. The Flatworm Planaria as a Toxicology and Behavioral Pharmacology Animal Model in Undergraduate Research Experiences. The Journal of Undergraduate Neuroscience Education 7(2):A48-A52
- Clarke S, Elwess NL, Jones K. 2002. Paramecium: An Excitable Cell with Great Potential. The American Biology Teacher 64(5): 369-375.
- De Ondarza J, Elwess NL. 2002. A matter of taste: Investigating sensory modalities in the protozoan Paramecium. The Science Teacher69(9): 36-40
- Elwess NL, Latourelle SM, Murphy M. 2017. Developing Scientists: Authentic Research in Genetics Using Paramecia. The American Biology Teacher 79(4):272-279.
- Bohrer KE. 2006. Effects of drugs on pulsation rate of Lumbricoides variegatus (blackworms). Association for Biology Laboratory Education 2005 Proceedings 27:127-146
- Ryan AB, Elwess NL. 2017. A New Approach in Examining the Influence of Drugs on Pulsation Rates in Blackworms (Lumbriculus variegatus). Bioscene 43(2):38-43
- Baird DJ, Barber I, Bradley M, Calow P, Soares AMVM. 1989. The Daphnia bioassay: a critique. Hydrobiologia 188(1):403-406
- Martins J, Teles O, Vasconcelos V. 2007. Assays with Daphnia magna and Danio rerio as alert systems in aquatic toxicology. Environment International. 33(3):414-425
- Corotto F, Ceballos D, Lee A, Vinson L. 2010. Making the most of the Daphnia heart rate lab: Optimizing the use of Ethanol, Nicotine, & Caffeine. The American Biology Teacher 72(3):176-179
- Wang JTH. 2017. Course-based undergraduate research experiences in molecular biosciences- patterns, trends, and faculty support. FEMS Microbiology Letters 364(15), fnx157.
- Drew JC, Triplett EW. 2008. Whole genome sequencing in the undergraduate classroom: Outcomes and lessons from a pilot course. Journal of Microbiology biology education. 9(1):3-11
- Jordan TC, Burnett SH, Carson S, Caruso SM, Clase K, DeJong RJ, Denney JJ, Denvr DR, Dunbar D, Elgin SCR, Findley AM, Gissendanner CR, Golebiewska UP, Guild N, Hartzog GA, Grillo WH, HollowellGP, Hughes LE, Johnson A, King RA, Lewis LO, Li W, Rosenzweig F, Rubin MR, Shah MS, Sandoz J, Shaffer CD, Taylor B, Temple L, Vazquez E, Ware VC, Barker LP, Bradley KW, Jacobs-Sera D, Pope WH, Russell DA, Cresawn SG, Lopatto D, Bailey CP, Hatfull GF. 2014. A broadly implementable research course in phage discovery and genomics for first-year undergraduate students. mBio 5(1):e01051-13
- Gardner SM, Adedokun OA, Weaver GC, Bartlett EL. 2011. Human Brains Engaged in Rat Brains: Student-driven neuroanatomy research in an introductory biology lab course. J Undergrad Neuroscience Education 10(1):A24-A36
- Rivers DB. 2002. Using a course-long theme for inquiry-based laboratories in a comparative physiology course. Advances in physiology education 26(4):317-326
- Kloser MJ, Brownell SE, Chiariello NR, Fukami T. 2011. Integrating Teaching and Research in Undergraduate Biology Laboratory Education. PLOS Biology 9(11):e1001174
- Oberhauser K, LeBuhn G. 2012. Insects and Plants: Engaging undergraduates in authentic research through citizen science. Frontiers in Ecology 10(6):318-320.
- Kloser MJ, Brownell SE, Shavelson RJ, Fukami T. 2013. Effects of a Resaerch-Based Ecology Lab Course: A study of Nonvolunteer Achievement, Self-Confidence, and Perception of Lab Course Purpose. Journal of College Science Teaching 42(3): 72-82
- Freeman, S., Eddy, S. L., McDonough, M., Smith, M. K., Okoroafor, N., Jordt, H., & Wenderoth, M. P. (2014). Active learning increases student performance in science, engineering, and mathematics. Proceedings of the National Academy of Sciences, 111(23), 8410-8415.https://doi.org/10.1073/pnas.1319030111
- Fitzgerald, D. (2013). Employing think-pair-share in associate degree nursing curriculum. Teaching and Learning in Nursing, 8(3), 88-90.https://doi.org/10.1016/j.teln.2013.01.006
- Angra, A., & Gardner, S. M. (2017). Reflecting on graphs: Attributes of graph choice and construction practices in biology. CBE Life Sciences Education, 16(3), 1-15. https://doi.org/10.1187/cbe.16-08-0245
- Dasgupta, A. P., Anderson, T. R., & Pelaez, N. (2014). Development and validation of a rubric for diagnosing students' experimental design knowledge and difficulties. CBE Life Sciences Education, 13(2), 265-284. https://doi.org/10.1187/cbe.13-09-0192
- Lie R, Abdullah C, He W, Tour E. 2016 Perceived Challenges in Primary Literature in a Master's Class: Effects of Experience and Instruction. CBE-Life Sci Educ;1-12. Available from: http://www.lifescied.org/content/15/4/ar77.short
- Abdullah C, Parris J, Lie R, Guzdar A, Tour E. 2015 Critical Analysis of Primary Literature in a Master's-Level Class: Effects on Self-Efficacy and Science-Process Skills. Cell Biol Educ;14(3):ar34-ar34. Available from: http://www.lifescied.org/cgi/doi/10.1187/cbe.14-10-0180
- Hoskins SG, Lopatto D, Stevens LM. 2011. The C . R . E . A . T . E . Approach to Primary Literature Shifts Undergraduates ' Self-Assessed Ability to Read and Analyze Journal Articles , Attitudes about Science , and Epistemological Beliefs. 2011;10:368-78.
- Bunner HC, Halcrow K. 1977. Experimental Induction of the Production of Ephippia by Daphnia magna (Cladocera). Crustaceana 32(1);77-86
Article Files
Login to access supporting documents
Gleichsner-Dynamic Daphnia- An inquiry-based research experience in ecology that teaches the scientific process to first-year biologists.pdf(PDF | 231 KB)
S1.Dynamic_Daphnia_LabManual_0.pdf(PDF | 2 MB)
S2.Dynamic_Daphnia_Glossary_0.docx(DOCX | 20 KB)
S3.Dynamic_Daphnia_DaphniaCare_1.docx(DOCX | 25 KB)
S4_Dynamic_Daphnia_PreLabQuizzes_0.docx(DOCX | 26 KB)
S5_Dynamic_Daphnia_ExcelTemplate_0.xlsx(XLSX | 21 KB)
S6.Dynamic_Daphnia_SampleLiterature_0.docx(DOCX | 140 KB)
S7.Dynamic_Daphnia_HowToReadScientificPapers_0.doc(DOC | 30 KB)
S8a_Dynamic_Daphnia_PosterTemplate1_0.pptx(PPTX | 39 KB)
S8b_Dynamic_Daphnia_Poster_Template2_0.pptx(PPTX | 41 KB)
S9.Dynamic_Daphnia_PosterRubric_0.docx(DOCX | 17 KB)
S10.Dynamic_Daphnia_Alignment_Table_0.xlsx(XLSX | 13 KB)
- License terms

Comments
Comments
There are no comments on this resource.