Using a Sequential Interpretation of Data in Envelopes (SIDE) approach to identify a mystery TRP channel
Published online:
Abstract
This lesson sought to engage students in data interpretation and to encourage critical thinking about neurophysiology in the context of temperature sensation. A family of receptors called Transient Receptor Potential (TRP) channels are activated in response to specific temperatures. Upon activation, TRP channels can trigger sensory neurons to signal the perception of temperature. In this lesson, that we have tested with nearly 1000 students in 12 class sections over four years, students worked in groups of three to identify a "mystery" TRP channel by interpreting five different sets of data. In this activity, we used an approach that we call Sequential Interpretation of Data in an Envelope (SIDE), where students sequentially analyze primary source-like data that are on folded pieces of paper in an envelope. Students analyzed data and hypothesized which TRP channel(s) might be their mystery TRP channel. Students then analyzed four additional sets of data from different experiments and revised their hypotheses about their mystery TRP channel after each experiment. There are four different mystery TRP channels so different groups of three students each analyzed different data and came to different conclusions. This lesson gave students the opportunity to analyze data from multiple experiments. We assessed learning through an in-class worksheet, which students completed as they interpreted each data set and a post-class homework assignment, which students completed online.
Citation
Cala, J.M., Cooper, K.M., and Brownell, S.E. 2018. Using a Sequential Interpretation of Data in Envelopes (SIDE) approach to identify a mystery TRP channel. CourseSource. https://doi.org/10.24918/cs.2018.7Society Learning Goals
Cell Biology
- Cell Communication
- How do cells send, receive, and respond to signals from their environment, including other cells?
- Methods & Tools of Cell Biology
- How do the methods and tools of cell biology enable and limit our understanding of the cell?
Lesson Learning Goals
- Students will further their understanding of the physiology of TRP channels.
- Students will understand information flow in the context of temperature sensation/neuronal signaling.
- Students will understand how structure impacts function in the context of ligand and temperature specificity and ion channel function.
- Students will value the need for replication in science and have a better understanding of the nature of science.
Lesson Learning Objectives
- Students will be able to analyze data from multiple experimental methodologies to determine the identity of their "mystery" TRP channel.
- Students will be able to interpret the results of individual experiments and from multiple experiments simultaneously to identify their "mystery" TRP channel.
- Students will be able to evaluate the advantages and limitations of experimental methodologies presented in this lesson.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
The national report Vision and Change calls for students to be able to apply the process of science (1). Students need to be able to analyze and interpret data in order to derive working hypotheses about scientific phenomena. They also need to understand how to interpret results from multiple experiments to refine and revise their hypotheses. Simultaneously, students need to understand the limitations of their ability to form conclusions from a single experiment and how there is a need to replicate experiments. While we often think of lab-based courses and course-based undergraduate research experiences as the places for students to be able to engage in the process of science and understand the nature of science (2-4), there is evidence that we can bring these practices into our lecture-based courses to increase the exposure of students to the skills of thinking like a scientist (5,6). However, to facilitate the implementation of these practices in a large-enrollment, lecture-based course, we may need to be able to do this in a low-cost way with no specialized equipment.
Building on these ideas, we have designed an approach to allow students, in the context of a large-enrollment biology lecture course, to interpret primary source-like data from multiple experiments and to collectively interpret those data to come to a working hypothesis. We call this approach Sequential Interpretation of Data in an Envelope (SIDE). In this approach, groups of students are given an envelope with multiple experiments inside on folded pieces of paper. When given the cue by the instructor, students open the envelope then analyze and interpret the results from the first experiment on the respective piece of paper. Only when the instructor gives the next cue do the students move on to analyze and interpret the results of the next experiment on the corresponding piece of paper. As they interpret each set of data, they refine and revise their hypotheses so that their ultimate hypothesis is built on multiple sets of data from different experiments. Students complete a worksheet simultaneously to help scaffold the activity and ensure that they are generating hypotheses at each step.
This SIDE activity was developed and tested with almost 1000 students in groups of three in 12 different sections over 4 years. Students are guided through the analysis of data from in vitro, ex vivo, and in vivo research methodologies that measure activation of Transient Receptor Potential (TRP) channels and temperature sensation. In this activity, students work in groups of three to come to a hypothesis about the most likely identity of a mystery TRP channel. Using known information about TRP channel stimulation and physiology, students sequentially analyze and interpret primary source-like data using electrophysiology, calcium imaging, and behavioral assays to narrow down the identity of their mystery TRP channel.
This SIDE approach promotes analytical thinking by giving students the opportunity to engage in the process of science in the lecture classroom. We argue that this approach could be used for other topics in undergraduate biology courses.
INTENDED AUDIENCE
This activity was developed for upper-division life sciences majors, but the SIDE method could be used for non-majors or lower-division courses with different content. The course is a large-enrollment, upper-division Animal Physiology course at a large research university.
REQUIRED LEARNING TIME
This activity is designed for a 60-minute lesson, but can be modified for a 50-minute class by removing the last 10 minute discussion. There are 5-10 minutes of introduction to the activity; 5-10 minutes of group analysis of each data set, with 2 minutes of class discussion (five data sets); and a 10-minute discussion at the end.
PRE-REQUISITE STUDENT KNOWLEDGE
Students need to understand the basic principles of receptors, ion channels, and action potentials. Specifically, they need to understand that TRP channels are non-specific cation channels on sensory neurons that allow calcium ions to flow into the neuron causing depolarization in the sensory neuron, which could lead to an action potential in that sensory neuron. Students should also recognize that TRP channels respond to specific temperatures and chemical agonists, and ultimately signal temperature sensation to the brain, which could lead to behavioral responses.
PRE-REQUISITE TEACHER KNOWLEDGE
Instructors need to have a clear understanding of basic neurobiology, TRP channel signaling, and the nature of science, specifically, how only tentative hypotheses can be derived from a single experiment. An instructor should also be familiar with the specifics of the experimental approaches used, namely electrophysiology, in vitro cell culture, transgenic animals, chemical agonists and antagonists, and calcium imaging. Teaching assistants (TAs) and undergraduate learning assistants (LAs) were introduced to the complete lesson by the instructor during TA meeting a week before the lesson was taught to students. The TAs and LAs were instructed to guide students through the activity by asking questions about the data in envelopes to scaffold students' thinking. They were explicitly told not to provide students with the correct answers. The TAs and LAs were also given all the material for the lesson a week beforehand to ensure that they were familiar enough with the content and activity.
SCIENTIFIC TEACHING THEMES
Active Learning
Students use the SIDE approach to analyze data and identify a mystery TRP channel. Students work in small groups of three to analyze and interpret data to better understand TRP channel function. The group size of three is useful because it provides three opinions on the analysis of complex data, but it is not so large so that students do not get to participate in the activity.
We have developed different sets of envelopes for four mystery TRP channels so different student groups analyze different data (See Supporting File S1: Using a SIDE approach - Data set). This can promote student ownership over "their" mystery TRP channel.
Students actively construct their knowledge in small groups, and groups do not report out to the whole class. This approach maximizes the amount of time students actively engage with the data in their groups.
Assessment
A pre-class assignment on TRP channels: students read a review article (7) about TRP channels and answer questions about the article before coming to class (Supporting File S2: Using a SIDE approach - Pre-class assignment key).
An in-class worksheet: students answer questions about each data set and provide a detailed justification for which channels can be excluded from or included in their working hypothesis about which TRP channel they have (Supporting File S3: Using a SIDE approach - In-class worksheet key).
A post-class homework assignment on TRP channels: students answer an exam-like question about concepts that were emphasized in the TRP channel SIDE activity, and a question about how TRP channel signaling is an example of the core concept of information flow (Supporting File S4: Using a SIDE approach - Post-class homework assignment key).
Inclusive Teaching
Having students work in small groups encourages more involvement with the material and creates a collaborative learning environment. To ensure that each student in the group has a chance to engage with the activity and handle the pieces of paper, we recommend that instructors limit groups to three students each.
Each student has their own worksheet so they are able to write down their own ideas.
The end-of-class discussion highlights the need for diversity in science, emphasizing how more diverse scientists minimize the potential for bias in scientific reasoning (8). The importance of repeating experiments is also emphasized.

Figure 1. Images of students participating in the SIDE activity.
LESSON PLAN
The lesson plan includes the course context, materials required, instructor preparation, instructor instructions, student instructions, student assessment, descriptions of experimental techniques, a description of the data sets for each mystery TRP channel, and a timeline - which outlines pre-class, during class, and post class instructor preparation (Table 1).
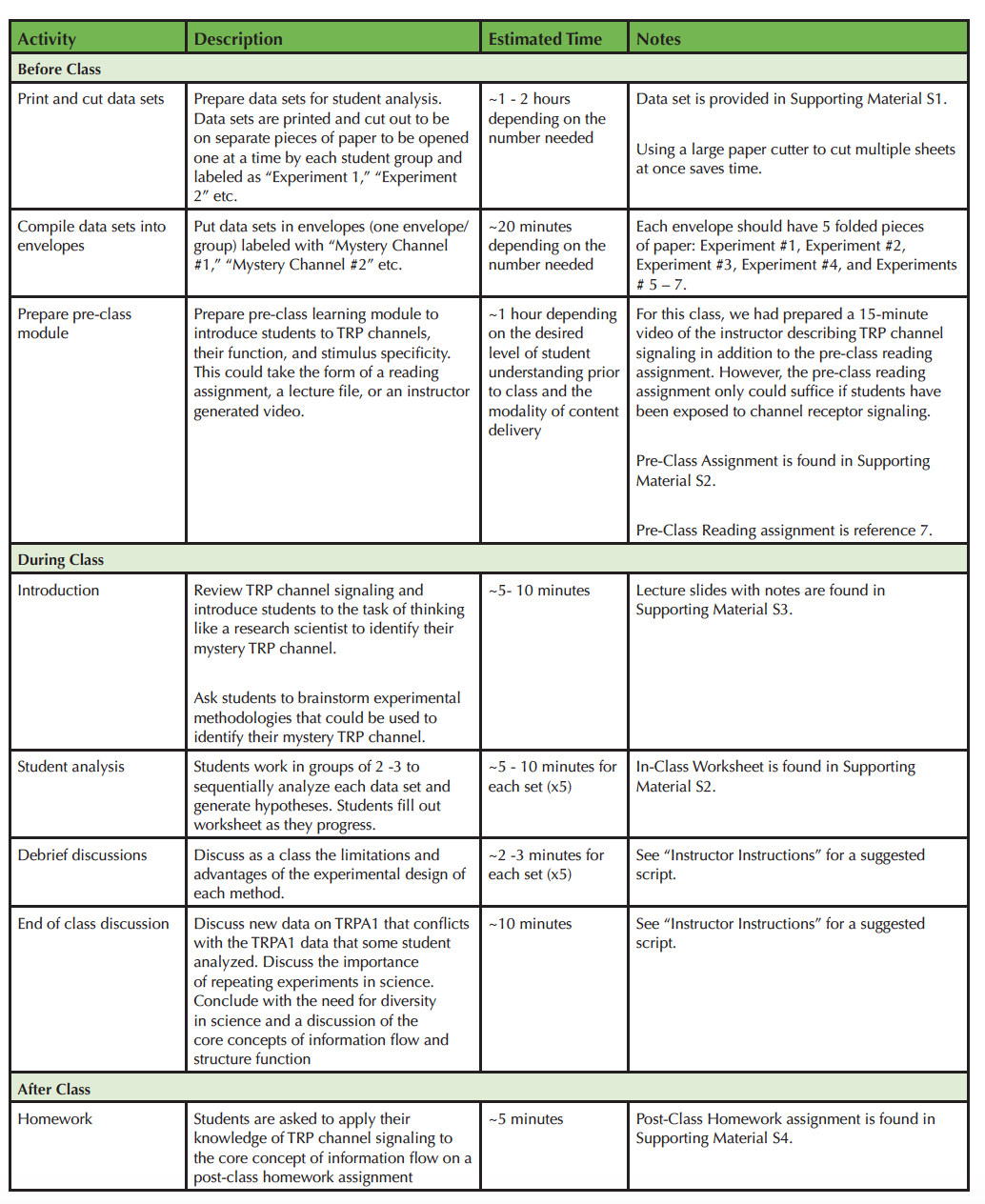
Table 1. Teaching Timeline
Course Context
This lesson was taught in a discussion/recitation section for an upper-division Animal Physiology class of ~300 students. Discussion/recitation sections are three medium sized classes (~90 students) that meet in a scale-up classroom with tables of six students. During the activity, the instructor, one or two graduate TAs and one or two undergraduate LAs were circulating around the room, guiding individual groups and answering questions.
Materials Required
- Envelopes (number of students in your class divided by three is the number of envelopes you need)
- Scissors or paper cutter
- Access to a colored printer
Instructor Preparation
Take the number of students in your class and divide by three. This is the total number of envelopes that you will create. If the total number of students is not evenly divisible by three, then we would suggest creating two groups of two students (if one student remains) or a single group of two students (if two students remain). There are four different mystery TRP channels, so you will divide the number of groups by four to determine how many sets of data you need to prepare for each mystery TRP channel. Print that number of data sets (Supporting File S1: Using a SIDE approach - Data set) for each mystery TRP channel on separate pieces of one-sided paper and cut out each individual experiment. Each piece of paper should be folded and labeled with the corresponding experiment number on the outside. Each envelope should have five pieces of folded paper, each labeled on the outside of the fold with the appropriate experiment number. Label the outside of the envelope with the mystery channel number (Figure 2). Do not seal the envelope.
Immediately before the class, set out the envelopes. We carried out this SIDE activity in an active learning classroom with tables of six students, so we put two envelopes in the center of the table. We made sure that the envelopes were of different mystery TRP channels so students at the same table worked with different data. Placing the envelopes before class saves time, but you could also pass out the envelopes to students during class if you do not have the opportunity to put out the envelopes before class.
Instructors need to print out worksheets for each student in the class. Although students will be working in groups, we have our students complete individual worksheets to promote accountability and student learning.

Figure 2. Example of the envelope set up that students will view. A. Envelope of Mystery Channel #1. B. Experiments #1, #2, #3, #4, and #5 – 7 folded in the envelope for Mystery Channel #1. C. Experiment #1 opened (indicating how students should open one experiment at a time). D. All experiments opened for Mystery Channel #1.
Instructor Instructions
1. Use a PowerPoint presentation to guide the class discussion and SIDE activity (Supporting File S8: Using a SIDE approach - Lecture). Begin the lesson by reviewing the steps of TRP channel signaling. This is meant to be a brief review since the students should have already been exposed to the basics of TRP channel signaling in previous course periods.
2. Present the following scenario to students: "You are research scientists attempting to identify a TRP channel. Your worksheet has a list of known TRP channels and characteristics of each TRP channel. Your task is to analyze data to identify which channel you have. Each group will be exploring a different channel." Inform students that they will be working in groups of three and that their mystery TRP channel data are in an envelope. You will need to tell students explicitly to not open the envelope at this point and that when they do open the envelope, to only unfold the first experiment. Students are often eager to look at all of the data at once, so this instruction about the sequential interpretation of data is critical. Ask students not to write on the pieces of paper so that the data papers can be re-used with a different section or class.
3. To help students begin thinking about what experiments could be done to determine a specific TRP channel, ask them to brainstorm in their groups what experiments could be used to measure the activation of TRP channels. Allow students ~2 minutes to brainstorm experiment ideas with their partners and walk around the classroom to hear students' ideas during this discussion time. After, you should not specifically debrief any of these experiments, but perhaps mention that you heard a lot of really good ideas.
4. You will then present the experimental methodology for experiment #1, which is electrophysiology using primary sensory neurons isolated from an organism. The figures presented in this exercise were generated based, in part, on primary source data. Describe the experimental approach of measuring membrane potential changes and some of the advantages and disadvantages of this approach. You could say "sensory neurons that primarily express your TRP channel were isolated from an organism. The advantage of this type of experiment is that you are working with sensory neurons, making it more authentic because that is the cell type that expresses TRP channels in organisms. However, as you analyze your results, keep in mind that other ion channels may also be expressed in these neurons. The isolated sensory neurons were incubated at specific temperatures and probes were inserted into the membrane to measure membrane potential. Please now open experiment #1 in your envelopes and evaluate the results." Allow approximately 5 - 10 minutes for students to analyze the data and answer question #1 on the worksheet. Debrief this experiment by saying "I heard a lot of good thinking and a lot of you were able to eliminate some TRP channels, but we need to do more experiments to be able to continue to narrow down which TRP channel might be your mystery TRP channel. Further analysis will allow us to be more confident in our conclusions because these sensory neurons could be expressing other channels that are causing the effects."
5. Then guide students into experiment #2 by asking the following question: "Now that we have seen how an isolated sensory neuron primarily expressing your channel responds at specific temperatures, how can we take a reductionist approach to be more confident that your channel is responsible for eliciting that response?" Present the experimental methodology for using a cell line that only expresses that mystery TRP channel to determine activation of TRP channels using calcium imaging. Because TRP channels are non-specific cation channels, their activation can be measured by using an intracellular calcium indicator. Specifically, you can say: "A non-neuronal cell line that does not ordinarily express TRP channels has been transfected with your mystery TRP channel gene. These cells were then injected with a calcium indicator and incubated at specific temperatures. The method of calcium imaging allows for the detection of intracellular calcium. The calcium indicator binds to intracellular calcium and fluoresces. Thus, if your channel opens and calcium influxes, cells will fluoresce. Please now open experiment #2 and evaluate the results." Allow 5-10 minutes for students to analyze this experiment and answer question #2 on the worksheet. Debrief this experiment by asking the rhetorical questions: "How does experiment #2 compare to experiment #1? Does the more reductionist approach support your hypothesis from experiment #1?"
6. You will then transition students to experiment #3 by asking the following question: "So far, we've been evaluating the effects of temperature and chemicals on your TRP channel in isolated cells, but whole organisms experience temperature sensation. How can we evaluate the effects of TRP channel signaling in a whole organism?" You can introduce students to the research methodology of experiment #3 by saying: "Transgenic knock-out mice were generated by deleting your mystery TRP channel gene, thus your channel is not expressed anywhere in the mouse. This can help us see if your mystery TRP channel is essential for sensation of a specific temperature. These mice are placed on plates that are held at a specific temperature and behavioral responses are observed in comparison to a wild-type mouse that has your functional mystery TRP channel. Please now open experiment #3 and evaluate the results." Allow 5-10 minutes for students to analyze and answer question 3 on the worksheet. Debrief by saying that these experiments should be building on each other, but we want to continue to probe this system to increase our confidence in our interpretation of the data.
7. Highlight that there are established chemical antagonists that are specific for TRP channels that can block signaling and briefly describe the definition of a chemical antagonist. Direct students to the table on their worksheet that outlines the known TRP antagonists. Remind students of the methodology of calcium imaging and inform students that experiment #4 is replicating calcium imaging in the transfected cells, but with the addition of specific chemical antagonists. Allow 5-10 minutes for students to analyze and answer question #4 on the worksheet. Note: many of these chemical antagonists are meant to be distractors for students to help them understand how to evaluate data using chemical antagonists. Some of the antagonists chosen for this experiment produce null findings because using other chemical antagonists would direct students to only one TRP channel prematurely.
8. You can then rhetorically ask the students if they are able to conclusively identify their TRP channel. At this point, all groups should still have two possible TRP channels that their mystery TRP channel could be. Ask the students what could be done next. Allow 2 minutes for students to answer question #5 on the worksheet.
9. Many of the students should have come up with the idea of using chemical agonists so you should introduce students to experiments #5-#7, which are repeated methods of experiments #1-3, but now in the presence of chemical agonists. Allow 5-10 minutes for students to analyze and answer questions #6 and #7 on the worksheet. At this point, comparing all of the data from the experiments, students should have identified their mystery TRP channel.
10. Poll the class for the identity of each mystery channel using choral responses where all of the students in the class simultaneously say the answer. You should ask students who had Mystery TRP channel #1 what they think it is and should subsequently ask about Mystery TRP channel #2, #3, and #4. The identity of each Mystery TRP channel is listed on slide 27 (in Supporting File S8: Using a SIDE approach - Lecture) and additional information about each channel is outlined in the Description of the Data Sets for Each Mystery TRP Channel section. The students are often excited about these conclusions and very confident. You should ask students if they are 100% certain of their conclusions based on these sets of experiments. Highlight that although different experimental approaches were used, the students only analyzed one set of data for each experiment. Experiments need to be repeated in science to be more confident of one's findings.
11. As a whole class, introduce students to new TRPA1 data that conflicts with the TRPA1 data that some students analyzed. Ask students to draw conclusions from these conflicting data. Allow 3 minutes for students to answer question #8 on the worksheet.
12. As a class, debrief question #8 on the worksheet by highlighting how two different research groups came to different findings. Based on only these two results, one can't conclude anything because they are contradictory. This implies that more experiments need to be done to determine the role of TRPA1 in temperature sensation. Describe that what is known to be "fact" is often changing based on new and emerging data until so much evidence has accumulated that it is unlikely to change. Highlight that every researcher has their own background, experiences, values, and interests that may influence how they tackle scientific problems and thus, science is often subjective. However, when we have a diverse group of scientists working together to solve a problem, science becomes more objective, which supports the argument for why we need diverse scientists from different backgrounds (8). Further, you can highlight the need to replicate research findings and discuss how the TRP channel community is still exploring how TRPA1 relates to temperature sensation.
13. Conclude the class with a discussion/overview of the core concepts of information flow and structure function in the context of TRP channel signaling.
14. Ask students to fold their pieces of paper back up and put them back into their envelopes. Students should leave the envelopes on the tables and turn in their individual worksheets on their way out of class.
Student Instructions
Most instructions are given to students verbally by the instructor and on the written worksheet. The instructions provided by the instructor to students are presented under Instructor Instructions.
Student Assessment
We evaluated student achievement of the learning objectives by grading each individual's in-class worksheet (Supporting File S3: Using a SIDE approach - In-class worksheet key) and a post-class homework assignment (Supporting File S4: Using a SIDE approach - Post-class homework assignment key) that required students to apply their knowledge obtained from this lesson to the core concept of information flow. The instructors also circulate around the room when students are completing the activity to check in on student understanding in real-time. For example, if an instructor notices that students seemed to be confused about an experimental approach or if a student misconception is revealed, then the instructor can address the concern or misconception with the whole class in real-time.
Descriptions of Experimental Techniques
Data sets were generated for the purpose of the lesson based on known information about TRP channel signaling and based, in part, on primary source data. There are four different mystery TRP channels so neighboring groups are not working with the same channel (Supporting File S1: Using a SIDE approach - Data set).
- Experiment #1: Ex vivo electrophysiology data from sensory neurons primarily expressing TRPA1, TRPV1, TRPM8, or TRPV1b while incubated at specific temperatures (we generated data based on figures from reference 9 and known information about TRP channels).
- Experiment 2: In vitro calcium imaging from HEK293 cells transfected with only TRPA1, TRPV1, TRPM8, or TRPV1b and incubated at specific temperatures (we generated data based on figures from reference 10 and known information about TRP channels).
- Experiment 3: In vivo experiment with transgenic mice that have a deletion of the TRPA1, TRPV1, TRPM8, or TRPV1b gene. Mice are placed on surfaces set to the specified temperatures and behavior is recorded (we generated data based on known information about TRP channels).
- Experiment 4: In vitro calcium imaging from HEK293 cells transfected with TRPA1, TRPV1, TRPM8, or TRPV1b and incubated at the specified temperature while applying TRP channel antagonists (we generated data based on figures from reference 10 and information in reference 11).
- Experiments 5-7: Ex vivo electrophysiology, in vitro calcium imaging, and in vivo transgenic mice experiments with the chemical ligands: capsaicin, menthol, and allicin (we generated data based on figures from references 9 and 10, information in reference 11, and known information about TRP channels).
New data for whole class discussion:
- TRPA1 data showing contradictory results of the calcium imaging data students analyzed (we generated data based on reference 12)
- TRPA1 in rodents and humans in vitro demonstrating uncertainty regarding whether TRPA1 is cold sensitive (reference 13)
- TRPA1 and snake pit organs (reference 14)
Description of the Data Sets for Each Mystery TRP Channel (Figure 3)
Mystery TRP Channel #1 = TRPV1: moderate frequency of action potentials are seen at 35°C and the frequency of action potentials further increased at 50°C in primary sensory neurons; moderate calcium influx is seen at 35°C and further increased at 50°C in transfected HEK293 cells; transgenic knock-out mice experience a less aversive response to 50°C (but the response is not completely absent because other channels such as TRPV1b also respond to high temperatures); antagonist data demonstrate that out of the antagonists administered, only I-RTX prevents calcium influx at 35°C and 50°C in HEK293 cells; and transgenic knock-out mice show no response to capsaicin as TRPV1 is the only known channel responsive to capsaicin and a reduced response to allicin (as TRPA1 is also responsive to allicin).
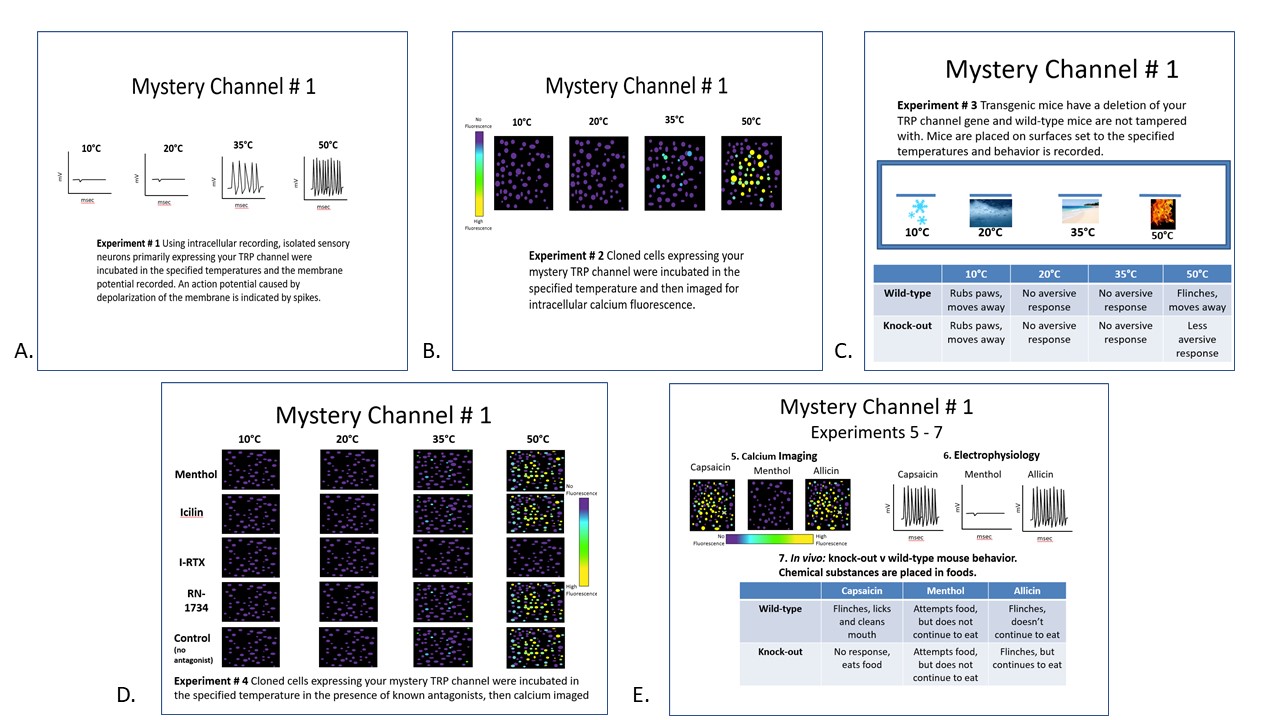
Figure 3. Example data set: Mystery TRP Channel #1 (TRPV1). A. Experiment 1: shows that in sensory neurons primarily expressing mystery TRP channel #1, action potentials are recorded at 35°C and at an increased frequency 50°C. B. In HEK 293 cells transfected with TRPV1, calcium influx is observed at 35°C and a substantial influx at 50°C. C. Experiment 3 reveals that knock-out mystery TRP channel #1 mice have a less aversive response at 50°C than wild-type mice. D. Experiment 4 shows antagonist data demonstrate that only I-RTX prevents calcium influx at 35°C and 50°C in transfected HEK293 cells. E. Experiments # 5- 7 show calcium influx, and neuronal firing to capsaicin and allicin. In the transgenic knock-out mice, there is an absence of response to capsaicin, and a reduced response to allicin. From the cumulative data, students can conclude their mystery channel is TRPV1.
Mystery TRP Channel #2 = TRPV1b: moderate frequency of action potentials are seen at 35°C and the frequency of action potentials further increased at 50°C in primary sensory neurons; moderate calcium influx is seen at 35°C and further increased at 50°C in transfected HEK 293 cells; transgenic knock-out mice experience a less aversive response to 50°C (as TRPV1 also responds to high temperatures); antagonist data demonstrate that out of the antagonists administered; only I-RTX prevents calcium influx at 35°C and 50°C in transfected HEK 293 cells; and transgenic knock-out mice show no change compared to wild-type in response to any of the chemical agonists.
Mystery TRP Channel #3 = TRPA1: action potentials are seen at 10°C and at a decreased rate at 20°C in primary sensory neurons; calcium influx is seen at 10°C and minimal influx is seen at 20°C in transfected HEK 293 cells; transgenic knock-out mice experience a less aversive response to 10°C (as other channels such as TRPV3 also respond to cold temperatures); antagonist data demonstrate that none of the antagonists administered prevent calcium influx at 10°C and 20°C; and transgenic knock-out mice show a reduced response to allicin (as TRPV1 is another channel responsive to allicin).
Mystery TRP Channel #4 = TRPM8: action potentials are seen at 10°C and at an increased rate at 20°C in primary sensory neurons; calcium influx is seen at 10°C and at an increased rate at 20°C in transfected HEK 293 cells; transgenic knock-out mice experience no changes compared to wild-type mice at each temperature (as 20°C does not elicit an aversive response); antagonist data demonstrate that none of the antagonists administered prevent calcium influx at 10°C and 20°C; and transgenic knock-out mice show a less aversive response to menthol (as TRPV3 is another channel responsive to menthol).
TEACHING DISCUSSION
In this activity, we used what we are calling the Sequential Interpretation of Data in Envelopes (SIDE) as a way to engage students in the process of science. Students were able to analyze multiple sets of data and collectively interpret them. The process of having students compare and contrast data from different experiments makes it a more complex task that better mimics the process of research (15).
Students appear to be highly engaged with this task, treating it like a puzzle to solve. The complexity of the data does not allow for much time for students to get off-task if the instructor is attentive to when the majority of students have completed the worksheet question. Students are often frustrated by the contradictory TRPA1 data because they struggle with not being able to come to a conclusion about a dataset. However, the instructor can alleviate student concerns by acknowledging that frustration and highlighting how messy data is an inherent component of scientific research.
Instructors, graduate TAs, and undergraduate LAs circulated the room to ensure that students were on task, to probe into student understanding, and be available for student questions. The number of instructors allowed us to nudge groups of students to think more critically about the data and the limitations of experiments. A common issue was that students were too confident in their conclusions and did not acknowledge that for some experiments there could be more than one explanation for the data. Often the instructor identified common issues that students were having and debriefed these issues with the class as a whole. Thus, in-class formative assessment was used to gauge student progress towards the learning goals. Students appeared to enjoy being challenged and many students expressed benefitting from the "real-world" application of scientific discovery to understand temperature sensation/neuronal signaling. Students demonstrated improvement in their critical thinking skills using data interpretation and problem-solving skills to include or eliminate channels after each experimental analysis.
We had the benefit of having between four and five instructors for 90 students and we acknowledge that other instructors may not have the same level of instructional support team. We think that this activity would still be possible to do with only one or two instructors circulating around the room, but it would be more challenging to assess student progress and clarify student misunderstandings.
The data chosen were tailored to the needs of our class; however, different data sets that are more or less challenging could also be used. For example, we added the chemical antagonist experiments to increase the challenge of the activity when it seemed like it was too easy for some students. We conducted this activity in a scale-up classroom with large tables for six students each. While this setup was helpful for students to display the pieces of paper, we think that this activity could be implemented in a lecture hall. However, there may be greater numbers of logistical challenges, namely passing out the envelopes and students potentially losing pieces of paper.
This SIDE activity promoted analytical thinking by giving students the opportunity to engage in the process of science in the lecture classroom by analyzing data in a low-cost way. We encourage other instructors to consider this approach, even with different topics and data, if they want students to learn how to interpret complex datasets.
SUPPORTING MATERIALS
- S1. Using a SIDE approach - Data set with each set on separated slides for easy printing.
- S2. Using a SIDE approach - Pre-class assignment key to be given to students before class.
- S3. Using a SIDE approach - In-class worksheet key for students to complete during the mystery TRP channel activity.
- S4. Using a SIDE approach - Post-class homework assignment key containing questions students completed online after class.
- S5. Using a SIDE approach - Figure 1: Students participating in the SIDE activity
- S6. Using a SIDE approach - Table 1: Lesson Plan Outline describing before, during, and after class preparation.
- S7. Using a SIDE approach - Figure 2: Envelope set up for mystery TRP channel #1
- S8. Using a SIDE approach - Lecture file with notes for this SIDE activity.
- S9. Using a SIDE approach - Figure 3: Data experiments for mystery TRP channel #1 - A figure showing an example of the compiled data sets.
ACKNOWLEDGMENTS
We would like to thank the co-instructor of this course, Miles Orchinik. We also thank Daniel Grunspan, Samiksha Raut, and Michelle Stephens for their valuable feedback, and the graduate teaching assistants and the undergraduate learning assistants for their aid in facilitating the activity.
References
- American Association for the Advancement of Science (AAAS) (2010). Vision and Change: A Call to Action, Washington, DC.
- Myers MJ, Burgess AB. 2003. Inquiry-based laboratory course improves students' ability to design experiments and interpret data. Adv Physiol Ed.27:26-33.
- Gasper BJ, Gardner SM. 2013. Engaging students in authentic microbiology research in an introductory biology laboratory course is correlated with gains in student understanding of the nature of authentic research and critical thinking. J Microbiol Biol Educ. 14:25-34.
- Cooper, K. M., Soneral, P. A., & Brownell, S. E. (2017). Define Your Goals Before You Design a CURE: A Call to Use Backward Design in Planning Course-Based Undergraduate Research Experiences. Journal of microbiology & biology education, 18(2).
- DebBurman SK. 2002. Learning How Scientists Work: Experiential Research Projects to Promote Cell Biology Learning and Scientific Process Skills. Cell Biol Educ. 1: 154-172.
- Round, J. E., & Campbell, A. M. (2013). Figure facts: encouraging undergraduates to take a data-centered approach to reading primary literature. CBE-Life Sciences Education, 12(1), 39-46.
- Story, G., & Cruz-Orengo, L. (2007). Feel the Burn: The linked sensations of temperature and pain come from a family of membrane proteins that can tell neurons to fire when heated or hot-peppered. American Scientist, 95(4): 326-333.
- Intemann, K. (2009). Why diversity matters: Understanding and applying the diversity component of the National Science Foundation's broader impacts criterion. Social Epistemology, 23(3-4), 249-266.
- Perez-Reyes E, 2003. Molecular Physiology of Low-Voltage-Activated T-type Calcium Channels, Phy Rev. 83:117-161
- Hinman A, Chuang H, Bautista D, Julius D. TRP channel activation by reversible covalent modification. 2006. PNAS. 103: 19564-19568.
- Fern?ndez-Carvajal, A., Fern?ndez-Ballester, G., Gonz?lez-Mu?iz, R., & Ferrer-Montiel, A. (2015). Pharmacology of TRP channels. In TRP Channels in sensory transduction (pp. 41-71). Springer International Publishing.
- Jordt, S. E., Bautista, D. M., Chuang, H. H., McKemy, D. D., Zygmunt, P. M., H?gest?tt, E. D., & Julius, D. (2004). Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature, 427(6971), 260-265.
- Caspani, O., & Heppenstall, P. A. (2009). TRPA1 and cold transduction: an unresolved issue? The Journal of general physiology, 133(3), 245-249.
- Gracheva, E. O., Ingolia, N. T., Kelly, Y. M., Cordero-Morales, J. F., Hollopeter, G., Chesler, A. T., & Julius, D. (2010). Molecular basis of infrared detection by snakes. Nature, 464(7291), 1006-1011.
- Brownell, S. E., Hekmat-Scafe, D. S., Singla, V., Seawell, P. C., Imam, J. F. C., Eddy, S. L., & Cyert, M. S. (2015). A high-enrollment course-based undergraduate research experience improves student conceptions of scientific thinking and ability to interpret data. CBE-Life Sciences Education, 14(2), ar21.
Article Files
Login to access supporting documents
Using a Sequential Interpretation of Data in Envelopes (SIDE) approach to identify a mystery TRP channel(PDF | 965 KB)
S1. Using a SIDE approach - Data set .pptx(PPTX | 425 KB)
S2. Using a SIDE approach - Pre-class assignment key.docx(DOCX | 21 KB)
S3. Using a SIDE approach - In-class worksheet key.docx(DOCX | 29 KB)
S4. Using a SIDE approach - Post-class homework assignment key .docx(DOCX | 17 KB)
S5. Using a SIDE approach - Figure 1.jpg(JPG | 292 KB)
S6. Using a SIDE approach - Table 1.docx(DOCX | 24 KB)
S7. Using a SIDE approach - Figure 2.jpg(JPG | 187 KB)
S8. Using a SIDE approach - Lecture .pptx(PPTX | 2 MB)
S9. Using a SIDE approach - Figure 3.jpg(JPG | 302 KB)
- License terms

Comments
Comments
There are no comments on this resource.