Using Zebrafish in a Developmental Biology Lab Course to Explore Interactions Between Development and the Environment
Editor: William J. Anderson
Published online:
Abstract
Important learning outcomes for biology students include the ability to develop experiments as well as pull together concepts across their coursework. The field of developmental biology, especially environmental influences on development (eco-devo), provides a framework for connecting concepts including tissue dynamics, cell signaling, and physiology. An eco-devo framework also provides opportunities for experiments that are relevant to student interests and/or experiences by encompassing topics such as the impact of environmental contamination or maternal health on development. Here we present a guided course-based undergraduate research experience (CURE) for students to work with zebrafish embryos as a foundation for the design and execution of their own novel research project. The guided experiment that is performed first in this lesson explores how the weed killer atrazine might affect development of zebrafish, even though atrazine would not be expected to impact animals. The student-developed independent experiment is planned during the guided experiment and then performed in subsequent weeks by students in the second part of this lesson. The independent experiment allows students to investigate a research question related to their own interests. These experiments can be modified for a variety of courses depending on the instructor's curriculum, time constraints, and goals for the experiment. Students are particularly engaged in the lesson because it enables them to investigate their ideas and interests.
Citation
Cresiski RH, Lenkowski JR. 2020. Using zebrafish in a developmental biology lab course to explore interactions between development and the environment. CourseSource. https://doi.org/10.24918/cs.2020.20
Society Learning Goals
Developmental Biology
- Gene Networks
- How does the control of gene regulation contribute to development?
- Organogenesis
- How do extracellular factors control organ and tissue growth?
- Experimental Approaches
- How do different organisms help us understand development? And what are their strengths and limitations?
Science Process Skills
- Process of Science
- Pose testable questions and hypotheses to address gaps in knowledge
- Plan, evaluate, and implement scientific investigations
- Interpret, evaluate, and draw conclusions from data
- Construct explanations and make evidence-based arguments about the natural world
- Address novel questions through authentic research experiences
- Modeling/ Developing and Using Models
- Build and evaluate models of biological systems
- Quantitative Reasoning/ Using Mathematics and Computational Thinking
- Apply the tools of graphing, statistics, and data science to analyze biological data
- Communication and Collaboration
- Share ideas, data, and findings with others clearly and accurately
Lesson Learning Goals
At the end of this lesson, students will:
- Appreciate that environmental conditions can impact development of a vertebrate animal.
- Understand the connection between cell signaling and the morphological outcomes of developmental processes.
- Value the use of animals in scientific inquiry.
Lesson Learning Objectives
At the end of this lesson, students will be able to:
- Design, execute, and analyze a novel, controlled experiment to examine how environmental conditions affect developmental outcomes.
- Apply quantitative reasoning skills (e.g. data analysis, serial dilutions, generating data tables and/or graphs, etc.).
- Perform basic morphometric analysis.
- Present experimental findings orally in the form of a scientific meeting presentation.
- Present findings using standard scientific writing conventions.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
There is overwhelming consensus that undergraduate research experiences have positive benefits for student learning gains (1-4) as well as student acquisition of skills and lasting learning (summarized in (5) and (6)). Importantly, these opportunities result in higher retention in STEM majors, graduation from college, and pursuit of STEM graduate degrees (5,7-10). Documented gains for underrepresented populations in STEM fields, including women and students of color, are especially encouraging (11). This has led to a call from the American Association for the Advancement of Science (AAAS) to increase access to research experiences for undergraduate students (12). Unfortunately, traditional apprentice models of undergraduate research are expensive (both faculty and students usually receive financial compensation) and are inefficient (faculty work with a small number of undergraduates at a time) (13-15), so new approaches to increasing research experiences for undergraduates are necessary.
In recent years, an emergent model of course-based undergraduate research experiences, or CUREs, circumvents these barriers to increase access to and benefits of participating in research, and may be a critical mechanism to increase diversity in STEM (16). Auchincloss et al. evaluated existing CUREs and defined common characteristics as having students engage with: 1) scientific practices, 2) discovery, or the investigation of the unknown, 3) work that is broadly relevant/important, 4) collaboration with others, and 5) iteration (17). CUREs are being developed and implemented at individual universities, as large-scale national projects (such as Howard Hughes Medical Institute's SEA-Phages program, www.hhmi.org/developing-scientists/science-education-alliance), and even international consortiums (for example, Yale's Small World Initiative, www.smallworldinitiative.org). Online resources that highlight or curate CURE projects are also being developed, including CureNet (https://serc.carleton.edu/curenet/index.html), but these resources often do not equip interested faculty with details and materials to recreate or execute a similar CURE. Here, a course-based research experience using zebrafish embryos is presented as a mechanism to teach students about environmental impacts on development.
Many research studies have shown that animal development is affected by the environment (eco-devo), whether development occurs internally or externally. Developmental biology textbooks can use this as a principle for teaching developmental biology (18); once a specific environmental effect is observed, scientists can explore the molecular and cellular events that underlie the developmental outcome. Although the current lesson begins with a guided experiment that explores the weed-killer atrazine's effects on development in zebrafish embryos, there are many other eco-devo examples that underscore the relevance of environment on development. For instance, in various reptilian species, if eggs are incubated at a female-producing temperature, estrogen levels in the gonad increase to promote ovary differentiation (19). Interestingly, different reptilian species have different female-producing temperatures, so one species may produce females when eggs are incubated at low temperatures and another species may produce females when eggs are incubated at higher temperatures (20,21). Another well-known example of environment influencing development is the case of thalidomide. Pregnant women were prescribed the drug in the late 1950s through the 1970s in Europe to treat morning sickness. Unfortunately, a concomitant increase in extreme limb malformations resulted in children exposed in utero (22). While the exact mechanism by which thalidomide causes limb defects is still not completely known, it is likely that it is partially due to inhibition of capillary formation that results in cell death (23), a defect that can be rescued by administering nitric oxide to promote angiogenesis in the presence of thalidomide (24).
This lesson uses zebrafish to study development. Frogs (Xenopus) and zebrafish (Danio) are model vertebrate animals that are commonly used in research labs to study toxicology and the interactions between the environment and development in general (25-31). These animals develop externally, and thus can be easily observed throughout all developmental stages; reliably produce large numbers of embryos; develop quickly; and are relatively easy and cost-effective to maintain or acquire. The ease of using zebrafish in the classroom is so recognized that resources are available for their use in K-12 classrooms, i.e. Zebrafish in the Classroom (www.zfic.org), BioEYES (www.bioeyes.org), and Zebrafish K-12 (www.uoneuro.uoregon.edu/k12/zfk12.html). Collegiate classrooms are also recognizing zebrafish as a model organism for guided inquiry, with several publications in the past decade using zebrafish as a tool to teach developmental biology. Zebrafish have been used in course modules to demonstrate second messenger systems (32), oxygen consumption (33), and nervous system development (34). Here we use zebrafish as an accessible system in which students can explore the effects of chemical exposure or other environmental parameters (temperature, pH, etc.) in a classroom laboratory. Students can first observe malformations (or lack of malformations) after a chemical exposure, then hypothesize and research the mechanisms underlying those malformations.
Several studies have used zebrafish as a model system to study the toxicology and teratology of exposure to the weed-killer atrazine. These studies demonstrate the important developmental toxicology concept that duration of exposure and the developmental stage at which exposure occurs should be considered when studying environmental impacts on animal development. For example, in early developmental stages, atrazine can rapidly cross the outer membrane (chorion) that protects the developing zebrafish embryo (35). A study examining the toxicity of atrazine during embryonic, larval, and juvenile stages showed that juvenile and larval stages were more sensitive to constant atrazine exposure than embryonic stages (36). This study reported that atrazine-induced death was apparent within 48 hours of exposure, but atrazine-induced malformations were not specifically monitored (36). At later larval stages, atrazine and its break-down products disrupt swimming behavior in zebrafish larvae, probably due to disruption of neuronal signaling (37,38). At the molecular and cellular level, atrazine exposure changes the activity of a group of enzymes, glutathione s-transferases (GSTs), that add reduced glutathione (a tripeptide antioxidant) to environmental chemicals (xenobiotics) to decrease the toxicity of the chemical. In zebrafish, the atrazine-induced change in GST activity varies depending on the stage of development (18,35). Therefore, the ability of zebrafish embryos to detoxify xenobiotics using GSTs depends on the stage of development (35,39). It is important to note that if zebrafish embryos are removed from an atrazine-contaminated environment, they can clear 75% of the atrazine within 3 hours and nearly all of the atrazine within 48 hours (35), although this study did not screen for subsequent malformations or death as a result of a short-term atrazine exposure. As a result, researchers, including those in a classroom lab, can choose to expose zebrafish embryos to atrazine for discreet periods of time during development to focus on the impact during gastrulation or neurulation, for example, and be confident that atrazine is subsequently detoxified and cleared. Published studies on atrazine exposure in zebrafish typically study the impact of exposure to multiple atrazine concentrations at one stage of development (35,37-41). We know of no published study that has provided a time course of malformations and death as a result of exposure to different atrazine concentrations across zebrafish development.
To date, very few CURE eco-devo projects using zebrafish have been described. A recently published CURE uses zebrafish to study the impacts of nicotine and caffeine on heart development over a 3-semester biology course sequence (42). The lesson plan described here is similar in principle, but it is designed to be completed in a single-semester. During an initial Guided Experiment, students gain experience with the techniques and skills they will need to address their own research question in an Independent Experiment. In the Guided Experiment, students work as a class to determine the developmental outcome when zebrafish embryos are exposed to different concentrations of the weed-killer atrazine during different developmental stages. This lesson also demonstrates how small research groups (lab partners) contribute to a larger study (whole class). Student groups are assigned different distinct windows of development to study, and then data are consolidated to generate a more complete time course so that all groups have contributed to the final result. As a result, students have the background information and skills needed to explore the potential effects of atrazine on different developmental processes, such as early cleavage, gastrulation, and organogenesis. While students are performing atrazine exposures, collecting data, and consolidating and analyzing those data, they are also planning their own Independent Experiment. Lab partners can choose a particular developmental process or stage that is most interesting to them and an environmental condition or chemical that may impact that developmental process.
Overall, both experiments outlined in this lesson meet the criteria Auchincloss et al. established for CUREs (17). Throughout the lesson, students engage with scientific practices, learn laboratory techniques to make observations, generate hypotheses, collect and analyze novel data, and present their research findings. Students are engaged in discovery centered around broadly relevant/important questions because impacts of environmental atrazine exposure are unknown and widely debated. Students collaborate with others, working in partners to design and execute experiments and collecting and analyzing data as a class. Finally, students experience iteration in the repetition of their own experiment, and in the investigation of reproducibility among the class as pairs all complete identical experiments.
Intended Audience
The full implementation of this lesson was used in an upper level developmental biology course at a residential small liberal arts college. The class had 12-16 students, and students worked in pairs. The students were in their third or fourth year and were required to be enrolled in a companion lecture course. The lecture course uses a textbook and includes journal clubs that focus on environmental impacts on development. All students in the course were majoring in biology or biochemistry and molecular biology and had completed the prerequisite core coursework in introductory animal biology, cell biology, and genetics. Students were proficient at collecting data, basic microscopy, performing descriptive statistics, and generating graphs from data.
This lesson could be modified into a capstone or culminating experience, as advanced coursework would allow for deeper interpretation of the experimental results. The instructor can adjust expectations about the preparation for the student-designed experiment and interpretation of the data to fit the goals of the course and skill/knowledge level of the students.
This lesson plan also could be modified to be appropriate for an introductory environmental studies course or introductory biology course (Figure 1). The authors have used the Guided Experiment approach with introductory students at the end of the semester when students had been articulating hypotheses and performing experiments with protocols for several weeks. Students were provided the staging chart for zebrafish (43) and ideas about environmental conditions fish are exposed to in nature such as pesticides and road salt runoff. Students then designed their own experiment and presented their results to the class. At the introductory level, students may already have experience collecting data, performing descriptive statistics, and generating graphs from data, or the instructor could use this experiment to teach students about these aspects of scientific experimentation.
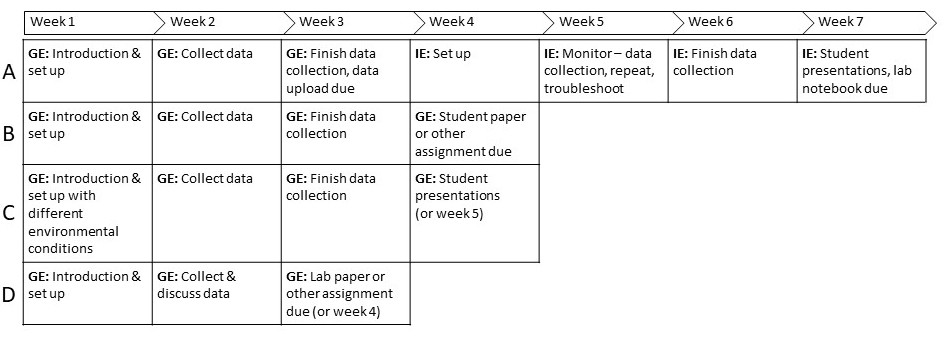
Figure 1. Possible modifications for the lesson. (A) Full 7-week lesson plan. (B) Modification that could be appropriate for an upper level developmental biology course if you are not performing the Independent Experiment. (C) Modification for environmental science course in which student groups may expose fish to different environmental conditions, then compare results with student presentations in Week 4 (or Week 5). (D) Modification that streamlines the experiment for an introductory course. In this modification, the instructor should explicitly state what data students will collect so that class wide data can be compiled and students can complete a lab paper or assignment. GE – Guided Experiment, IE – Independent Experiment
Required Learning Time
This lesson spans seven weeks of class, with a three-hour lab each week. Students are also expected to monitor their experiments outside of the scheduled class time. The lesson is broken into two slightly overlapping components outlined in the lesson timeline (Table 1):
- Guided Experiment: students expose zebrafish embryos to specific concentrations of atrazine. Student groups are given different starting developmental stages with which to perform their experiment. As a class, students then generate a time course by pooling the data they collected at different starting stages of development. This part of the lesson can be performed without including the Independent Experiment.
- Independent Experiment: after gaining experience working with zebrafish embryos in the Guided Experiment, students develop and perform their own project.
If the instructor or other faculty at the institution do not already have approved protocols for working with zebrafish, the instructor will need to submit protocols to their institutional animal use committee. It is important for anyone using this lesson to understand how long this process takes at their institution in order to have approval prior to beginning the lesson.
Prerequisite Student Knowledge
This lesson is adaptable to introductory or advanced students. Lab skills that will facilitate performing the described experiments include light microscopy, dilution calculations, basic spreadsheet management, descriptive statistics, generation of figures with raw data, observation, team work, and controlled experimentation. Students also should know how to use lab equipment including micropipettes, pipets, and dissecting microscopes. Second year students and above would ideally be comfortable with these skills and use the research experience to apply and practice them. In an introductory biology course, lab skills could be developed in the same course prior to and during the lesson.
Prerequisite Teacher Knowledge
The instructor should have content knowledge in developmental processes from early cleavage through organogenesis and be comfortable with experimental design, troubleshooting, and management of teaching laboratory group work. Experience with zebrafish is important.
SCIENTIFIC TEACHING THEMES
Active Learning
Because this lesson is based in wet lab experimentation, it is inherently an active learning experience. Lab partners meet regularly to plan and perform their experiment in and out of class. Students engage with technology, interpretation and execution of laboratory protocols, as well as data collection and analysis. In addition, even when students are not conducting experiments, they are doing much more than passive listening; they discuss ideas, make proposals, explore and compare collected data, come to consensus on interpretation of results, and present their findings to classmates. Importantly, this lesson allows students to apply their life experiences related to environmental issues and/or previous coursework in a research environment while they develop and practice valuable critical thinking and lab skills.
Assessment
Items used for assessment of student learning for this lesson include:
- A report of data collected in the Guided Experiment (Weeks 1-3) modeled after the Results section of a research publication and evaluated for proper formatting of figures and accompanying results text.
- A report of experimental design, protocols, and list of required resources for the Independent Experiment (Weeks 4-6).
- Student research notebook (maintained during the whole lesson) evaluated for detailed notes on experimental design and planning, experimental set up, troubleshooting notes, data collection, observational notes, and conclusions.
- Student presentations evaluated on clarity and organization of the background, experimental design, data, conclusions, knowledge of the project by both partners, and scientific thoughtfulness in answers to peers' questions.
Steps in the lesson that can be used for formative and summative assessment are indicated in the Lesson Timeline with an asterisk (Table 1).
Inclusive Teaching
Laboratory courses in which students design and perform experiments in small groups likely garner many additional benefits for students than do courses in which students work independently. Studies have shown that problem solving and work done in groups enhances learning (44-47) and benefits from diverse contributing perspectives (48-50). In particular, an examination of collegiate student group work demonstrated that racio-ethnic diversity was positively related to group efficacy (51).
Additionally, educational research has shown that applied laboratory experiences increase interest in science by traditionally underrepresented populations in the sciences by demonstrating the relevance of the course material (11). In particular, including content related to social justice issues such as health disparities has been shown to increase minority engagement and persistence (52), which may be one of the many reasons the AAAS encourages courses to connect science to society more directly (12). This lesson has very overt relevance and highlights the importance of considering developmental biology in discussions about environmental issues when students observe the impact of pesticide exposure on organogenesis (followed by more focused and personally-relevant experiments in the Independent Experiment portion). It also has the opportunity to be connected to issues of social justice around access to quality water and the link between this access and public health outcomes. Readings from the 2010 book, Living Downstream: An ecologist's personal investigation of cancer and the environment (53) or scenes from the documentary based on this book, could be a way to stimulate conversation about this connection. This CURE reinforces the value of a course project and demonstrates how coursework can be connected to broader issues students may care about. In fact, CUREs are recommended as a mechanism for making scientific research more inclusive because they grant access to meaningful research experiences for all students (16). While some particularly recommend establishing CURE programs at minority serving institutions (54), establishing CUREs at any undergraduate institution has the potential to enhance the interest and persistence of students from underrepresented groups in STEM fields.
LESSON PLAN
This lesson has two parts: 1) the Guided Experiment in which lab groups gather data to contribute to a class-wide time course study; and 2) the Independent Experiments that are planned and performed in pairs.
Pre-lesson Preparation
Reagents and handouts
All stock solutions and imaging plates can be prepared in sufficient quantity before week 1 to last through the Guided Experiment (Supporting File S1. Eco Devo Zfish – Required supplies and reagent protocols). Working dilutions can be prepared in large quantities prior to the start of the Guided Experiment, but the following reagents may need to be made again as students use them: working dilution of E3 medium, fry media, and 0.1M phosphate buffer with 5% sucrose. The fixative (4% paraformaldehyde, 0.1M Phosphate buffer with 5% sucrose solution) needs to be made every 2 weeks. Handouts can be prepared and distributed together at the start of the lesson. Within the student handouts, the text that the instructors should modify for their course is highlighted in red, although other details may need to be modified depending on the resources available (See the individual student handouts, Supporting Files S2-S6). See Supporting File S7. Eco Devo Zfish – Planning calendar for independent experiment, for a blank calendar to use in planning instructor preparation for the Independent Experiment. The Lesson Plan Timeline (Table 1) provides guidance on when to prepare reagents and handouts, assuming the instructor is using the entire lesson plan.
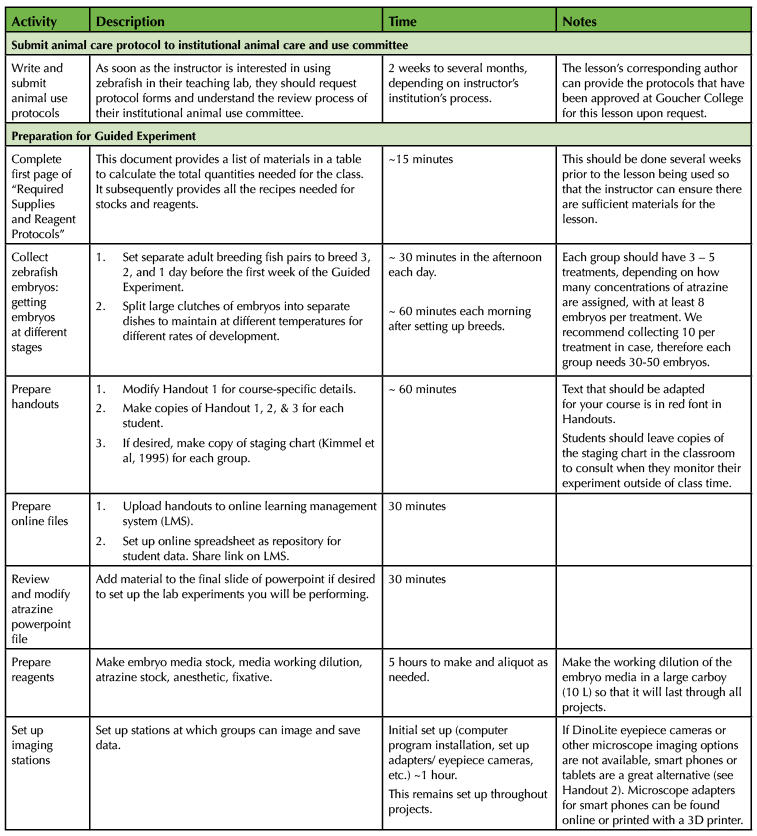
Table 1. Lesson teaching timeline

Table 1. Lesson teaching timeline (continued)

Table 1. Lesson teaching timeline (continued)
Zebrafish embryos
Ideally, the instructor will have access to a breeding zebrafish colony to collect embryos at different stages of development. Zebrafish embryos will need to be collected in the days leading up to the first lab session (Table 1). If there is not a zebrafish colony available at the instructor's institution, research labs at a neighboring institution may be willing to provide embryos. Alternatively, the Zebrafish International Resource Center (ZIRC) can supply embryos at a reduced cost to academic institutions (www.zebrafish.org); it is important to note that these embryos would be at later stages of development due to shipping time. Both the Guided Experiment and the Independent Experiment could be performed using embryos starting at 24 hours post fertilization acquired from ZIRC. In the absence of having breeding fish available, ordering embryos from ZIRC and limiting students to starting at a later stage will streamline the preparation needed to collect embryos. The experiments described in this lesson can all be performed with wild-type embryos. For general references on zebrafish care at all life stages, the instructor can find open access protocols through The Zebrafish International Resource Center (ZIRC, https://zebrafish.org) including the online 4th edition of The Zebrafish Book and the ZFIN Protocol Wiki.
Imaging
A learning goal of this lesson involves morphometric analysis. We used an eyepiece camera (Dino-Lite AM4023X, BigC Dino-Lite Digital Microscopes) inserted into the eye piece of a teaching stereo microscope. The camera has a USB connection and software compatible with multiple computer platforms for image collection for analysis using the camera software or with ImageJ (imagej.nih.gov/ij/, National Institutes of Health) (Supporting File S6. Eco Devo Zfish – Handout 5. Using ImageJ to analyze images). If eyepiece cameras are available, the instructor should aim to have one for every two or three groups during the experiment. Smart phone adapters that stabilize the phone to facilitate imaging with a phone can be purchased or printed with a 3D printer, although we have not used this approach. In addition, guidelines are available for consistent approaches using a handheld smartphone (55,56). The instructor should modify Handout 5 (Supporting File S6. Eco Devo Zfish – Handout 5. Using ImageJ to analyze images) to include only the imaging options available to their students. Alternatively, the instructor can leave all options in the handout so that students learn about multiple approaches to methods, but make sure that students are clear about the options available to them.
There are several ways to quantify morphology. If using a cell phone, pictures should be taken through an eyepiece fitted with a micrometer if possible. The Dino-Lite eyepiece camera includes a micrometer that students can take a picture of at the same magnification as their experiment. In both the camera software and ImageJ, students can open their images and set the scale in the program using the image of the micrometer. Students can then record measurements with units. If students do not have access to a micrometer, they can open their images in ImageJ, measure structures in pixels, and calculate ratios of two measurements taken, for example length of the embryo:width of head cartilage. There are links to tutorials for the eyepiece camera, imaging, and analysis in Handout 2 (Supporting File S3. Eco Devo Zfish – Handout 2. Reference links for stages of embryonic development of the zebrafish, software, imaging methods and measurements).
Module 1: Guided Experiment
Collecting fish embryos prior to the first class
The goal is to have embryos at different stages of development for the Guided Experiment. Each group should have a minimum of eight embryos per treatment at the same stage of development determined using the zebrafish staging chart by Kimmel et al (43), so we recommend collecting 10 embryos per treatment per group to provide a buffer in sample sizes. Therefore, each student group will need 30 healthy embryos if three treatments will be studied or 50 embryos if five treatments will be studied (for planning see Table 1 and Supporting File S1. Eco Devo Zfish – Required supplies and reagent protocols). In the three days prior to the first lab meeting, the instructor should breed zebrafish daily. The collected embryos then should be divided and raised at 28°C (the recommended temperature for zebrafish growth) and 22°C, to slow development. If incubators are not available, this temperature difference can be accomplished by putting dishes of embryos in rooms at higher or lower temperatures. Just before the first class, the instructor should divide embryos so that each group gets at least the minimum number of embryos required for their experiment that are all at the same stage; separate groups will receive different developmental stages.
Week 1
During the first week, the instructor introduces the background on atrazine (or another chemical if desired) and the scope of the project (Supporting File S8. Eco Devo Zfish – Lecture slides. Background on atrazine). Students then receive a dish of zebrafish embryos and a staging chart (43). Students first determine the stage of development they are starting with using the staging chart. Students will then follow the instructions on Handout 1 (Supporting File S2. Eco Devo Zfish – Handout 1. Student instructions for guided experiment) to set up their experiment. Students will use class time to: 1) perform calculations for the treatment conditions; 2) write a detailed protocol for experimental set up in their lab notebook; 3) create a table to collect data for 24 hours of treatment; 4) set up their experiment; 5) label tubes for ending the experiment; and 6) become familiar with the shared online spreadsheet in which they will post their data. To ensure visible malformations and compare the effects of different concentrations of atrazine on development, we recommend that students should test (at a minimum) 10 and 30 mg/L atrazine as described in Handout 1. If extra embryos are available, students may also include 1 mg/L atrazine to have one dose that results in little to no visible effect.
Students will need to monitor their experiment at 24 hours of treatment and then end the experiment by putting their embryos in a fixative at three or four days of treatment, depending on timing of the course and when students can have access to the classroom. Therefore, the instructor should walk students through the fixation protocol on Handout 3 (Supporting File S4. Eco Devo Zfish – Handout 3. Fixation protocol) during lab session 1, so that students know how to properly handle the fixative.
Weeks 2 & 3
All of the student groups should have their embryos in fixative by the second week. At the beginning of Week 2, the instructor reviews the proper washing procedure and handling of fixative waste. Students will wash the fixative from their embryos, take extensive observational and quantitative notes in their research notebook on the Guided Experiment, and input their data into the shared online spreadsheet. Students should also image embryos to record malformations and determine a quantitative way to measure those malformations using ImageJ. Examples of formats for the online shared spreadsheet are in Supporting File S9. Eco Devo Zfish – Screenshots of shared online spreadsheet. Sample rubrics are provided in Supporting File S10. Eco Devo Zfish – Sample rubrics for evaluation of student notebooks from the Guided Experiment. If the Independent Experiment (Supporting File S5. Eco Devo Zfish. – Handout 4. Independent experiment basic guidelines) will be performed, students should plan their experiment during and outside of class time during Weeks 2 & 3 of the Guided Experiment.
Module 2: Independent Experiment
Prior to Week 4
Week 4 is the beginning of the Independent Experiment. While the students are planning their Independent Experiment during the Guided Experiment, the instructor should plan the reagents and fish embryos needed for the Independent Experiment. This will include planning fish breeding to have the embryos needed for the student Independent Experiment and may include ordering chemicals or refreshing the working dilutions of the embryo and fry media, fixative, and wash solutions that were also used in the Guided Experiment. Depending on students' previous training, they can be expected to develop their own experimental design and protocols based on published and publicly available resources or can be provided more guidance to facilitate productive lab time. The instructor can define options for the experimental parameters (developmental stage and environmental factor) as narrowly as necessary based on available resources and the course level.
Week 4
Students begin setting up their Independent Experiments. This week will likely be the most hectic as the instructor distributes embryos and supplies for the different projects. Students are expected to carefully record their experimental protocol in their lab notebooks as they set up their experiment during Week 4. The instructor will monitor and consult with all groups throughout the class period.
Weeks 5 & 6
The instructor should remain in close communication with students during class time and between class meetings to ensure that projects are progressing, students have access to their experiments as appropriate, have help to manage any problems that arise, and are provided additional embryos for biological replicates or experimental improvements. Students are expected to monitor experiments and put embryos in fixative between class periods as necessary, but to use the class meetings to perform the bulk of imaging and analysis. As students are collecting data, they should be generating figures and discussing the interpretation of the data within their group and with the instructor.
Week 7
Students will clean up their lab projects and give research presentations during class. Sample rubrics are provided in Supporting File S10. Eco Devo Zfish – Sample rubrics for evaluation of student performance in the Independent Experiment.
TEACHING DISCUSSION
After gaining the needed experience/skills in the guided experiment, students were able to develop and perform their own novel experiment. In general, students enjoyed planning their own experiment and performing an experiment that did not fit solely within the class meeting times, and thus more closely mimicked authentic original research. Given the small class size, we used the instructor's research lab for some of the independent experiment. Student comments in course evaluations reflected their appreciation of this experience and the desire for even more independent work, including:
- "I really liked the independent project at the end because it was really nice to do a project without strict guidelines and on your own time so it was more interesting instead of just something I had to do like in some previous labs"
- "...was able to practice good lab techniques and designing experiments"
- "I wish there had been more time for independent experiments to look at slightly more complicated outcomes. But I have no idea how the class could have been condensed at all to allow for this"
- "...I learned a lot about how to use microscopes, image larva, and keep a lab notebook."
These comments are consistent with literature about the importance of CUREs, namely that students use scientific processes in a novel discovery process instead of employing formulaic techniques to replicate an already determined outcome (17).
We ran the Independent Experiment with up to six projects and one instructor, with each project addressing very different developmental biology questions (Table 2). Some challenges can arise when ensuring that students have adequately planned their work during the first week of the Independent Experiment. However, it was exciting to discuss the experimental design, hypotheses, and data with students, particularly because none of us had previously performed the experiments they were proposing. This allowed the instructor and students to talk about their experiments as novel research, a somewhat rare opportunity at a small liberal arts college. The instructor should consider the size of the class, the availability of embryos, and other support (staff, teaching assistants, etc.) when deciding the scope of the Independent Experiments. For example, we were able to have students perform the Independent Experiment in pairs, but groups of 3-4 students would allow an instructor to more easily adapt this lesson for a larger class.
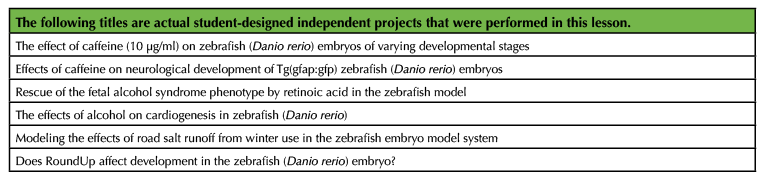
Table 2. Example titles of independent projects.
Even at the advanced level, some student groups have vague ideas about the experiment they want to perform. Because of this, the instructor expected students to have their protocols, endpoint, and hypothesis documented in their lab notebook prior to the distribution of embryos for their experiment. Much of the formative assessment in this lesson was through in-class conversations with student groups as they finalized their experimental design, followed by check-ins during class and lab sessions that included confirmation that the students were checking on their experiment and documenting quantitative and qualitative data. We recommend informal check-ins with students that explicitly include any problems or concerns they have with their experiment.
We believe this lesson could easily be modified for use in an introductory course. We have used zebrafish development as a research topic for introductory biology students in 2-week long student-designed group research projects. Examples include effect of temperature and salinity on zebrafish development. The latter example was a model for spring road run-off after salt application in the winter and the impact that additional salt in waterways may have on animals that develop in those waters. Here we had group sizes of three to five students. This allowed us to implement a small CURE in a larger lab course (up to 24 students per section) and found that it was beneficial to have more students in the group to better plan and perform an experiment. We found it helpful to ensure two weeks between the end of the experiment and student presentations. During this time, students generate figures that include basic descriptive statistics (mean and standard error of the mean) and have group meetings with the instructor to discuss their figures and the interpretation of the data. The timing of these projects is provided below and in Table 1 as a modification for introductory biology.
Students are not evaluated on the quality of their data, but rather their ability to perform and analyze what they have observed. Because this is a learning experience, the instructor emphasizes to students that what they deem 'bad data' due to procedural problems is still part of their analysis. Students are required to provide biological explanations for outcomes of all experiments.
Comments on responsible and humane use of zebrafish embryos in this lesson
A statement on the use of animals in research is included in Handout 1 (Supporting File S2. Eco Devo Zfish – Handout 1. Student instructions for guided experiment), and the instructor should consider adding to or modifying this document to best reflect their institution and student population. We have found that students greatly appreciate having a conversation about the use of animals in the laboratory and knowing that responsible and humane treatment of animals has been considered. Furthermore, explaining how animal protocols are approved and the implications for the institution if they do not follow the policies also conveys the responsibility of working with vertebrate animals. Researchers and instructors who work with vertebrate animals must first have their procedures approved by their institutional animal care committee. Institutions that receive federal grant funding are required to work with the National Institutes of Health Office of Laboratory Animal Welfare and establish an Institutional Animal Care and Use Committee to ensure the treatment of animals in grant funded research and teaching labs at the institution is humane. If an institution does not fulfill their responsibilities in ensuring the welfare of research animals at the institution, they can lose their federal grant funding.
In these experiments, all zebrafish larvae are euthanized prior to eight days post fertilization, and while MS-222 is not as effective at these stages, immersion in fixative is an acceptable mode of euthanasia (57). We recommend using MS-222 to reduce movement in the zebrafish larva at all stages followed by immersion in fixative as outlined in the protocols.
Modifications on the lesson plan
For introductory courses
A condensed version of this lesson was incorporated into introductory biology spanning four weekly three-hour labs. Students submit their experimental plan in week 1, perform their experiment in weeks 2 and 3, and give a group presentation of their results in week 4. Ideally there are two weeks between completion of the experiment and presentations so that student groups can generate figures and meet with the instructor to discuss interpretation of their results and presentation preparation.
Alternative animal models
Although this lesson is designed to use an available breeding zebrafish colony, other animal models would also provide students with a comparable experience. The Guided Experiment was modeled after a research publication using African claw-toed frog embryos (Xenopus laevis) (59), and thus early stages of frog embryos can be used in place of zebrafish. Students could also be provided with a variety of organisms to compare effects across biological processes and organisms.
SUPPORTING MATERIALS
- S1. Eco Devo Zfish – Required supplies and reagent protocols. Includes protocols and materials for instructor preparation.
- S2. Eco Devo Zfish – Handout 1. Student instructions for guided experiment. To be modified and handed out to students
- S3. Eco Devo Zfish – Handout 2. Reference links for stages of embryonic development of the zebrafish, software, imaging methods and measurements. To be modified and handed out to students
- S4. Eco Devo Zfish – Handout 3. Fixation protocol. To be modified and handed out to students
- S5. Eco Devo Zfish. – Handout 4. Independent experiment basic guidelines. To be modified and handed out to students.
- S6. Eco Devo Zfish – Handout 5. Using ImageJ to analyze images. To be handed out to students.
- S7. Eco Devo Zfish – Planning calendar for independent experiment. For instructor to map out instructor preparation to support student independent experiments.
- S8. Eco Devo Zfish – Lecture slides. Background on atrazine. For instructor to use to introduce the topic of the guided experiment to the students.
- S9. Eco Devo Zfish – Screenshots of shared online spreadsheet. Examples of how students have documented their data for the Guided Experiment.
- S10. Eco Devo Zfish – Sample rubrics. For assessing the different components of the lesson.
References
- Denofrio LA, Russell B, Lopatto D, Lu Y. 2007. Mentoring. Linking student interests to science curricula. Science 318:1872-3.
- Russell SH, Hancock MP, McCullough J. 2007. The pipeline. Benefits of undergraduate research experiences. Science 316:548-9.
- Hunter A-B, Laursen SL, Seymour E. 2007. Becoming a Scientist: The Role of Undergraduate Research in Students' Cognitive, Personal, and Professional Development. Science Education 91:36-74.
- Lopatto D, Alvarez C, Barnard D, Chandrasekaran C, Chung HM, Du C, Eckdahl T, Goodman AL, Hauser C, Jones CJ, Kopp OR, Kuleck GA, McNeil G, Morris R, Myka JL, Nagengast A, Overvoorde PJ, Poet JL, Reed K, Regisford G, Revie D, Rosenwald A, Saville K, Shaw M, Skuse GR, Smith C, Smith M, Spratt M, Stamm J, Thompson JS, Wilson BA, Witkowski C, Youngblom J, Leung W, Shaffer CD, Buhler J, Mardis E, Elgin SC. 2008. Undergraduate research. Genomics Education Partnership. Science 322:684-5.
- Lopatto D. 2010 Science in Solution: The impact of undergraduate research on student learning. Research Corporation for Science Advancement, Tucson, AZ.
- Laursen S, Hunter A-B, Seymour E, Thiry H, Melton G. 2010. Undergraduate research in the sciences: Engaging students in real science. Jossey-Bass.
- Council NR. 2003. BIO 2010: Transforming undergraduate education for future research biologists. National Academies Press, Washington, D.C.
- Bauer KW, Bennett JS. 2003. Alumni Perceptions Used to Assess Undergraduate Research Experience. The Journal of Higher Education 74:210-230.
- Nagda BA, Gregerman SR, Jonides J, von Hippel W, Lerner JS. 1998. Undergraduate student-faculty research partnerships affect student retention. The Review of Higher Education 22:55-72.
- Eagan MK, Hurtado S, Chang MJ, Garcia GA, Herrera FA, Garibay JC. 2013. Making a Difference in Science Education: The Impact of Undergraduate Research Programs. Am Educ Res J 50:683-713.
- Hurtado S, Newman CB, Tran MC, Chang MJ. 2010. Improving the rate of success for underrepresented racial minorities in STEM fields: Insights from a national project. New Directions for Institutional Research 2010:5--15.
- Anonymous. 2011. Vision and change in undergraduate biology education: A call to action. American Association for the Advancement of Science (AAAS),
- Wood WB. 2003. Inquiry-based undergraduate teaching in the life sciences at large research universities: A perspective on the Boyer Commission Report. Cell Biol Educ 2:112-116.
- Desai KV, Gatson SN, Stiles TW, Stewart RH, Laine GA, Quick CM. 2008. Integrating research and education at research-extensive universities with research-intensive communities. Adv Physiology Educ 32:136-141.
- Eagan MK, Sharkness J, Hurtado S, Mosqueda CM, Chang MJ. 2011. Engaging undergraduates in science research: Not just about faculty willingness. Res High Educ 52:151.
- Bangera G, Brownell SE. 2014. Course-based undergraduate research experiences can make scientific research more inclusive. CBE Life Sci Educ 13:602-606.
- Auchincloss LC, Lauresen SL, Branchaw JL, Eagan K, Graham M, Hanauer DI, Lawrie G, McLinn CM, Pelaez N, Rowland S, Towns M, Trautmann NM, Varma-Nelson P, Weston TJ, Dolan EL. 2014. Assessment of course-based undergraduate research experiences: A meeting report. CBE Life Sci Educ 13 29-40.
- Gilbert SF, Epel D. 2015. Ecological Developmental Biology: The Environmental Regulation of Development, Health, and Evolution, 2nd ed doi:QH438.5.G55 2015. Sinauer Associates, Inc., Sunderland, MA.
- Pieau C, Dorizzi M. 2004. Oestrogens and temperature-dependent sex determination in reptiles: all is in the gonads. J Endocrinol 181:367-77.
- Ciofi C, Swingland IR. 1997 Environmental sex determination in reptiles. App Animal Behaviour Sci 51:251-265.
- Merchant-Larios H, Díaz-Hernández V. 2013. Environmental sex determination mechanisms in reptiles. Sex Dev 7:95-103.
- Vianna FS, Lopez-Camelo JS, Leite JC, Sanseverino MT, Dutra MaG, Castilla EE, Schüler-Faccini L. 2011. Epidemiological surveillance of birth defects compatible with thalidomide embryopathy in Brazil. PLoS One 6:e21735.
- Vargesson N. 2013. Thalidomide Embryopathy: An Enigmatic Challenge. ISRN Developmental Biology 2013:18.
- Siamwala JH, Veeriah V, Priya MK, Rajendran S, Saran U, Sinha S, Nagarajan S, Pradeep T, Chatterjee S. 2012. Nitric oxide rescues thalidomide mediated teratogenicity. Sci Rep 2:679.
- E1439-12 A. 2012. Annual Book of ASTM Standards, vol 11.06, p 826-836. ASTM International, West Conshohocken, PA.
- Hoke RA, Ankley GT. 2005. Application of frog embryo teratogenesis assay-Xenopus to ecological risk assessment. Environ Toxicol Chem 24:2677-90.
- Dumont JM, Schultz TW, Buchanan MV, Kao GL. 1983. Frog Embryo Teratogenesis Assay: Xenopus (FETAX) - A short-term assay applicable to complex environmental mixtures. In Waters MD, Sandhu S.S., Lewtas J., Claxton L., Chernoff N., Nesnow S. (ed), Short-Term Bioassays in the Analysis of Complex Environmental Mixtures III, vol 27. Springer, Boston, MA.
- Opitz R, Braunbeck T, Bögi C, Pickford DB, Nentwig G, Oehlmann J, Tooi O, Lutz I, Kloas W. 2005. Description and initial evaluation of a Xenopus metamorphosis assay for detection of thyroid system-disrupting activities of environmental compounds. Environ Toxicol Chem 24:653-64.
- Dai YJ, Jia YF, Chen N, Bian WP, Li QK, Ma YB, Chen YL, Pei DS. 2014. Zebrafish as a model system to study toxicology. Environ Toxicol Chem 33:11-7.
- Lee O, Green JM, Tyler CR. 2015. Transgenic fish systems and their application in ecotoxicology. Crit Rev Toxicol 45:124-41.
- Teraoka H, Dong W, Hiraga T. 2003. Zebrafish as a novel experimental model for developmental toxicology. Congenit Anom (Kyoto) 43:123-32.
- Jensen BH. 2016. Zebrafish Melanophores: A Model for Teaching Second Messenger Systems. Zebrafish 13:305-9.
- Bagatto B. 2009. Guided inquiry lab exercises in development and oxygen consumption using zebrafish. Zebrafish 6:161-8.
- Ross AW, Bonner J. 2012. Activation of Wnt signaling using lithium chloride: inquiry-based undergraduate laboratory exercises. Zebrafish 9:220-5.
- Wiegand C, Pflugmacher S, Giese M, Frank H, Steinberg C. 2000. Uptake, toxicity, and effects on detoxification enzymes of atrazine and trifluoroacetate in embryos of zebrafish. Ecotoxicol Environ Saf 45:122-31.
- Wang Y, Lv L, Yu Y, Yang G, Xu Z, Wang Q, Cai L. 2017. Single and joint toxic effects of five selected pesticides on the early life stages of zebrafish (Denio rerio). Chemosphere 170:61-67.
- Liu Z, Wang Y, Zhu Z, Yang E, Feng X, Fu Z, Jin Y. 2016. Atrazine and its main metabolites alter the locomotor activity of larval zebrafish (Danio rerio). Chemosphere 148:163-70.
- Wang H, Mu S, Zhang F, Wang H, Liu H, Zhang H, Kang X. 2015. Effects of Atrazine on the Development of Neural System of Zebrafish, Danio rerio. Biomed Res Int 2015:976068.
- Wiegand C, Krause E, Steinberg C, Pflugmacher S. 2001. Toxicokinetics of atrazine in embryos of the zebrafish (Danio rerio). Ecotoxicol Environ Saf 49:199-205.
- Adeyemi JA, da Cunha Martins-Junior A, Barbosa F, Jr. 2015. Teratogenicity, genotoxicity and oxidative stress in zebrafish embryos (Danio rerio) co-exposed to arsenic and atrazine. Comp Biochem Physiol C Toxicol Pharmacol 172-173:7-12.
- Wirbisky SE, Weber GJ, Schlotman KE, Sepulveda MS, Freeman JL. 2016. Embryonic atrazine exposure alters zebrafish and human miRNAs associated with angiogenesis, cancer, and neurodevelopment. Food Chem Toxicol doi:10.1016/j.fct.2016.03.027.
- Sarmah S, Chism GW, Vaughan MA, Muralidharan P, Marrs JA, Marrs KA. 2016. Using Zebrafish to Implement a Course-Based Undergraduate Research Experience to Study Teratogenesis in Two Biology Laboratory Courses. Zebrafish 13:293-304.
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. 1995. Stages of embryonic development of the zebrafish. Dev Dyn 203:253-310.
- Prince KJAH, Van Eijs PWLJ, Boshuizen HPA, Van Der Vleuten CPM, Scherpbier AJJA. 2005. General competencies of problem-based learning (PBL) and non-PBL graduates. Medical Education 39:394--401.
- Springer L, Stanne ME, Donovan SS. 1999. Effects of Small-Group Learning on Undergraduates in Science, Mathematics, Engineering, and Technology: A Meta-Analysis. Review of Educational Research 69:21-51.
- Terenzini PT, F. CA, L. CC, M. PJ, A. BS. 2001. Collaborative Learning vs. Lecture/Discussion: Students' Reported Learning Gains*. Journal of Engineering Education 90:123--130.
- Hmelo-Silver CE. 2004. Problem-Based Learning: What and How Do Students Learn? Educational Psychology Review 16:235-266.
- Watson WE, Kumar K, Michaelsen LK. 1993. Cultural diversity's impact on interaction process and performance: Comparing homogeneous and diverse task groups. The Academy of Management Journal 36:590-602.
- Guzzo RA, Dickson MW. 1996. Teams in organizations: recent research on performance and effectiveness. Annu Rev Psychol 47:307-38.
- van Knippenberg D, Schippers MC. 2007. Work group diversity. Annu Rev Psychol 58:515-41.
- Sargent LD, Sue-Chan C. 2001. Does Diversity Affect Group Efficacy?:The Intervening Role of Cohesion and Task Interdependence. Small Group Research 32:426-450.
- Benabentos R, Ray P, Kumar D. 2014. Addressing health disparities in the undergraduate curriculum: an approach to develop a knowledgeable biomedical workforce. CBE Life Sci Educ 13:636-40.
- Steingraber S. 2010. Living downstream: an ecologist's personal investigation of cancer and the environment, 2nd ed. Da Capo Press, Cambridge, MA.
- Malcom LE, Dowd AC, Yu T. 2010. Tapping HSI-STEM funds to improve Latina and Latino access to STEM professions. University of Southern California, Los Angeles, CA.
- Morrison AS, Gardner JM. 2014. Smart phone microscopic photography: a novel tool for physicians and trainees. Arch Pathol Lab Med 138:1002.
- Bellina L, Missoni E. 2016. The first description of how to take a picture from the microscope with an m-phone. J Cutan Pathol 43:1077-1078.
- Anonymous. 2016. Animal Research Advisory Committee Guidelines: Guidelines for use of zebrafish in the NIH intramural research program, p 1-3. NIH Intramural Research Program: Office of Animal Care and Use.
- Strykowski JL, Schech JM. 2015. Effectiveness of recommended euthanasia methods in larval zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 54:81-4.
- Lenkowski JR, Reed JM, Deininger L, McLaughlin KA. 2008. Perturbation of organogenesis by the herbicide atrazine in the amphibian Xenopus laevis. Environ Health Perspect 116:223-30.
Article Files
Login to access supporting documents
Using Zebrafish in a Developmental Biology Lab Course to Explore Interactions Between Development and the Environment(PDF | 267 KB)
S1. Eco Devo Zfish-Required supplies and reagent protocols.docx(DOCX | 29 KB)
S2. Eco Devo Zfish-Handout 1 Guided Experiment with instructor notes.docx(DOCX | 54 KB)
S3. Eco Devo Zfish-Handout 2 Reference Links.docx(DOCX | 18 KB)
S4. Eco Devo Zfish-Handout 3 Fixation Protocol.docx(DOCX | 22 KB)
S5. Eco Devo Zfish-Handout 4 Independent experiment.docx(DOCX | 30 KB)
S6.Eco Devo Zfish-Handout 5 Using ImageJ to analyze images.doc(DOC | 4 MB)
S7. Eco Devo Zfish-Planning Calendar for Independent Experiment.docx(DOCX | 20 KB)
S8.Eco Devo Zfish- Lecture slides_ Background on atrazine.pptx(PPTX | 986 KB)
S9. Eco Devo Zfish-Screenshots of shared online spreadsheet.docx(DOCX | 2 MB)
S10. Eco Devo Zfish-Sample Rubrics.docx(DOCX | 33 KB)
- License terms

Comments
Comments
There are no comments on this resource.