Taking the Hassle out of Hasselbalch
Published online:
Abstract
Mention pH in a classroom and you can almost see the inner yawn on the faces of your students. The definition may even roll easily from their lips. However, despite multiple exposures to the relationship of pH and pKa throughout a typical undergraduate science curriculum, students struggle to differentiate between a property of a molecule (pKa) and the impact of molecules, such as acids and bases, on the environmental conditions (pH). Even fewer are comfortable applying their knowledge to predict the effect of environmental pH on the ionized status of functional groups in biologically relevant molecules, groups that are behaving as weak acids and weak bases. The approach of this lesson is to review this foundational topic from an intuitive angle first and then connect qualitative to quantitative reasoning. It progresses through a series of clicker questions that are used to facilitate peer instruction in small groups. The clicker questions are structured by mini-lectures that provide support for the "math phobic"; therefore, this sequence is also suitable as a group work-sheet activity. This lesson is intended for an introductory biology or chemistry course, but can be adapted and expanded for advanced students to explore medical and pharmaceutical applications.
Citation
Rosenberg, M.J., Abel, E., Garver, W.S., and Osgood, M.P. 2016. Taking the Hassle out of Hasselbalch. CourseSource. https://doi.org/10.24918/cs.2016.17Society Learning Goals
Biochemistry and Molecular Biology
- Homeostasis
- What is the biological need for homeostasis?
- How are steady state processes and homeostasis linked?
- How is homeostasis quantified?
Science Process Skills
- Modeling/ Developing and Using Models
- Recognize the important roles that scientific models, of many different types (conceptual, mathematical, physical, etc.), play in predicting and communicating biological phenomena
- Make inferences and solve problems using models and simulations
Lesson Learning Goals
Students will understand the effect of environmental pH on the ionization status of weak acids and weak bases and be able to explain to a 'layperson' why this relationship matters in human health (unit goal).Lesson Learning Objectives
Students will be able to:- Characterize an aqueous environment as acidic or basic.
- Explain that pKa is a measure of how easy it is to remove a proton from a molecule.
- Predict ionization state of a molecule at a particular pH based on its pKa (qualitative use of the Henderson-Hasselbalch equation).
- Calculate the ratio of protonated/unprotonated forms of ionizable groups depending on chemical characteristics and /or environment pH (quantitative use of the Henderson-Hasselbalch equation).
- Apply this knowledge in a medical context.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
The origin of this teaching activity
Instructors and education researchers in the life sciences have long recognized the importance of helping learners understand the relationship between pH and pKa. Inclusion of this foundational concept in textbooks, assessment instruments, and concept inventories does not come as a surprise (i.e., 1–4).
Despite the foundational role in chemistry, biology, and connected disciplines, the pH/pKa topic remains a rich source for misconceptions (3, 5, 6). Students have difficulties with the distinction between pKa as a measurement of the ionization state for a solute, versus the impact of acids and bases on the environment, i.e. the pH of an aqueous solution. A summary of common incorrect mental models is provided in Table 1.
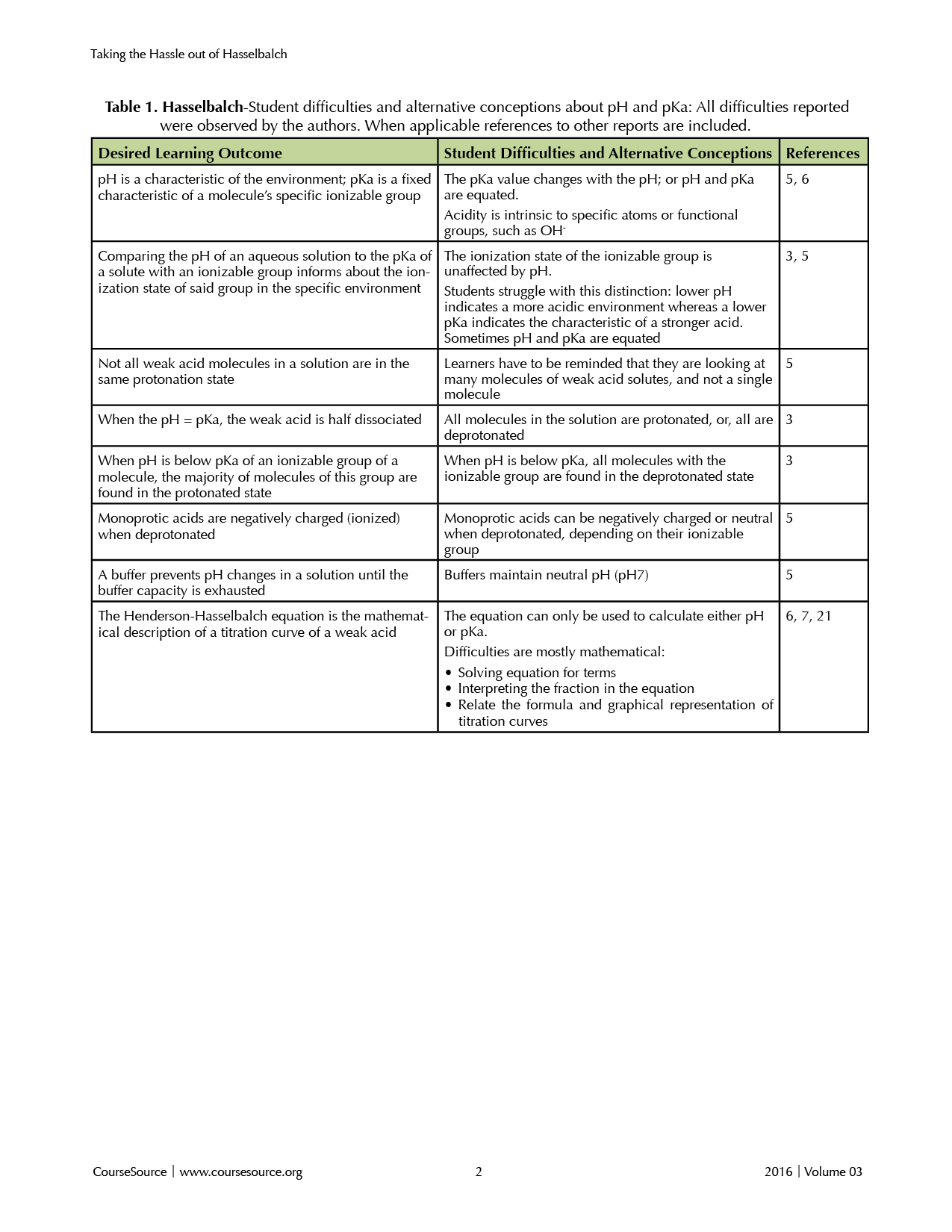
Table 1
Although students encounter pH and pKa on multiple occasions during their coursework, both upper and lower level students struggle with the concept (7). A group of national experts recognized “threshold concepts” that, when mastered, lead to a transformed understanding of a discipline (8). Among the top three threshold concepts in biochemistry is the relationship of pH and pKa. It appears that the topic is rarely put into a context that learners easily understand. As a result, student knowledge often does not go beyond reciting a definition or formula. It is not that resources or attempts to convey the concepts are scarce, but that the concept is not intuitive (9). Without guidance, the students may feel that the topic is entirely esoteric rather than bearing practical relevance. Therefore, instructors may find greater success in encouraging learners, if they can convey the fundamental importance of pH and pKa.
A Brief Review of the Major Concepts in pH and pKa
The following paragraph serves as a brief refresher for the instructor. For more detail Lehninger Principles of Biochemistry provides a good reference (4).
Acidity or alkalinity of an aqueous solution is measured as the negative decadic logarithm of the hydrogen ion concentration, also known as pH. A strong acid, such as HCl, ionizes essentially completely in water. In contrast, physiological relevant acids and bases do not dissociate completely. They are referred to as ‘weak’ acids or bases and their ability to ionize is described by the negative logarithm of their dissociation constant, pKa and pKb, respectively. These values are measures of the strengths of the acid or base using a logarithmic scale.
Addition of weak conjugate acid/base pairs to an aqueous solution will result in either adding to or subtracting from the population of protons in the solution. As a result, the pH will adjust until equilibrium is achieved. Cells produce weak acids of particular pKa values. In the cytosol and in different organelles of the cell the appropriate pH is maintained within a very narrow range. These weak conjugate acid/base pairs act as buffers against pH fluctuations that would otherwise occur due to changes in metabolism. Important examples of biological buffers include the inorganic phosphate system and the bicarbonate buffer systems. The maintenance of physiological pH in the blood by the bicarbonate buffer system is of clinical importance. The small pH difference of arterial blood (pH =7.44) and venous blood (pH = 7.35) facilitates the release of oxygen from oxyhemoglobin in peripheral tissue. This pH-dependent change of hemoglobin’s affinity for oxygen is known as the Bohr effect in biochemistry textbooks (4). A more drastic deviation outside of this pH range leads to coma and death (pH<6.95), or tetany and muscle spasms (pH>7.9) in animals. Maintenance of pH also prevents protonation of various functional groups within biological macromolecules, which otherwise would be drastically altered in structure and/or function within the cell.
The example of a biologically active compound, aspirin (acetylsalicylic acid), is used in this lesson. Many pharmaceutical drugs on the market today are weak acids or weak bases with ionization constants that affect their physiochemical properties, including solubility, distribution, and excretion (10). In addition to pharmacokinetics, biomedical examples involving the pH/pKa relationship include: maintaining cellular conditions for optimal enzyme activity; treating poison ivy rash with a mildly basic solution of sodium bicarbonate to increase the solubility of the reactive catechol (11); the debated use of cranberry juice to prevent urinary tract infections (12); and the role of pH on sperm motility (13). All of these examples reveal that a seemingly “dry” subject matter is immediately important to everyday living.
Intended Audience
This lesson has been used for the last six semesters at the beginning of a one-semester “Introduction to Biochemistry” class; a non-majors undergraduate course with an organic chemistry prerequisite. Participants are mostly juniors wanting to attend medical or other professional schools in the health sciences field. The class is part of a pipeline program and is relatively small (30-33 students) and has one undergraduate TA. However, the activities are easily scalable and the unit may be adjusted to the needs of instructors in introductory chemistry or biology courses, as well as in organic chemistry and physiology classes.
Learning Time
This lesson has been used for the last six semesters at the beginning of a one-semester “Introduction to Biochemistry” class; a non-majors undergraduate course with an organic chemistry prerequisite. Participants are mostly juniors wanting to attend medical or other professional schools in the health sciences field. The class is part of a pipeline program and is relatively small (30-33 students) and has one undergraduate TA. However, the activities are easily scalable and the unit may be adjusted to the needs of instructors in introductory chemistry or biology courses, as well as in organic chemistry and physiology classes.
Prerequisite Student Knowledge
We will have reviewed water as biological solvent, definitions of acids/bases in biosystems, and amino acids as weak polyprotic acid/base entities. This session also requires that students understand the difference between the natural and decadic logarithm and be able to solve a logarithmic equation for terms. Assigning a review tutorial on logarithmic functions before this class may provide additional support (see S1 slides for a suggested resource).
SCIENTIFIC TEACHING THEMES
Active Learning
Student pre-class textbook review of basic information is required to limit the time spent on transmission-style teaching. During class, the students sit in groups of three at individual tables with small whiteboards. Other ways of arranging seating and small groups are feasible. Group and classroom-wide discussions are encouraged as part of a vote-discuss-revote pattern during questions using an audience response system (referred to as 'clicker questions'), think-pair-shares, and a drawing activity.
Assessment
In our course, students are given a low-stakes quiz about the assigned material at the beginning of each class period. The quiz is peer-graded as part of the class, which provides another chance for students to think about these questions. Independent thought after clicker questions allows for discussion, which serves as both a tool of engagement and formative assessment. The results allow the instructor to gauge student knowledge levels and clarify if necessary. The group assignment prompts students to discuss the context and to apply the content. The summative exam example question uses another familiar drug (ibuprofen) with a different chemical structure and pKa value (S4) and can be administered in multiple choice or short answer format.
Inclusive Teaching
The pH/pKa concept is presented with an intuitive as well as a mathematical solution to accommodate students who have never built a 'scaffold' around the topic of pH, thus facilitating transfer. Unnecessary jargon, chemical names, or use of color schemes that are difficult to distinguish by students with most types of colorblindness are avoided in the presentation slides to support inclusion of all learners (14). Peer instruction elements support engagement, student-appropriate language, and opportunities for questions.
LESSON PLAN
The format presented here will take about 75 minutes, including a 10-minute readiness quiz, 15-minute peer grading/class discussion/ instructor clarification activity, and a 50-minute interactive lecture and group activity. Table 1 shows a sample teaching timeline with options.
A slide presentation containing all clicker questions, activities, notes for educators and student prompts is available (S1). Table 2 provides an overview of the timeline and student reactions to the lesson.
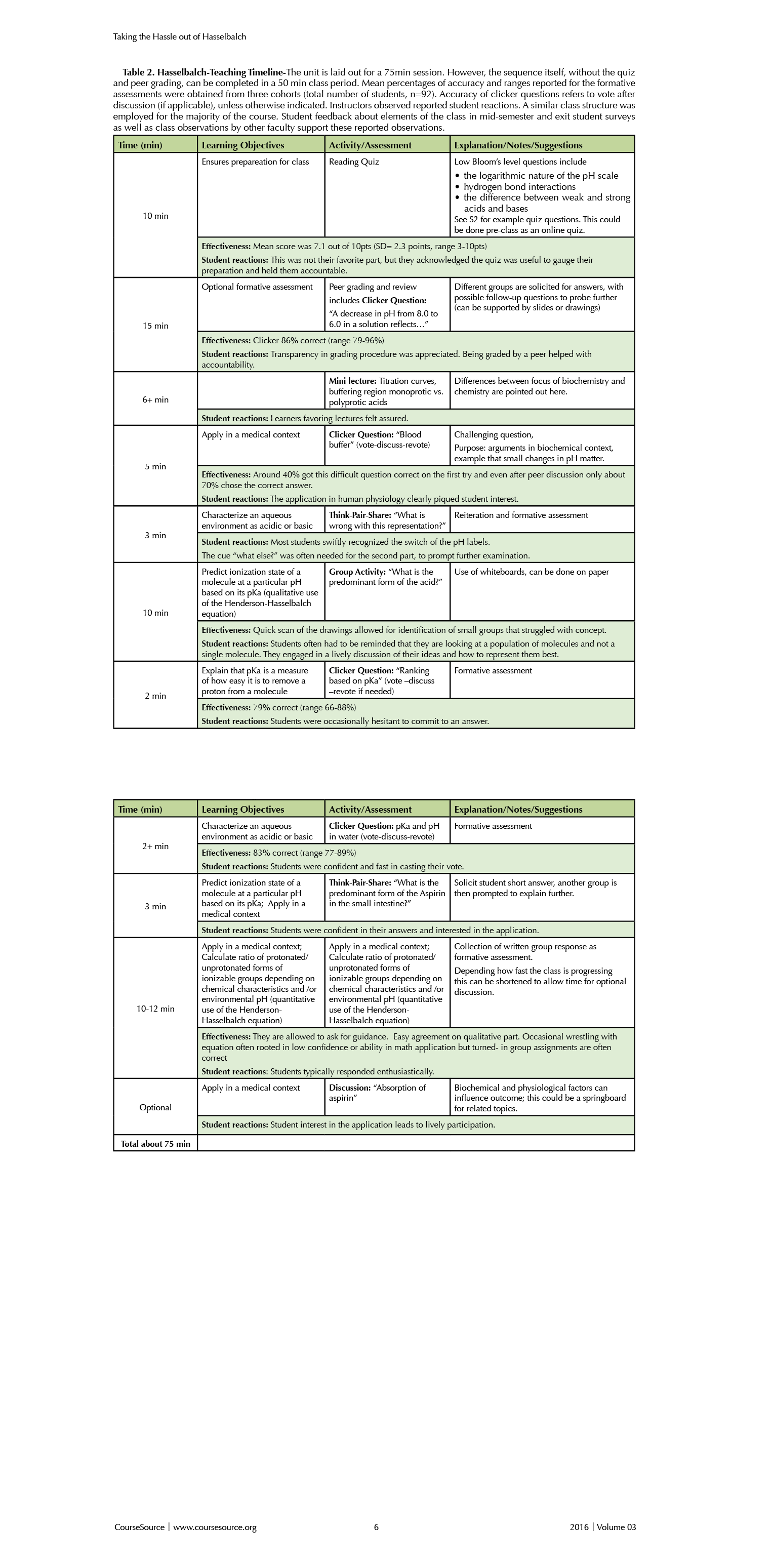
Table 2
Before Class cc
Students review assigned textbook chapters before class and are quizzed on the content. For this lesson the reading contains information and context about weak interactions of molecules in aqueous solutions, pH, and buffers. We use a commonly-used biochemistry textbook (15) that offers an optional online self-test learning platform, but most science textbooks provide adequate information on the topic.
There are many additional pH/pKa online tutorials available to students, either as stand alone options or integrated with textbooks. Some suggestions for review outside of class (pre- or post) are included in the slide presentation (S1).
In-Class Activities
List of Materials as field tested (and alternatives):
- Clicker response system (or note cards with letters, hand gestures, marks on folders that students hold up and can point to)
- Whiteboards (or paper)
- Pencil and paper (or submission via electronic devices as email or to online learning platform)
1. Reading Quiz
The reading quiz is closed book and completed individually. S2 provides an example quiz and highlights typical student difficulties. Students then exchange quizzes with other small groups for peer grading of the quiz. The peer graders are encouraged to work together in their small group and are instructed to identify and correct misconceptions evident in other students' answers, consulting the textbook as necessary. After grading, which usually takes about 4-5 min, the instructor solicits answers from different small groups, discusses responses revealing misconceptions, and emphasizes key concepts, including:
- the fact that water is the solvent in a physiological context.
- the importance of hydrogen bonding capacity, the logarithmic nature of the pH scale, why logarithmic representation makes sense (covering a large range); optional: use of clicker question #1 for quick additional check on understanding.
- the difference between weak and strong acids and bases.
- the fact that pH does not measure the strength of an acid, but the acidity of a given solution.
- the definition of equilibrium and Le Chatelier's principle (mass action) and how it applies to weak acids and bases in solution.
Explanations can be illustrated with slides from any standard textbook for visual support (4, 15). We included an optional clicker question that probes for understanding of the logarithmic nature of the pH scale.
2. Understanding Titration Curves
The slides for the next activity are provided in S1, but visuals could also be created by drawing on a board or using a document camera in class. Table 3 summarizes concepts connected to titration curves. Students are asked to explain how they would create a simple titration curve, what information they can derive from it, and if the curves are the same or different between different weak acids. Learners typically do not think about how to set up an experiment that will collect these kinds of data.
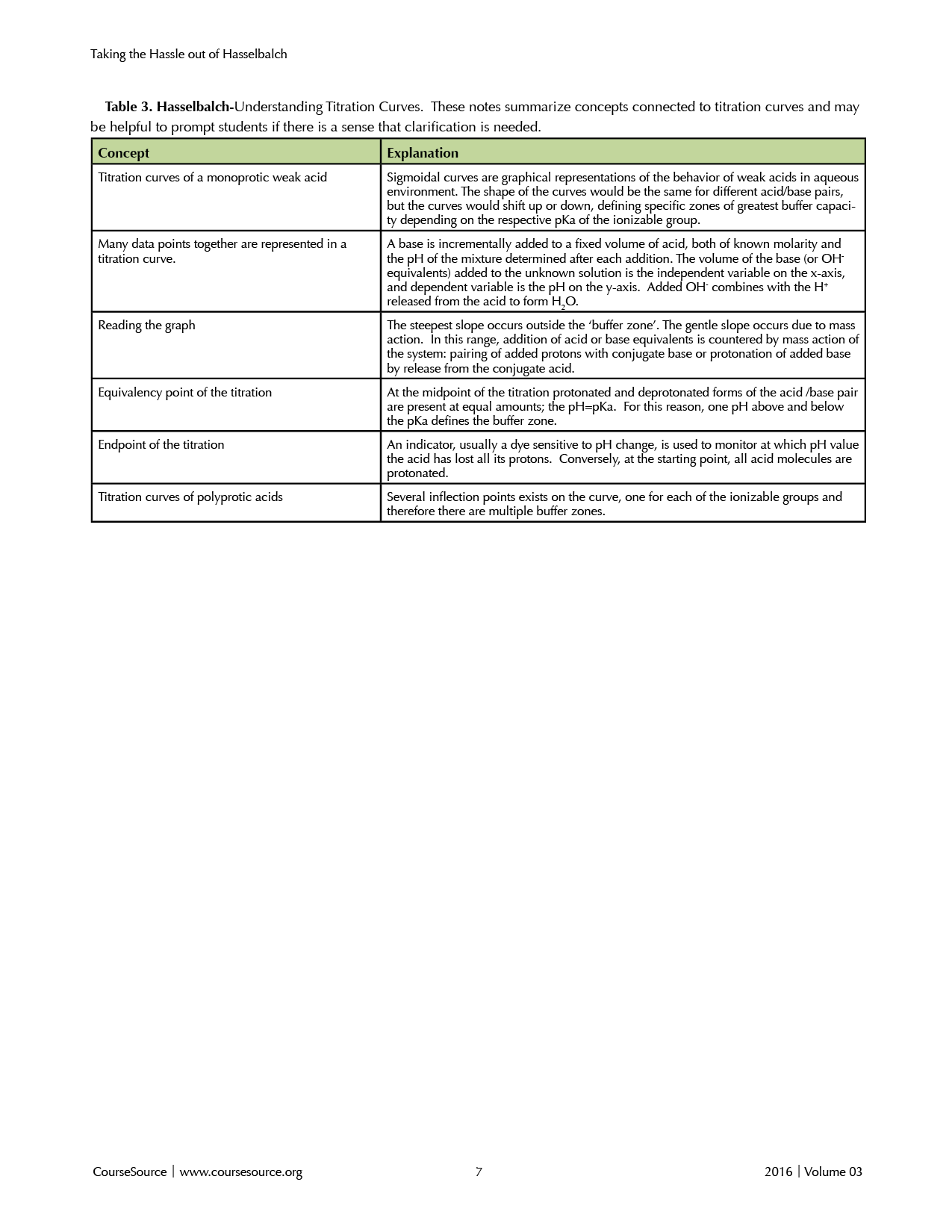
Table 3
It is helpful to have students verbalize what is plotted on the x- and y-axes of a titration plot and the reasons for changes in the slope of the curve. Before moving to a comparison of curves, ensure that students understand the reason for steep versus gentle slope portions of a single curve. Students should be able to articulate the relationship between the inflection point in the curves, the ratio of protonated:deprotonated species in solution, and the pKa of the ionizable group. Proceed asking students to identify why various curves for different weak acids fall within different regions of the plot (different buffer zones). Ask: "If you needed to buffer a solution at pH 7 what would the ideal buffer's titration curve look like?" The answer should indicate that the pKa would be around 7 as well.
If desired and time allows, curves of diprotic acids (such as amino acids) or triprotic acids may be introduced. Have students identify why these titration curves have varying numbers of inflection points (answer: multiple ionizable groups, multiple buffer zones).
3. Optional Clicker Question 2: "Blood buffer"
Vote-discuss-revote: This activity builds on reading quiz questions #4 and 5 (transfer and combination of concepts) and can be guided after the initial vote by prompting "We will soon talk about the catabolism of fuels when the generation of organic acids will elevate the concentration of H+. What would happen to the equilibrium during exercise? Choose the BEST answer" (answer: A. H2CO3 is an unstable intermediate; more CO2 will be exhaled, in an effort to remove the protons and rebalance the system at equilibrium, per Le Chatelier's principle). Starting with the last option, solicit arguments "Why might somebody have voted for..." and give an explanation for each option ("Is this a possible answer?"). If it has not already come up as a question from the groups explain that we are using as a simplification the apparent pKa of two combined sets of reactions with an unstable intermediate H2CO3 (4). Point out, “This is a challenging question and to think on that level in this class, we will now review fundamentals of pH to make sure everybody is on the same page.”
Instructors of classes that lack a health focus may omit this blood buffer question. In some classes, full understanding of the answer may require a review of physiologic function. For example, students may have forgotten or never been exposed to kidney function. The renal tubular cells reabsorb filtered bicarbonate and excrete acid (H+). Students may also be unaware that vigorous exercise and associated metabolism of reduced carbon fuels can lower the pH in tissues and blood. The instructor may wish to follow up this discussion with a question about the predicted effect of hyperventilation on blood pH (answer: elevated blood pH due to depletion of CO2 through increased exhalation and resultant mass action reduction of [H+]).
Expansion option: Here a discussion on physiological functions of buffers will hook your pre-meds. You could ask for suggestions as to why the blood buffer system is needed. Example answers may include:
- The pH of healthy arterial blood, or extracellular fluids in general, is maintained in a narrow range and you may point out that "later in the course we will see how pH affects oxygen binding to hemoglobin."
- Enzymes, including carbonic anhydrase, evolved to work best at physiological pH. On the other hand, tumor cells are able function in more acidic environments. Their higher metabolic rate may lead to acidosis (16).
- Changes in pH influences calcium homeostasis in cells (17).
4. Think-Pair-Share: "What is wrong with this representation?"
Prompt with "Let's make sure everyone is on board with what we talked about so far: Identify what is wrong with this representation of two solutions" (answer: 1. Relative pH is swapped, 2. The difference in the number of H+ is 105 not 2). Student should use whiteboards and display them when done.
5. Group Activity: "Which molecular form will predominate at a given pH?"
Make sure to emphasize that, unlike the previous slide, the number of H+ shown is NOT proportional to each other for the pH values shown. Ask why that would be impractical to show (answer: remind students about the logarithmic relation of pH values). Prompt with: "If you put a weak acid, HA, with a pKa of 7, into the solution at the indicated pH values, what form of the acid would be predominantly found in each situation?" Instruct students to use their whiteboards to draw the relative proportions of the protonated form (i.e. undissociated weak acid HA) and its deprotonated form (e.g. H+, i.e. the acid, and its conjugate base, A-).
Students discuss this question in their groups and can explain their thinking to the instructor or their TAs/ facilitators. Conclude by revealing the answer slide, pointing out that even at the pH 6, with predominantly protonated HA, you still have some dissociated molecules (hence the fraction in the Henderson-Hasselbalch equation). This fact is often overlooked during the discussions.
6. Clicker Question 3: "Ranking based on pKa"
Check for understanding by evaluating the number of correct answers. If necessary, ask students to discuss and revote or solicit an answer as a cold call. The goal is to have students recognize the relationship between pKa and the 'ease' with which the weak acid releases a proton into the aqueous solution. Emphasize that it is the pKa that is used to discuss relative strength of acids with each other. Clicker questions that achieved less than 70% accuracy were followed by a brief peer discussion and revote.
7. Clicker Question 4: "pKa and pH in water"
Check for understanding. If necessary, have students discuss and revote or solicit an answer as cold call. The goal is to extend the students' conceptual understanding from question #3 to include realization that more 'easily' donating a proton means that the pH is more substantially impacted (lowered).
8. Think-Pair-Share: "What is the predominant form of the weak acid aspirin in the small intestine?"
To answer this series of questions, students must recall that in lower pH (more acidic) environments like the stomach, the higher [H+] will dictate that the proportion of protonated aspirin molecules will be higher than in a less acidic environment. This follows Le Chatelier's principle and is described in mathematical terms by the Henderson-Hasselbalch equation. On the other hand, in higher pH (less acidic) environments like the small intestine, the lower [H+] will dictate that most of the aspirin will be deprotonated.
In other words, if the pKa of an acid is lower than the pH of environment, deprotonating of the acid is favored.
9. Group activity: "What is the predominant form of aspirin in the stomach?"
This problem will be solved both qualitatively (answer: protonated; pKa>pH of environment) and mathematically. Each group submits their answer, which can be graded for credit and allows for feedback (see S3 for key).
10. Optional Discussion
If time permits use the prompt: "Given that neutral (uncharged) molecules are better able to cross membranes, predict where aspirin will be better absorbed (cross the organ's membranes), the stomach or small intestine." This is a great opportunity for integration and expansion of the topic! Based on charge alone, the answer to question would be the stomach. However, with aspirin, charge is not the whole story. Acetylsalicylic acid is poorly soluble in the acidic conditions of the stomach, but very soluble at the pH in the small intestine. Once in the small intestine, a small percentage of neutral aspirin can passively move through the membrane and is then absorbed via the portal vein into the blood. In response to loss of the neutral aspirin molecules, more neutral molecules are formed in the small intestine to reestablish the equilibrium, which then allows more to pass across the membrane, etc. So, really, it is in the small intestine where the majority of aspirin absorption happens. As a side note, aspirin is associated with damage to the gastric mucosa of the stomach, elevating the risk of developing ulcers and bleeding. This is not directly related to pH, but due to inhibition of epithelial cell autophagy.
Post class options
Questions that are similar to those used in class can be designed as either formative or summative assessments to probe for transfer of the concepts. For instance, instead of the carboxylic acid aspirin discussed above, the nervous system amine stimulant amphetamine has a functional group (ammonium ion, pKa=10) that carries a positive charge at pH 7.4, the context of normal physiological conditions (18). Yet, while the carboxylic acid functional group (as found in aspirin) is neutral when protonated, an amine is protonated to a positive ammonium ion and therefore carries a charge. In other words, a deprotonated carboxylic acid molecule is negatively charged and therefore tends to be better soluble in water, but less suited to cross the lipid bilayer of a membrane. Whereas, increased solubility and diminished ability to cross membranes of a protonated base such as ammonium ion is due to its positive charge.
The antimalarial drug pyrimethamine (19) is another example of an amine functional group acting as a weak base and demonstrating the impact ionization processes have on the distribution of drugs among different compartments in the body. Pyrimethamine is a diaminopyrimidine derivative and the amino functional groups may exist in either the protonated (charged) or unprotonated (neutral) state. The protonation status of pyrimethamine influences the rate of its elimination from the body.
During a process called ‘ion trapping’, a drug in its ionized state gets trapped on one side of a membrane that separates two compartments with different pH values. In this case, pyrimethamine is cleared from the blood by the kidney to be excreted in urine. Pyrimethamine, a weak base with a pKa of 7.3, is trapped in the urine, which is more acidic than the blood. At pH 6, lower than the pKa of the drug, pyrimethamine is mostly protonated/ionized and unable to get back across the membrane. This prevents its reabsorption by the blood. To promote the excretion of pyrimethamine, administration of a urine acidifier would lower the pH of the urine and will shift the equilibrium even further towards the ionized, water soluble form of the drug (20).
Typically, the pKa is used to discuss the strength of ionizable groups of weak acid/base systems and comparing pKa values relative to each other. Despite this convention, characterizing a molecule using the pKb and probing for the relationship between pKa and pKb would be an interesting extension of this lesson.
TEACHING DISCUSSION
Observations about the Lesson's effectiveness and student reactions
Overall, the structure of this lesson promotes active participation and several opportunities for students to check their understanding. Effectiveness in achieving the stated learning goals and objectives is detailed in Table 2, which also contains typical student reactions to individual components of this lesson. The data on effectiveness were collected during three semesters. In general, students are substantially more engaged and interested in the topic when it is presented in the context of the human body (stomach, small intestine, and a pharmaceutical agent). Additionally, the traditional method of presenting only the mathematical/graphical representations of the Henderson-Hasselbalch relationship alienates students for whom mathematics is intimidating (21). In presenting the relationships in a more concept-oriented approach (i.e. the pictures of beakers with acid and conjugate base figures), the learning environment is more inclusive. Numerous students have reported an "ah-ha!" moment later or verbalized this during the module.
Possible Adaptions
Although this lesson was used in a junior-level introductory class for non-biochemistry majors in a single class period, the fundamental yet interdisciplinary nature of this topic lends itself to easy adaptation and possible expansion. Depending on the audience, the angle on human physiology can be emphasized more, or less. For instance, questions of membrane transport depending on charge can focus the class on pharmacological aspects of the protonation state. Prompting the students to predict absorption of a drug in different organ systems, as demonstrated with aspirin in this lesson as an example, is guiding the learner to consider other factors that could impact drug action, such as distribution, half-life, route of administration, and nutritional state. This angle may be more suitable for a class focused on drug action. A pharmacokinetics text, e.g. 22, may be helpful for developing a more extensive active learning exercise or case study scenario, which could take two or more class periods.
In a traditional biochemistry course sequence, expanding this lesson to predicting charge of amino acids or small peptides within a given environmental pH is a logical progression and provides the foundation for a unit on protein folding and pH-dependent catalytic activity of enzymes.
SUPPORTING MATERIALS
S1.Hasselbalch-Power point slides for lesson
S2.Hasselbalch-Pre-class reading quiz: Example questions
S3.Hasselbalch-Answer key and rubric of group activity
S4.Hasselbalch-Summative assessment: Example question
ACKNOWLEDGMENTS
This lesson was the result of the 2013 Mountain West Summer Institute in Boulder, CO.
The authors would like to thank the following people for their contributions and discussions: Aaron Snead, BPS Bioscience Inc., San Diego; Vanessa Castleberry, Rizalia Klausmeyer, Baylor University; Graciela Unguez, New Mexico State University.
References
- Bretz SL, McClary L. 2014. Students’ Understandings of Acid Strength: How Meaningful Is Reliability When Measuring Alternative Conceptions? J Chem Educ 92:212–219.
- Howitt S, Anderson T, Costa M, Hamilton S, Wright T. 2008. A concept inventory for molecular life sciences: how will it help your teaching practice. Aust Biochem 39:14–17.
- Villafañe SM, Bailey CP, Loertscher J, Minderhout V, Lewis JE. 2011. Development and analysis of an instrument to assess student understanding of foundational concepts before biochemistry coursework. Biochem Mol Biol Educ 39:102–109.
- Nelson DL, Lehninger AL, Cox MM. 2013. Lehninger Principles of Biochemistry, 6th Edition. W.H. Freeman.
- Orgill M, Sutherland A. 2008. Undergraduate chemistry students’ perceptions of and misconceptions about buffers and buffer problems. Chem Educ Res Pract 9:131–143.
- McClary L, Talanquer V. 2011. College chemistry students’ mental models of acids and acid strength. J Res Sci Teach 48:396–413.
- Chickering AW, Gamson ZF. 1987. Seven principles for good practice in undergraduate education. AAHE Bull 3:7.
- Loertscher J, Green D, Lewis JE, Lin S, Minderhout V. 2014. Identification of Threshold Concepts for Biochemistry. Cell Biol Educ 13:516–528.
- Brown BS. 1982. How I finally came to terms with pKa. Biochem Educ 10:143–144.
- Manallack DT, Prankerd RJ, Yuriev E, Oprea TI, Chalmers DK. 2013. The Significance of Acid/Base Properties in Drug Discovery. Chem Soc Rev 42:485–496.
- Hays M. 1916. The treatment of ivy poisoning. N Y Med J 104:902–904.
- Feliciano RP, Krueger CG, Reed JD. 2015. Methods to determine effects of cranberry proanthocyanidins on extraintestinal infections: Relevance for urinary tract health. Mol Nutr Food Res 59:1292–1306.
- Matsuzaki M, Mizushima S, Hiyama G, Hirohashi N, Shiba K, Inaba K, Suzuki T, Dohra H, Ohnishi T, Sato Y, Kohsaka T, Ichikawa Y, Atsumi Y, Yoshimura T, Sasanami T. 2015. Lactic acid is a sperm motility inactivation factor in the sperm storage tubules. Sci Rep 5:17643.
- Okabe M, Ito K. 2002. Color Universal Design (CUD) - How to make figures and presentations that are friendly to Colorblind people -.
- Tymoczko JL, Berg JM, Stryer L. 2011. Biochemistry: A Short Course, 2nd EditionSecond Edition edition. W. H. Freeman, New York.
- Neri D, Supuran CT. 2011. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 10:767–777.
- Hickam JB, Wilson WP, Frayser R. 1956. Observations on the early elevation of serum potassium during respiratory alkalosis. J Clin Invest 35:601.
- National Center for Biotechnology. AMPHETAMINE | C9H13N - PubChem. Openchemistry Database.
- National Center for Biotechnology. pyrimethamine | C12H13ClN4 - PubChem. Openchemistry Database.
- Maria A. Hernandez, Appu Rathinavelu. 2006. Basic Pharmacology: Understanding Drug Actions and Reactions. CRC Press.
- Watters DJ, Watters JJ. 2006. Student understanding of pH:“i don’t know what the log actually is, i only know where the button is on my calculator.” Biochem Mol Biol Educ 34:278–284.
- Sara E. Rosenbaum. Wiley: Basic Pharmacokinetics and Pharmacodynamics: An Integrated Textbook and Computer Simulations - Sara E. Rosenbaum.
Article Files
Login to access supporting documents
Taking the Hassle out of Hasselbalch(PDF | 168 KB)
S1. Hasselbalch-Power point slides for lesson.pptx(PPTX | 2 MB)
S2. Hasselbalch-Pre-class reading quiz-Example questions.docx(DOCX | 20 KB)
S3. Hasselbalch-Answer key and rubric of group activity.docx(DOCX | 61 KB)
S4. Hasselbalch-Summative assessment-Example question.docx(DOCX | 82 KB)
Table 1.png(PNG | 111 KB)
Table 2.png(PNG | 291 KB)
Table 3.png(PNG | 83 KB)
- License terms

Comments
Comments
There are no comments on this resource.