Cell Signaling Pathways - a Case Study Approach
Published online:
Abstract
Signaling and gene expression are fundamental to cell biology and developmental biology. Although these topics are highly interrelated, they typically appear in separate units in a course. We use a series of short problem-based learning exercises for two complementary purposes: 1) to promote a better understanding of the mechanisms of signal transduction; and 2) to reinforce students' understanding of cell- and tissue-specific gene expression. Moreover, the exercises promote synthesis of these two topics in the context of real biological problems. The first small-group exercise that we present poses questions about the implications of cell- or tissue-specific expression of signaling molecules, encouraging students to synthesize information when thinking about biological systems. The second exercise asks students to apply the principles of signal transduction to interpret data presented in a case study based on mutations in a MAP kinase pathway that cause Noonan syndrome. Both in-class exercises present opportunities for the students and the instructor to assess the students' understanding of signaling mechanisms. Finally, we include a set of guiding questions on the Wnt signaling pathway as an out-of-class assignment, to be followed by a quiz on Wnt signaling as a summative assessment.
Citation
Emtage, L., Bradbury, L., Coleman, N., Devenport, D., Nietel, A., and Grew, J. 2016. Cell Signaling Pathways: A case study approach. CourseSource. https://doi.org/10.24918/cs.2016.9Society Learning Goals
Cell Biology
- Cell Communication
- How do cells send, receive, and respond to signals from their environment, including other cells?
Lesson Learning Goals
- Know how components of a signaling pathway relay a signal, using both positive and negative mechanisms of regulation.
- Understand the results of inhibiting or activating components of signaling pathways.
- Appreciate how defects in signaling pathways underlie congenital diseases that affect the development of specific tissues.
- Building on previous knowledge of how gene function and expression combine to create a phenotype, understand how function or expression of signaling genes might be altered in a specific disease given a set of symptoms.
Lesson Learning Objectives
- Use knowledge of positive and negative regulation of signaling pathways to predict the outcome of genetic modifications or pharmaceutical manipulation.
- From phenotypic data, predict whether a mutation is in a coding or a regulatory region of a gene involved in signaling.
- Use data, combined with knowledge of pathways, to make reasonable predictions about the genetic basis of altered signaling pathways.
- Interpret and use pathway diagrams.
- Synthesize information by applying prior knowledge on gene expression when considering congenital syndromes.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
All cells receive information about the outside world through signaling pathways. Responsiveness of single-celled organisms to external conditions helps determine metabolic responses to environmental changes, allows them to sense population density, is required for mating in organisms such as yeast, and may coordinate colony formation and response to external threats in species that form biofilms (1-3). In metazoans, communication between cells and tissues is critical during development (4), to regulate cell division (5), and to coordinate the functions of tissues and organs in everyday maintenance of the body.
Molecular mechanisms of signal transduction are therefore fundamental to biology and are taught in a variety of settings, including introductory biology, cell biology, biochemistry, genetics, physiology, and developmental biology. Here we present a set of exercises that can be incorporated into a lecture, in which students analyze genetic and pharmaceutical manipulations of signaling pathway components. The activities in this lesson are designed with two goals in mind: (1) to help students understand positive and negative signal relay; and (2) to prompt students to think analytically about the relationship between gene expression and protein function in determining phenotype. These questions synthesize aspects of the two topics to promote a better understanding of both concepts.
In a cell or developmental biology course that follows a textbook, mechanisms of gene expression and cell signaling are likely to be taught as separate chapters. Because textbook chapters are typically devoted to a particular subject, students are generally assigned to do problems on that topic, leading to "massed" or "blocked" learning. However, many studies have shown that two practice techniques, mixing up practice questions (shuffling, or interleaving) and spacing out questions over time (spacing, or distributed practice) result in better long-term mastery than blocked learning, even when they result in worse short-term performance (6-8). Interleaving has been shown to increase performance for diverse cognitive skills involving memory, judgment, and problem-solving (6-10). Similarly, spacing practice sessions over days or weeks, known as distributed practice, has long been shown to increase retention over periods of weeks to months (6,11) (for a meta-analysis, see Cepeda et al., 2006).
Because the mechanisms of gene expression are usually taught as a block, students typically learn about transcriptional and post-transcriptional mechanisms for turning on or off gene expression in a manner that isolates that information from other relevant topics. When signaling pathways are covered, some emphasis is placed on their function in modulating the activity of transcription factors. However, little attention is paid, for example, to the tissue-specific expression of the signaling molecules themselves, which is critical to their function. In contrast to the blocked presentation in texts, scientists and medical practitioners must combine information on expression and function when thinking about biological problems.
Accordingly, we include a series of questions that link the tissue-specific expression of signaling components to the phenotypic outcome of mutations. The questions give the students an opportunity to think about the implications of tissue-specific gene expression in the context of signaling pathways prior to an instructor-led discussion of the topic. The instructor then has the opportunity to review and extend coverage of molecular genetics and the mechanisms of gene expression. An added benefit of this reconsideration is that spacing questions and interleaving subjects is more effective for learning than presenting uninterrupted blocks of content on a single topic (6-11). This set of exercises encourages students to synthesize information that is normally presented at different times.
Many cell and developmental biology textbooks present the molecular details of important signaling pathways. Although knowledge of common signal transduction pathway components is important, in our experience, memorization of pathway components does not guarantee that students will be able to predict the outcome of manipulations of a pathway. We have developed a small-group, case-study exercise in which students interpret data on a genetic disorder, Noonan syndrome, and a hypothetical related syndrome. The activity is student-centered, cooperative- and problem-based, qualities that have been shown to improve understanding and critical-thinking skills (12). In a trial implementation (L. Emtage, Fall 2015), one of us found that these exercises prompted students to ask more questions of the instructor about transduction pathways and led to better performance on quizzes, in contrast to presentation of the same information in traditional lectures. Additionally, in-class problem-solving and data analysis have also been shown to reduce the achievement gap between disadvantaged and non-disadvantaged students (13). In settings with a high proportion of first-generation college students, achieving this performance equity may be especially critical.
The signaling pathways that we have chosen for these exercises are three highly conserved and centrally important signaling pathways: receptor tyrosine kinase (RTK), MAP kinase, and Wnt/β-catenin. All are critical to development and are frequently mutated in cancers. Our in-class exercises focus on the RTK and MAP kinase transduction pathways. We follow up with an out-of-class assignment that uses guided questions to help the students analyze transduction through the Wnt/β-catenin signaling pathway. In the following class meeting, we quiz students on the mechanics of the β-catenin signaling pathway and on their understanding of the connection between expression and phenotype. For the instructor, we have included additional information on the RTK pathways in the presentation slides (Supporting File S1), and on the Wnt/β-catenin pathway in the instructor notes for the homework exercise (Supporting File S7).
In a developmental biology course, these topics will naturally be revisited and expanded upon during the course of the semester. Wnt signaling is fundamental to initiating gastrulation and important in early embryonic patterning. Migrating cell populations such as the neural crest, hematopoietic stem cells (HSCs), or limb myoblasts express RTKs. RTK activity is critical for the survival and differentiation of many adult stem cells. Beyond individual pathways, which will be referenced in connection with particular tissues, mutations in signaling proteins can have pleiotropic effects. Understanding the function and complex expression patterns of many signaling proteins provides the key to understanding real-world examples of their action and the consequence of loss-of-function mutations.
A solid understanding of RTK and Wnt signaling is also helpful when covering cancer, since many of the proteins in these two pathways can be mutated, resulting in commonly encountered oncogenes. Personalized medicine, in the form of chemotherapeutics that target the specific signaling pathways that are up-regulated in a particular cancer, cannot be understood without a firm grasp of the mechanics of signal transduction. For example, approximately 90% of colorectal cancers have mutations in the canonical Wnt pathway, leading to the search for inhibitors of β-catenin (14). About half of all current drugs used for treatment target RTKs or the MAPK pathway (15). Thus, the active-learning exercises described here can be extended and revisited in a later unit on cell cycle regulation, differentiation, tumorigenesis, or, indeed, a separate course in cancer biology.
INTENDED AUDIENCE AND PRE-REQUISITE KNOWLEDGE
These activities were created for either a cell biology or developmental biology course at the sophomore level or above. To make use of the first small-group activity, the students need to understand the basics of regulation of transcription, including that regulatory sequences surrounding a gene determine the time and place of transcription. They should also understand that mutations may result in either loss of function or gain of function of the mutated protein. The instructor should have general knowledge of signal transduction and gene expression.
REQUIRED LEARNING TIME
The total time allotted to the in-class exercises should be about an hour and a quarter, not including the homework assignment. Time allotted to the quiz should be approximately 15 minutes at the beginning of the next lecture session.
SCIENTIFIC TEACHING THEMES
Active Learning
- In-class: Think/pair/share approach in interleaving exercise. The students will work in small groups to answer the case study questions. Full class discussion of small group answers.
- Outside of class: The assignment on the Wnt/β-catenin pathway asks the students to interpret a diagram of the pathway without text, although the students are encouraged to find any resource they can to answer the guiding questions.
Assessment
- Two in-class discussions, one led by the lecturer, and one led by the students, will provide formative assessment of student understanding.
- A follow-up quiz will encourage students to do the out-of-class assignment and provide summative assessment.
Inclusive Teaching
- This assignment includes mixed learning strategies: visual (slides), aural, textual, discussion and diagrammatically presented information.
- The instructor may form groups of mixed ability in order to promote peer-to-peer teaching and learning.
LESSON PLAN
PRE-CLASS PREPARATION
This lesson is intended to be used in conjunction with a chapter or unit on cell signaling, as is commonly found in cell biology or developmental biology textbooks. The students should also be familiar with basic molecular genetics, including simple mechanisms of transcriptional regulation, and loss-of-function versus activating (gain-of-function) mutations. To understand the first set of questions, students must know that a gene contains both regulatory sequences and coding sequences. They should be introduced to positive and negative mechanisms of signal transduction in the period prior to this lesson, including phosphorylation, guanine nucleotide exchange and hydrolysis, ubiquitination and proteolysis, and binding-induced conformational changes. Many textbooks cover this material, so students may be assigned readings to cover this material.
The instructor should prepare the handouts. Please see Table 1 for a teaching timeline of this Lesson.
IN-CLASS ACTIVITIES
Review by instructor
The class should begin with a brief review of positive and negative mechanisms of signal transduction, followed by a detailed explanation of positive and negative regulation in RTK pathways. Examples of negative regulation of the MAPK pathway are introduced through the example provided by the RTK/Akt-cell survival pathway, which provides an excellent introduction to negative regulation by sequestration and inhibition. While knowledge of the cell survival signaling pathway is not strictly necessary for these exercises, it is commonly covered in units on signaling and provides a good overview of mechanisms of negative regulation that the students will encounter in the Wnt/β-catenin signaling pathway. See Supporting File S1 for examples of pathways illustrating these principles.
During the lecture on the MAPK and cell survival pathways, we use the clicker questions and/or direct questioning to assess students' understanding of the logic of the pathways. In addition to the sample clicker questions that we have provided, we have found it helpful to elaborate on the repercussions of loss-of-function or gain-of-function mutations throughout the pathway. The logic of sequential activation as found in the MAPK pathway, and the logic of a pathway that operates through inhibition, such as the cell survival pathway, may be covered by asking questions of the students and guiding them through the answers. A brief guided analysis will be necessary for the students to tackle the applied questions found in the case study.
Exercise on gene expression
The exercise on gene expression reinforces and extends previous knowledge of how gene expression and protein function combine to produce a phenotype. This exercise covers mechanisms of gene expression that are not necessarily covered in a chapter on cell signaling. This exercise is intended to apply previously learned material to cell signaling pathways.
Let the students know before beginning that the quiz at the beginning of the next class session will test their understanding of the material in both in-class exercises and the out-of-class assignment.
The instructor should distribute the handout (Supporting File S2), which includes a description of an RTK pathway found in melanoblasts (Kit-mediated activation of MITF), followed by several guiding questions that prompt students to apply what they have learned about gene expression to signaling pathways. The instructor should ask students to read the handout and attempt to answer the guiding questions on their own. After students have attempted to answer the questions on their own, the instructor should pair students with their neighbors to compare answers (think/pair/share). After this discussion, the instructor can solicit individuals who are willing to share answers with the class.
There is not enough information in the handout to answer the first guiding question with certainty. The students are unlikely to know, although they should suspect based on what they have heard about the role of signal transduction proteins in survival pathways, that complete loss-of-function of the Kit receptor or its ligand, SCF, will cause the loss of all three cell types in which it is expressed (16,17). Students can be prompted to consider what is the likely outcome of loss of each of these cell populations separately for the organism. In this case, two of the affected populations are dispensable for development, and is the loss of hematopoietic stem cells that produces the embryonic lethal phenotype of deletion of Kit or SCF.
This discussion sets the stage for understanding why loss of MITF produces a different phenotype from loss of Kit or SCF. The transcription factor MITF is required for the differentiation of melanocytes and retinal pigment cells. However, germ cells and hematopoietic stem cells (HSCs) do not express MITF, but rather express other Kit targets instead. Therefore, loss of MITF has a different, more restricted phenotype than loss of Kit or SCF. This example simultaneously illustrates the modularity of signaling and the importance of tissue-specific gene expression in determining cell identity and behavior.
The second question tests the students' grasp of this idea. The third question asks for a more sophisticated analysis on the part of the students. Light pigmentation is typically recessive, and homozygous mutations in the SCF coding region would be expected to cause pleiotropic effects. There is no evidence, however, that individuals with blonde hair have defects in hematopoiesis or germ cell production. Therefore, a mutation in a melanoblast-specific enhancer is the most likely answer. A brief review of mechanisms of transcriptional control would be appropriate at this point in the discussion. Other possible topics for review include the molecular bases for loss-of-function mutations and pleiotropy.
It should take about 10 minutes total for students to read the questions and do the think/pair/share exercise, and the student-instructor discussion should take another 10 minutes, primarily to review mechanisms of tissue-specific regulation of transcription and the architecture of a gene locus.
Case study
The instructor should organize the pairs into small groups of approximately 4-6 students, and give everyone a copy of the case study and diagram (Supporting File S4). Beyond basic instructions, the instructor does not need to intervene. Each group should have about 20 minutes to read the case study and propose and discuss answers within the group.
After most of the groups have finished, the instructor should let the students argue it out in a whole-class discussion of the questions, in which the instructor serves as moderator but does not supply answers. The instructor may then intervene at the last moment and confirm the correct answers if necessary. The whole class discussion serves as a formative assessment for the students and the instructor. It is very important that the instructor not intervene until the students have debated the answers. Immediately after the students have had their discussion and are engaged with the material, the instructor has an excellent opportunity to review epistasis and hierarchical relationships in signaling.
The first question in the case study challenges the students to connect an abstract understanding of pathway activation with concrete molecular mechanisms of activation. Although Tiger syndrome is fictional, the activating mutation given here (T266K) is just one of many mutations in the MAPK pathway that are known to give rise to Noonan syndrome, or the related LEOPARD syndrome; for lists of other mutations, see the online catalogue of genes and genetic disorders, Online Mendelian Inheritance in Man (http://www.omim.org/).
The case study's second question is designed to assess the students understanding of the hierarchical nature of signaling pathways. Constitutive activation of Sos will activate downstream components of the pathway, regardless of the activation state of upstream molecules. This principle is akin to genetic epistasis and is fundamental to understanding how somatic mutations can cause cancer in specific tissues, for example.
The third question builds on the first and second questions, and requires the students to recall that Raf inhibitors are effective in both Noonan and the hypothetical Tiger syndrome. If we know that disease-causing mutations activate this pathway, and that inhibition of Raf is effective, then we know that the other disease-causing mutations must be in Raf itself, or components upstream of Raf. This question illustrates how information about a pathway can be obtained from inhibitors, and, further suggests how information about signaling pathways can be used when designing therapeutic agents.
Out-of-class assignment
The instructor should give the students the guided questions for the Wnt/β-catenin pathway. It should be pointed out that the pathway is unlike the MAPK pathway in that activation of the pathway occurs through inhibition of a negative regulator of β-catenin (Supporting File S6). The questions on the homework assignment are designed to review the hierarchical nature of signaling pathways, and to prompt the students to work through the logic of inhibitory mechanisms. This assignment asks the student to answer questions similar to those posed by the instructor in class during the initial introduction to RTK pathways (see above), but without the instructors' direct guidance. Students should be told that they may use any resource to figure out the answers to the questions, but that the quiz questions will not be identical to the guiding questions.
Assessment
During the in-class exercises, students' answers to the questions will provide feedback on student learning and difficulties. The quiz questions should be used to evaluate whether the students have mastered the material (Supporting File S8).
TEACHING DISCUSSION
The in-class exercises have been used in a Developmental Biology class (L. Emtage, Fall 2015). The exercises in Supporting Files S2 and S4 were given in class in the week after two lectures on regulation of gene expression and a lecture on signaling mechanisms. The instructor fielded many more questions on the actual material of the lesson (signaling pathways) after the case study exercise than after more traditional lectures.
OUTCOMES
The effect of these exercises on student understanding were measured in two ways. First, we compared students in a traditionally-taught Cell Biology course (Emtage, Spring 2015) with students in the Developmental Biology course described above (Emtage, Fall 2015). The Cell Biology course covered both gene expression and signaling pathways in a traditional lecture format. In the session following the lecture on signaling mechanisms and signaling pathway components, the students were given a quiz question similar to the question included here (Supporting File S8), but on G protein-coupled receptors. Only one out of 35 was able to correctly answer the question (Figure 1). In Developmental Biology, the students participated in the active learning exercises given in Supporting Files S2 and S4. In the class session after the active-learning exercises S2 and S4, they were given a quiz on the Wnt signaling pathway. Three out of fourteen students received full credit for the first quiz question given in S8 (Figure 1), while a further six students received partial credit (not shown).
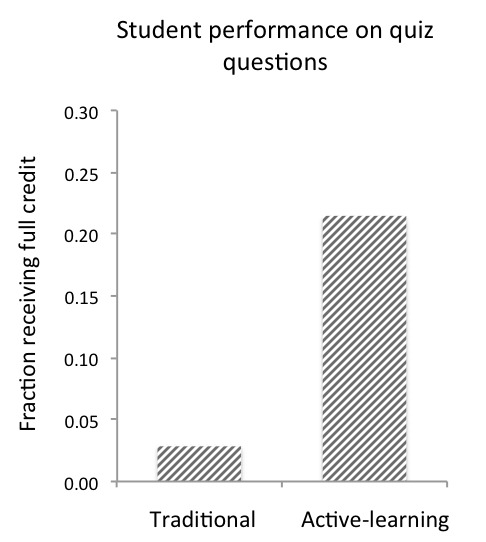
Figure 1. Student improvement with case study. The fraction of students receiving full credit on a quiz question requiring analysis of a signaling pathway after a traditional lecture (35 students), and after the active-learning activity described here (14 students).
In the following year's Developmental Biology class (Fall 2016), the students again participated in the in-class exercises S2 and S4, were assigned the homework exercise S6, and were given the quiz in S8. We did not discuss the homework assignment prior to the quiz; the 2016 cohort performed similarly to their peers from the previous year on the quiz. However, we did review the homework and quiz during the next lecture. Three weeks later, the students were given an exam including two short-answer questions on the β-catenin signaling pathway. One was a simple question on the mechanics of the pathway. Eleven out of 18 students were able to answer this question correctly (Figure 2, Simple exam question). The second question was analytical; it asked the students to predict the probable outcome of a manipulation of the pathway. Six out of 18 students were able to answer the question correctly and give a reasonable explanation for their answer (Figure 2, Analytical exam question).
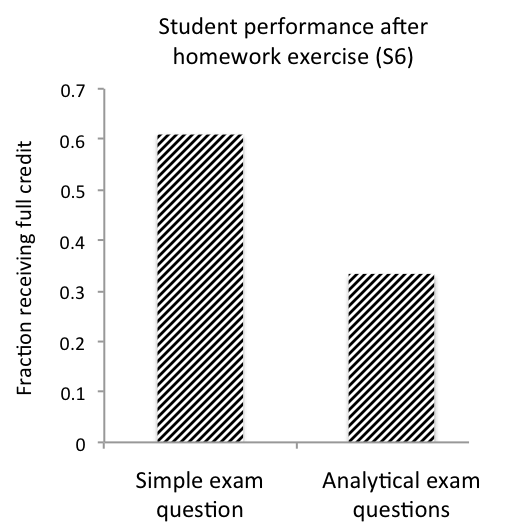
Figure 2. Student performance with case study and homework. Effect of an analytical, challenging homework exercise on student performance in active learning setting.
The fraction of students able to answer analytical questions does gradually increase with practice (Figures 1 and 2). These results indicate that the combination of active-learning exercises and the creation of opportunities to discuss challenging questions can improve both the students' basic understanding of signaling pathways and their ability to analyze and manipulate the information that they have learned.
MODIFICATIONS
The out-of-class exercise was developed to give the students an opportunity to apply the principles that they used in analyzing the MAP kinase pathway to the Wnt signaling pathway, which is more complicated than the MAP kinase pathway. The exercise includes guiding questions and a diagram of the pathway, but no explanatory text. However, the students are free to find additional materials to help them understand the diagram. Our goal is to challenge students to continue to develop build on their problem-solving skills and to encourage them to interpret information that is presented diagrammatically.
The lesson can be pared down to only the Noonan Case Study, to suit an introductory course covering signaling, or expanded upon in a variety of directions for a more in-depth analysis suitable to upper level courses in cellular, molecular genetics or developmental biology. One possible expansion would be to connect mutations that affect signaling and congenital disorders of development. If an instructor prefers, the out-of-class assignment could be altered or expanded to cover a different signaling pathway, such as G protein-coupled receptors; Essential Cell Biology, for example, covers GPCRs but not Wnt signaling( )(18). The small group exercise could be expanded with a discussion of oncogenes and tumor suppressors, and chemotherapeutics that target particular oncogenes (personalized medicine). Finally, the instructor could reverse the order of the case study and interleaving exercise if the unit on regulation of gene expression comes after the unit on signaling.
SUPPORTING MATERIALS
- S1. Cell Signaling: Introduction to the MAPK pathway.pptx (Supporting File S1 contains the PowerPoint slides introducing the MAPK and cell survival pathways, along with instructor notes on those pathways)
- S2. Cell Signaling: Interleaving exercise on gene expression.docx (Supporting File S2 contains the first student handout for this activity. See Supporting File S3 for the instructor version with a key and explanation)
- S3. Cell Signaling: Interleaving exercise on gene expression instructor notes.docx
- S4. Cell Signaling: Noonan Case Study.docx (Supporting File S4 contains the second student handout for this activity. See Supporting File S5 for the instructor version with a key and explanation)
- S5. Cell Signaling: Noonan Case Study instructor notes.docx
- S6. Cell Signaling: Beta-catenin pathway homework.docx (Supporting File S6 contains the homework (guiding questions). See Supporting File S7 for the instructor version with a key and explanation)
- S7. Cell Signaling: Beta-catenin pathway homework instructor notes.docx
- S8. Cell Signaling: Beta-catenin pathway quiz.docx (Supporting File S8 contains the quiz. See Supporting File S9 for the instructor version with a key and explanation)
- S9. Cell Signaling: Beta-catenin pathway quiz instructor notes.docx
ACKNOWLEDGMENTS
The authors developed the case study while attending the National Academies Summer Institute on Undergraduate Education in Science at Princeton University, 2015, sponsored by the National Academy of Sciences and the Howard Hughes Medical Institute. We thank David Gross of the Department of Biochemistry and Molecular Biology, University of Massachusetts, Amherst, as well as other participants in the Northeast Summer Institute, for valuable direction and feedback.
References
- Guzzo M, Agrebi R, Espinosa L, Baronian G, Molle V, Mauriello EMF, Brochier-Armanet C, Mignot T. Evolution and Design Governing Signal Precision and Amplification in a Bacterial Chemosensory Pathway. PLoS Genet [Internet] 2015 [cited 2016 Aug 24]; 11. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4546325/
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 2005; 21:319-46.
- Los DA, Zorina A, Sinetova M, Kryazhov S, Mironov K, Zinchenko VV. Stress Sensors and Signal Transducers in Cyanobacteria. Sensors 2010; 10:2386-415.
- Pires-daSilva A, Sommer RJ. The evolution of signalling pathways in animal development. Nat Rev Genet 2003; 4:39-49.
- Rhind N, Russell P. Signaling Pathways that Regulate Cell Division. Cold Spring Harb Perspect Biol 2012; 4:a005942.
- Rohrer D, Taylor K. The shuffling of mathematics problems improves learning. Instr Sci 2007; 35:481-98.
- Helsdingen A, van Gog T, van Merri?nboer J. The effects of practice schedule and critical thinking prompts on learning and transfer of a complex judgment task. J Educ Psychol 2011; 103:383-98.
- Carvalho PF, Goldstone RL. Effects of interleaved and blocked study on delayed test of category learning generalization. Front Psychol [Internet] 2014 [cited 2016 Aug 24]; 5. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4141442/
- Hatala RM, Brooks LR, Norman GR. Practice makes perfect: the critical role of mixed practice in the acquisition of ECG interpretation skills. Adv Health Sci Educ Theory Pract 2003; 8:17-26.
- Kornell N, Bjork RA. Learning concepts and categories: is spacing the "enemy of induction"? Psychol Sci 2008; 19:585-92.
- Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D. Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychol Bull 2006; 132:354-80.
- Vision and Change in Undergraduate Biology Education >> Final Report [Internet]. [cited 2016 Feb 9]; Available from: http://visionandchange.org/finalreport/
- Haak DC, HilleRisLambers J, Pitre E, Freeman S. Increased Structure and Active Learning Reduce the Achievement Gap in Introductory Biology. Science 2011; 332:1213-6.
- Zanni R, Galvez-Llompart M, Morell C, Rodr?guez-Henche N, D?az-Laviada I, Recio-Iglesias MC, Garcia-Domenech R, Galvez J. Novel Cancer Chemotherapy Hits by Molecular Topology: Dual Akt and Beta-Catenin Inhibitors. PLoS ONE 2015; 10:e0124244.
- Overview of Targeted Therapies for Cancer - My Cancer Genome [Internet]. [cited 2016 Feb 9]; Available from: http://www.mycancergenome.org/content/molecular-medicine/overview-of-targeted-therapies-for-cancer/
- Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J 1990; 9:1805-13.
- Brannan CI, Lyman SD, Williams DE, Eisenman J, Anderson DM, Cosman D, Bedell MA, Jenkins NA, Copeland NG. Steel-Dickie mutation encodes a c-kit ligand lacking transmembrane and cytoplasmic domains. Proc Natl Acad Sci U S A 1991; 88:4671-4.
- Alberts B, Bray D, Hopkin K, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Essential cell biology. Garland Science; 2013.
Article Files
Login to access supporting documents
Cell Signaling Pathways - a Case Study Approach(PDF | 173 KB)
S1.Cell Signaling-Introduction to the MAPK pathway.pptx(PPTX | 668 KB)
S2.Cell Signaling-Signaling and gene expresssion exercise.docx(DOCX | 410 KB)
S3.Cell Signaling-Signaling and gene expression instructor notes.docx(DOCX | 412 KB)
S4.Cell Signaling-Noonan Case Study.docx(DOCX | 496 KB)
S5.Cell Signaling-Noonan Case Study instructor notes.docx(DOCX | 488 KB)
S6.Cell Signaling--catenin pathway homework.docx(DOCX | 598 KB)
S7.Cell Signaling--catenin pathway homework instructor notes.docx(DOCX | 669 KB)
S8.Cell Signaling--catenin pathway quiz.docx(DOCX | 605 KB)
S9.Cell Signaling--catenin pathway quiz instructor notes.docx(DOCX | 636 KB)
- License terms

Comments
Comments
There are no comments on this resource.