A virtual laboratory on cell division using a publicly-available image database
Published online:
Abstract
Cell division is a key concept in cell biology. While there are many popular activities to teach students the stages of mitosis, most make use of simple schematics, cartoons, or textbook diagrams. Others engage students in acting out the stages, or modeling them with physical objects (i.e. noodles, pipe cleaners). These approaches are useful for developing student knowledge and comprehension of the stages of cell division, but do not readily convey the real-life processes of mitosis. Moreover, they do not teach students how cell biologists study these processes, nor the difficulties with imaging real cells. Here, we provide an activity to reinforce student knowledge of mitosis, demonstrate how data on mitosis and other dynamic cellular processes can be collected, and introduce methods of data analysis for real cellular images using research-quality digital images from a free public database. This activity guides students through a virtual experiment that can be easily scaled for large introductory classes or low-resource settings. The activity focuses on experimentally determining the timing of the stages of cell division, directing the attention of students to the tasks that are completed at each stage and promoting understanding of the underlying mechanisms. Before the experiment, the students generate testable predictions for the relative amount of time each step of mitosis takes, provide a mechanistic reason for their prediction, and explain how they will test their predictions using imaging data. Students then identify the stages of cell division in a curated set of digital images and determine how to convert their data into relative amount of time for each phase of mitosis. Finally, students are asked to relate their findings to their original predictions, reinforcing their increasing understanding of the cell cycle. Students praised the practical application of their knowledge and development of image interpretation skills that would be used in a cell biology research setting.
Citation
Shelden E.A., Offerdahl E.G., and Johnson G.T. 2019. A Virtual Laboratory on Cell Division Using a Publicly-Available Image Database. CourseSource. https://doi.org/10.24918/cs.2019.15Society Learning Goals
Cell Biology
- Cytoskeleton Structure and Function
- How do the different components of the cytoskeleton support a variety of cell functions, such as cell shape, division, movement, sensing the environment, and cell-cell communication?
- Cell Cycle and Cell Division
- How do cells conduct, coordinate, and regulate nuclear and cell division?
- Methods & Tools of Cell Biology
- How do the methods and tools of cell biology enable and limit our understanding of the cell?
Science Process Skills
- Process of Science
- Pose testable questions and hypotheses to address gaps in knowledge
- Interpret, evaluate, and draw conclusions from data
- Construct explanations and make evidence-based arguments about the natural world
- Modeling/ Developing and Using Models
- Build and evaluate models of biological systems
- Quantitative Reasoning/ Using Mathematics and Computational Thinking
- Apply the tools of graphing, statistics, and data science to analyze biological data
- Communication and Collaboration
- Share ideas, data, and findings with others clearly and accurately
Lesson Learning Goals
- Students will understand the events of mitosis and the cellular processes that occur during different stages of cell division.
- Students will understand how data on dynamic cellular events are collected and analyzed.
- Students will appreciate how cell biologists address experimental questions.
Lesson Learning Objectives
- Students will name and describe the salient features and cellular tasks for each stage of cell division.
- Students will predict the relative durations of the stages of cell division using prior knowledge and facts from assigned readings.
- Students will describe the relationship between duration of each stage of cell division and the frequency of cells present in each stage of cell division counted in a random sample of images of pluripotent stem cells.
- Students will identify the stages of cell division present in research-quality images of human pluripotent stem cells in various stages of cell division.
- Students will quantify, analyze and summarize data on the prevalence of cells at different stages of cell division in randomly sampled cell populations.
- Students will use data to reflect on and revise predictions.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
Cells are the fundamental unit of life and make up every living organism. All cells are produced by cell division, making the process one of the most important in all of biology. In addition to its role in reproduction, normal development and tissue maintenance, cell division underlies disease conditions, including cancer and hypertrophy. Not surprisingly, the mechanisms and control of cell division are central to the research efforts of many scientists and a description of cell division can be found in any biology textbook. The basic steps of cell division (prophase, prometaphase, metaphase, anaphase and telophase) are generally memorized by most biology students from textbook resources. With little first-hand experience in the underlying biology of these events, however, students (1,2) and even biology teachers (3,4) are known to struggle with memorizing and understanding the processes of cell division. Remarkably, problems associated with teaching cell division appeared in published literature well before the structure of DNA was established (5).
The teaching of concepts in cell division using texts, diagrams and models constructed from a variety of materials has been explored by numerous investigators (10) and offers students the opportunity to view stained chromosomes directly. Commercial and non-profit teaching kits for this activity are widely available. However, laboratory work with real biological samples is difficult to apply in introductory classes with large numbers of students, online teaching contexts, or settings with limited access to cell culture facilities and the necessary microscopes, cameras and computers (11). The complexities of working with real cells may limit the number of examples that students can view in a reasonable amount of time, reducing the potential for sampling cells in adequate numbers for statistical exploration. While plant cells are easy to obtain and manipulate, students may benefit from the opportunity to work with mammalian or even human cells, as they experience a more personal connection to the material. However, work with mammalian and human cells is technically difficult and involves specialized equipment needed to handle the exposure risks inherent to working with such cells. Moreover, current research in both academic and commercial settings is increasingly dependent on digital resources (12) and outsourcing of preparatory work to service laboratories (13). Thus, it can be argued that the use of digital images as the foundation of a data driven laboratory investigation offers potential insight into and training for real world research applications.
Analysis of digital images of cell populations containing dividing cells has the potential to address some of the limitations discussed above. The effectiveness of digital resources for teaching concepts in cell division has been previously assessed, and in general the available tools were shown to be as effective as hands-on laboratory exercises in promoting student understanding of key concepts. However, available digital and online resources are typically limited to small numbers of example images or videos (14,15). Recently, the Allen Institute for Cell Science (allencell.org) has provided public access to large sets of human cell images for research and educational purposes (16). The images were obtained by labeling human pluripotent stem cells with fluorescent markers for major cellular components including tubulin, the main building block of microtubules that form the mitotic spindle apparatus, and DNA. Images were obtained with high quality research-grade fluorescence microscopes at multiple image planes and are presented in an online viewer as both two- and three-dimensional data sets. Many thousands of examples are available (such as one shown in Figure 1). Some images represent individual cells suitable for high resolution structural analysis, while others show lower magnification fields containing twenty or more cells suitable for statistical analysis of cell populations. The image sets were obtained in a manner that permits rigorous scientific inquiry into cell morphology and intracellular organization and are therefore suitable for use in a hands-on virtual laboratory exercise that can provide insight into both the events of cell division and the sampling strategies that allow statistical analysis of dynamic cellular events. In addition to serving as a learning tool for the study of cell division, the activity can potentially provide students with exposure to a research method applicable to determining the mitotic index in a manner employed by scientists in both academic and commercial settings (17,18).
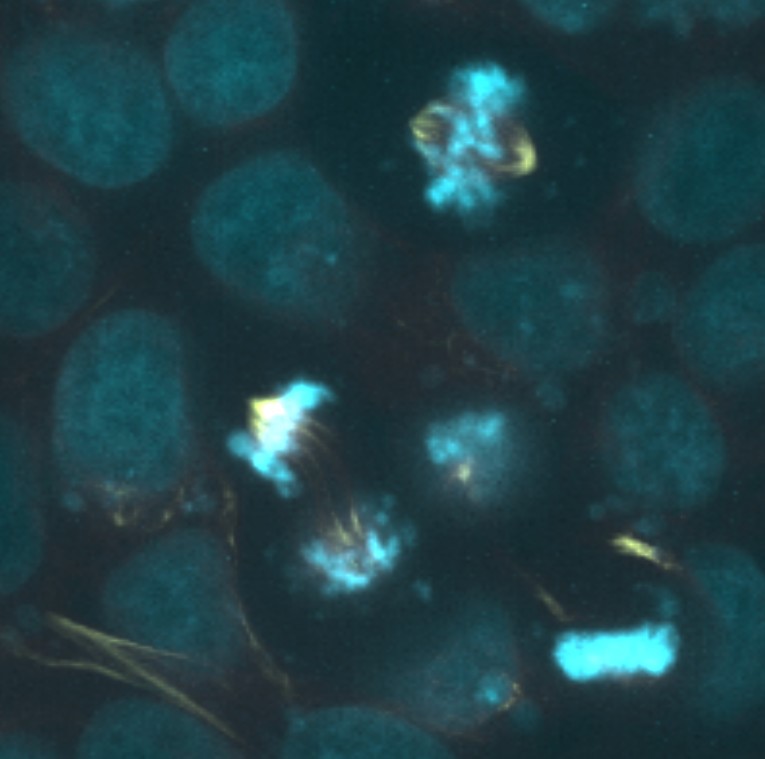
Figure 1. Example image of dividing cells obtained from the Allen Institute for Cell Science 3D Cell Viewer.
The activity described here was designed to improve engagement of students with the concepts of cell division by leveraging research resources provided by the Allen Institute for Cell Science. These are web-based resources, so this activity will require access to a computer with internet access. The activity is suitable for advanced undergraduate biology students but can be adapted for introductory biology for majors. This activity has been implemented as an extra credit assignment during an online web-based course as well as a take-home individual extra credit assignment in a face-to-face course. Potential adaptation for use as an in-class laboratory for individual students and small student groups is presented in the Discussion section.
In preparation for the activity, students should be provided with information about the stages of mitosis and the tasks performed by cells in each stage. They should be encouraged to consider the relationship between cellular tasks and duration of the mitotic stage. Instructors can present the material in the accompanying mini-lecture on cell division (Supporting File S1. A virtual laboratory on cell division-Mini-lecture) as well as the provided example images of cells at each stage of division (Supporting File S5. A virtual laboratory on cell vision-Example images of cells). The laboratory activity itself can be broken into two stages. In the first, students rationalize a prediction about the frequency of cells that will be present in each stage of mitosis in a random sample of pluripotent human stem cells. In Stage 2, students are provided with a random sample of real microscopic images of human pluripotent stem cells in various stages of the cell cycle. Students examine the images, quantify the number of cells in each stage of the cell cycle, and reflect on their predictions in light of individual and classroom aggregate data. Finally, students provide a written summary in which they consider how methods used to obtain, quantify and analyze data might influence the results obtained from their analysis. The activity was designed to promote both an understanding of mitosis and insight into the procedures used by scientists to obtain, quantify and analyze the behavior of cells.
INTENDED AUDIENCE
This activity has been used in an upper-level cell biology course at a large research-intensive university in both a face-to-face and an online course. Students in these courses include biology majors and first-year graduate students. The course is offered three times a year (fall, spring, and summer) with course enrollments ranging between 25 and 50. We anticipate this activity could be readily adapted for use in introductory biology classes in college and high school settings.
REQUIRED LEARNING TIME
The lesson takes ~2 hours to complete dispersed over two days of activity or instruction. The students spend ~30 minutes reviewing the events of cell division and producing a written hypothesis statement. Counting of the cells in images, tabulating, and summarizing results takes about an hour. Finally, producing a written summary and discussion takes about another 30 minutes.
PRE-REQUISITE STUDENT KNOWLEDGE
This lesson is intended for use after students have received instruction on mitosis. Students should be able to define the stages of mitosis and cellular structures that characterize the stages of cell division (e.g. mitotic spindle, microtubules, midbody, nuclear envelope). A good basic knowledge of cell division can be obtained by reading appropriate chapters in Alberts (19) , Lodish (20), Pollard (21), or an equivalent text. The Virtual Cell Animation collection has a helpful animation (www.vcell.science), and Scitable by Nature Education has a video of a mitotic cell (https://www.nature.com/scitable/content/mitosis-6656772). The Allen Institute for Cell Science also has example images and videos of cell division at various magnifications (22), and a brief series of lecture slides describing stages of cell division with images from this source is included (Supporting File S1: A virtual laboratory on cell division-Mini-lecture). Prior to conducting their analysis of cell images, students may also benefit from gaining some experience with the controls of the 3D Cell Viewer (23). We have included a handout that provides an introduction to the laboratory, instructions for using the 3D cell viewer and suggestions for efficient counting of cells (Supporting File S2: A virtual laboratory on cell division-Handout, assignment and instructions), which could be provided to students either in its entirety or in sections. Finally, students should also understand how to tabulate and graph data using Excel or similar software.
PRE-REQUISITE TEACHER KNOWLEDGE
The teacher should be familiar with the stages of cell division and the underlying mechanisms and processes that occur in them. For example, in prophase the cell condenses its chromosomes, disassembles the interphase microtubule array and begins to assemble an early mitotic spindle. The cell also changes shape, often rounding up which collects the chromosomes and minimizes the space containing them. This in turn aids the cell in attaching chromatids to the mitotic spindle in prometaphase and reduces the potential of them to get lost in the cytoplasm, which leads to improper numbers of chromosomes in the two daughter cells (known as aneuploidy) and which can result in developmental defects or cancer. The breakdown of the nuclear envelope marks the end of prophase and the beginning of prometaphase. In prometaphase, chromosomes are captured by microtubules and attached to the mitotic spindle, which takes on its final shape. In metaphase, the cell checks to determine that all chromosomes have equal attachments to both poles of the mitotic spindle. This is the stage where an important cell cycle checkpoint must be passed for the cell to proceed through the remainder of cell division. Anaphase comes after metaphase and consists of the movement of chromosomes from the center of the cell to one mitotic spindle pole. This process is called "anaphase A". The spindle poles also move away from each other using microtubules to push the opposite pole away in a process called "anaphase B". Because of anaphase B movement, the mitotic spindle is usually much longer in anaphase than in metaphase. Finally, telophase is characterized by decondensation of chromosomes, the reassembly of interphase cytoskeletal arrays and reformation of the nuclear membrane. In many cells, a "midbody" comprised of bundled microtubules connects the two daughter cells for some time in telophase. Cytokinesis, the process that divides the cytoplasm of cells into the two daughter cells, usually occurs at the end of anaphase B or start of telophase. For many cells, prophase may be the longest stage of cell division, followed by metaphase and telophase, while prometaphase is very short and anaphase slightly longer, but shorter than metaphase or telophase (24,25). However, different cell types may display longer or shorter relative durations of these stages.
To assist students and prepare for the activity, instructors should have basic skills working with Excel or a similar spreadsheet program. Teachers may also wish to be familiar with the operation of the 3D Cell Viewer (Supporting File S2: A virtual laboratory on cell division-Handout, assignment and instructions) provided by the Allen Institute for Cell Science. This activity requires preparing Excel spreadsheets for each student or group of students with links to human cell images. Instructions for how to embed links to cell images within Excel spreadsheets are below in the section "Lesson Plan, Pre-lesson/lab preparations:".
SCIENTIFIC TEACHING THEMES
ACTIVE LEARNING
Students are actively engaged in the activity through analysis of authentic data and performing higher-order tasks (i.e. predicting, analyzing, evaluating, justifying). After reviewing the tasks that cells must achieve to complete cell division, students generate mechanistic hypotheses of how long a cell needs to accomplish each task and then predict the frequency of human pluripotent stem cells in each phase within their random sample of real images. Students analyze the real images of human pluripotent stem cells and directly observe the diversity of morphologies that real cells display. Finally, students aggregate observation data, reflect on hypotheses, and consider the impact of technical issues and sampling methods on data collection, quantitative results and the validity of their initial hypothesis.
ASSESSMENT
Assessment of learning progress was measured by evaluation of two written documents generated during the first and second stage of the assignment. A possible grading rubric can be found below in the section "Lesson Plan, Grading rubric and notes". Emphasis was placed on understanding the process of obtaining data and the ability to support a line of reasoning with data obtained, rather than whether a student arrived at specific conclusions.
INCLUSIVE TEACHING
Our activity design contributes to inclusivity because it creates an environment where students' diverse backgrounds can be leveraged for learning. Specifically, students are encouraged to draw on their own existing cognitive resources (i.e. what they DO know) rather than engaging them from a deficit perspective by correcting or filling gaps in their knowledge (i.e. pointing out what students DON'T know). We further fostered inclusivity by using this activity as an alternative assessment (in contrast to high-stakes multiple choice or short answer exams) and incorporated multiple ways for students to demonstrate their understanding (e.g. written summary, group discussion). Our activity also requires students to use authentic data to answer authentic questions, and as such is a form of authentic assessment. Both alternative and authentic assessments have been identified as a best practice for inclusive teaching (26). Finally, this activity is beneficial to students with certain visual impairments because the images can be enlarged, and contrast can be enhanced.
LESSON PLAN
PRE-LESSON/LAB PREPARATIONS:
The emphasis of this lesson is to understand the tasks that a cell needs to perform during cell division, rather than just memorizing and identifying the names and characteristics of the stages. Before the lesson, the instructor prepares an Excel spreadsheet for each student or group of students containing links to the images of cells that will be used for analysis. We have provided several resources to facilitate this. An example student file is provided (Supporting File S3: A virtual laboratory on cell division-Example student Excel file) with links to 100 images, which can simply be duplicated for each student or group of students. Alternatively, individual links or groups of links can be copied from this file and pasted into separate Excel files. An additional file containing links to 927 3D image sets of cell groups stained for both DNA and microtubules available on the Allen Institute for Cell Science web site is also provided (Supporting File S4: A virtual laboratory on cell division-Complete Excel file). Instructors may wish to assign more or fewer images to each student or group depending on the availability of class time and the desired accuracy of the results. We suggest assigning 20 images which, depending on the number of cells in each image, has taken students between 20 and 40 minutes to analyze. We have included a file containing counts for total cells and dividing cells in 100 randomly selected links (Supporting File S6. A virtual laboratory on cell division-Example tallied results) sorted by the total number of cells in each image. Instructors may use this file to gain experience with counting and tallying the results of this analysis or to assign specific sets of images to students. For example, instructors may choose to assign images with low overall numbers of cells if class time is limited or assign sets of images that provide a known outcome. However, we believe the latter use should be adopted with caution and that the emphasis of this activity should be placed on the production of testable hypotheses and interpretation of obtained results, rather than that students obtain a specific result. Specifically, if small numbers of images are assigned for analysis to individual students, the probability that a small sample set will inaccurately represent the overall population behavior of cells is high. Instructors should also be aware that some students may find this file online through internet searches. Finally, we have created a java application available for download on sourceforge.net or by request from the authors. The application accepts as input a text file containing names of students or groups of students, with one name per line. It then generates (within a designated folder) named Excel files containing a desired number of randomly selected links to images on the Allen Institute for Cell Science web site. Each link accesses one of the 3D image sets labeled for DNA and tubulin, but provisions are made that would allow an instructor to generate links to other data sets. The application was written to facilitate the application of this exercise to large classroom sizes where creation of individual Excel files by copying and pasting would be impractical.
IN CLASS OR AT HOME WORK:
This activity is broken into two parts: (1) generating a written hypothesis and (2) collecting and analyzing data followed by summarizing the results. An alternative approach would be to combine hypothesis development and data collection in one session with analysis and summarization of the data reserved for a second session or assigned as homework.
STAGE ONE:
Students write down the events that occur at each stage of cell division. For example, a student may write that in prophase cells condense their chromosomes, disassemble the interphase microtubule array and begin construction of the mitotic spindle by separating their centrosomes. They then write a hypothesis regarding the length of time that cells will take to complete each stage of cell division and provide support for their hypothesis. For example, a student may hypothesize that telophase takes longer than anaphase and justify this statement by noting that anaphase only involves moving already attached chromosomes from the center of the cell to the spindle poles, while telophase involves assembly of the nuclear envelope, disassembly of the mitotic spindle, and relaxation of mitotic chromosomes. Based on their hypothesis, students predict what they will see in the real images of individual groups of cells in terms of frequencies of cells in each step of the cell cycle. For example, in a collection of photographs of a randomized population of dividing cells, longer stages of the cell cycle should be more common, while shorter stages should appear more rarely in the images. The students then submit their hypothesis and predictions for review. The instructor reviews the hypothesis and prediction; the hypothesis does not have to be correct. Students are asked to revise their statements if there is insufficient rationale supporting their hypothesis or if their predictions are not testable.
STAGE TWO:
Once the instructor approves the hypothesis and prediction, students are provided an overview of the controls for the 3D Cell Viewer they will use to view images of cells on allencell.org (Supporting File S2. A virtual laboratory on cell division-Handout, assignment and instructions) and an Excel spreadsheet (Supporting File S3: A virtual laboratory on cell division-Example student Excel file). In the first column of the spreadsheet there are clickable links to images of randomly selected fields of cells within the 3D Cell Viewer. Clicking on each cell will open the corresponding image in the student's default web browser. Students count the total number of cells in each linked image. Then they count the number of cells in each stage of mitotic cell division within that image and enter these values into the Excel spreadsheet. In our classes, students were instructed to count cells in a minimum number of images (twenty), but students were offered HTML links to 100 different images.
Students may encounter difficulty in identifying prophase cells because nuclear morphology of DNA in non-dividing (interphase) cells is much more heterogeneous than usually depicted in textbook images. Additionally, students may have trouble distinguishing prometaphase cells from metaphase cells because the orientation of cells in a cell monolayer is not always parallel to the image plane. A set of example images for cells at each stage of division has been included (Supporting File S5: A virtual laboratory on cell vision-Example images) which may assist students with recognizing cells in various stages of division. The 3D Cell Viewer controls allow students to move the image plane through the cell in order to get a more accurate picture of the cell's morphology (Supporting File S2. A virtual laboratory on cell division-Handout, assignment and instructions). An option is also available that allows students to rotate the entire volume in three dimensions to better understand the structure of cells. Exposing students to the diversity of morphologies present in real cells in culture is a strength of this lesson.
After students finish counting and recording cells in the Excel spreadsheet, they summarize their data in a graph or chart. Students then evaluate the data in light of their initial hypothesis. Students are required to write down and turn in a report containing a summary of their data and a discussion of how the data support their hypothesis or what the discrepancies are between their hypothesis and their data and what the basis of these differences might be.
TEACHING PROMPTS:
Instructors may wish to provide students verbally or in writing with prompts to assist them in developing a written summary. Examples can include:
- Do your results support your hypothesis?
- If your results did not support your hypothesis, what are possible explanations for this?
- Which stages of cell division were most and least common?
- How does the number of cells you counted for a stage of cell division relate to the duration of that stage?
- Why do you think cells took so long to complete the longest stage of cell division?
- Why do you think cells needed so little time to complete the shortest stage of cell division?
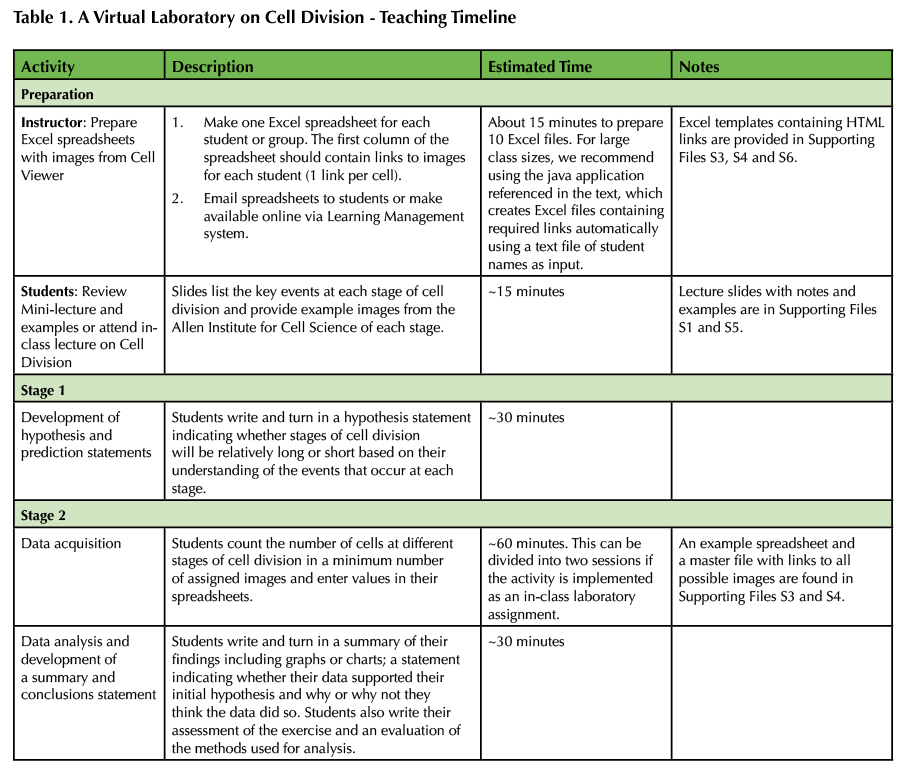
Table 1. A Virtual Laboratory on Cell Division - Teaching Timeline
GRADING RUBRIC AND NOTES:
The lesson was implemented as a ten-point extra credit exercise with points awarded for both stages of the project. In stage 1, student hypotheses were examined to ascertain whether they were mechanistic and predictive. The emphasis was not placed on whether a student's hypothesis was "right", but rather on whether the student could present a logical explanation for their hypothesis and make logical predictions. Three points were awarded for completion of this part of the lesson. Students who turned in work before the deadline were offered the opportunity to revise their hypotheses without penalty if their hypotheses failed to include a mechanistic discussion. Examples of hypothesis statements that were given full credit would include statements such as "I think prophase will be the longest stage of cell division because the cell has to condense its chromosomes, assemble the mitotic spindle and disassemble the interphase microtubule network". Examples of statement that would be given partial credit or returned for revision would include "I think prophase will be the longest stage of cell division" (lacks mechanistic explanation) or "Prophase is the stage of cell division where chromosomes condense" (lacks prediction about the relative length of time a cell needs to accomplish the task).
Evaluation of the second part of the lesson consisted of examining whether the student had correctly identified cells at different stages of cell division, and whether they could provide a logical discussion of why the data supported or failed to support their initial hypothesis. In practice, the instructor examined whether students were able to correctly identify the stages of cell division in a small sample of evaluated images, using the individualized Excel files for reference. Seven of 10 points were awarded for this component of the lesson, with 3 points awarded for data analysis and 4 points awarded for the written summary. Similar to part 1, students that completed the work before the completion deadline were offered an opportunity to revise their discussions as needed. Students that had difficulty identifying specific stages of the cell cycle were also provided with additional examples or training and the opportunity to revise their summaries.
Possible grading rubric:
Hypothesis: (0-3 points)
- Clearly written, logical, focus on mechanisms and dynamics, duration of events of cell division, makes predictions, discussion of methods and potential impact on data, 3 points
- Clearly written, focused on events, makes predictions, states a hypothesis, 2-3 points
- Poorly written, disorganized, does not make predictions, 0-1 points.
Data analysis and presentation: (0-3 points)
- Presentation of results in a well formatted and labeled graph or chart that illustrates major findings, 3 points
- Presentation of results in a graph that illustrates major findings but missing labels, not well formatted, hard to read, 2 points
- Submission of a graph that does not illustrate major findings, graphs the wrong items, incorrect data, but good formatting, carefully and clearly labeled, 1-2 points
- Submission of a graph that does not illustrate major findings and poor formatting, 0 points.
Conclusion: (0-4 points)
- Thoughtful and logical conclusion, accurate summary of data, focus on mechanisms, dynamics, methods, discussion of hypothesis and why data did or did not support it, 4 points.
- Logical conclusion, accurate summary, some discussion of hypothesis and why data did or did not support it, 3 points
- Accurate summary of results, 2 points
- Some discussion of results, 1-2 points
- Conclusion lacks logic, little discussion of results, 0 points
TEACHING DISCUSSION
GENERAL OBSERVATIONS
To date, we have offered this laboratory to students in four classes ranging in size from 21 to 59 students. Sixty percent (77 total) students have voluntarily engaged in the activity, with participation ranging from 45 to 72 percent. Students generally found the activity interesting and beneficial. Students had some difficulty with determining the difference between interphase and prophase cells, in part because the nuclear morphology of interphase cells in the images is considerably more granular and heterogeneous than expected. Determination of the differences between prometaphase and metaphase cells is also potentially difficult, but observation of the mitotic spindle morphology by checking the box to display alpha tubulin may help with this determination. Due to the small number of images analyzed by each student (~20) and the random assignment of images, students occasionally produced results with significant over or under representation of specific stages of mitosis. Students often recognized the potential sources of this artifact or were guided to an understanding of the effects of small samples size during feedback on their written summaries. Given sufficient time, it may be useful for the instructor to have students combine their results to obtain larger sample sizes and more representative results.
EXAMPLE RESPONSES
As expected by most students, interphase cells made up the majority of cells in each image. Metaphase cells were the second most prevalent stage observed, followed by prometaphase and telophase. Prometaphase and anaphase cells were the rarest of observed stages. Examples of graphs submitted by students are shown in Figure 2.
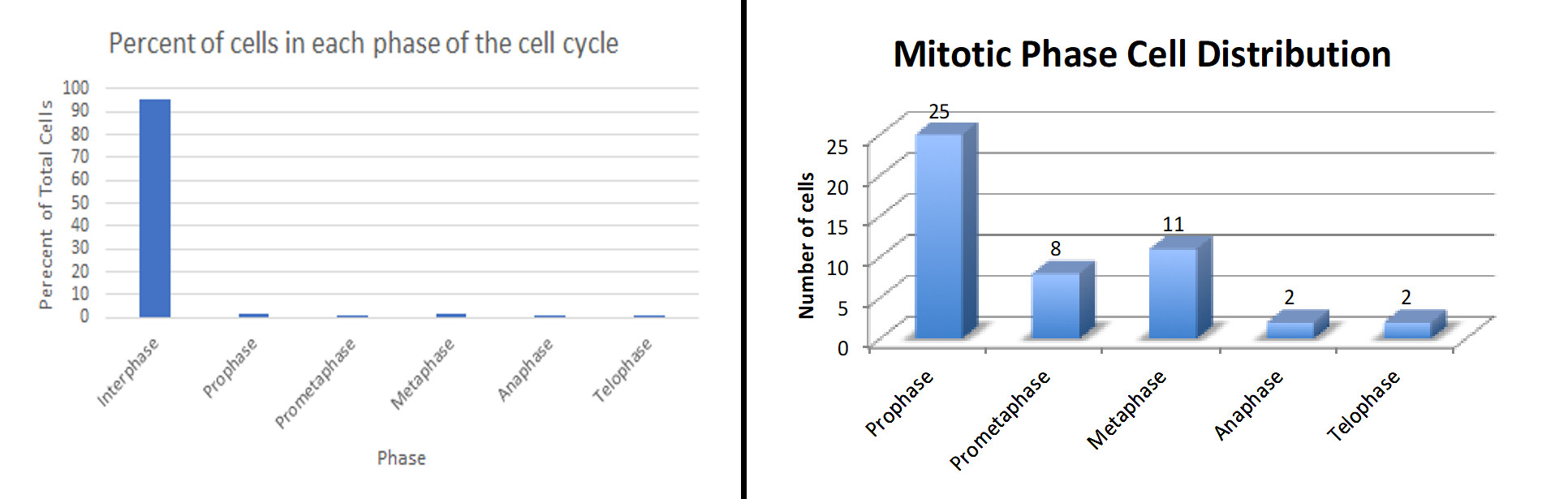
Figure 2. Examples of results submitted by students after analyzing the number of cells in each stage of the cell cycle and cell division.
Most students made positive comments about the lesson. For example, one student wrote "Most importantly, the exercise allowed me to look at the theory presented in this last section from a practical perspective. I have been really struggling with the theory in this class and I think that such practical exercises with other cell processes would have helped me gain a much, much better grasp of the material presented." Students did express some frustration with identifying the different stages in these images. For example, a student wrote "Cells in interphase and prophase looked very similar to me". It is likely that more training on the use of the 3D Cell Viewer and a larger number of example images would be helpful in addressing this problem. Our earliest implementation of this lesson used an image series that lacked alpha tubulin staining, however use of tubulin staining was added in a later version and facilitated identification of mitotic cells, especially those in prophase, which often displayed two separated microtubule organizing centers well before the appearance of a mitotic spindle. Telophase cells were also more easily defined by the presence of a mid-body of microtubules. In addition, students in face to face classes could benefit from the experience of the instructor and group discussion as they identify the stages of cell division. This is less easily implemented if the lesson is given as a take-home assignment and for online students. However, the difficulty in identifying images was also seen as a valuable experience and offered insight into the tasks faced by real biologists. For example, one response was "This assignment was useful in providing me with insight into cell biology methodology and data collection because it helped me to realize how difficult this research can be."
POTENTIAL VARIATIONS
Although this activity was developed as a take home extra credit exercise for in class and distance learning students, it could readily be adapted to an in-class laboratory exercise for individual students and small student groups if suitable computer and internet resources were available. The two stages of the activity could be completed in two classroom sessions by some student groups, or two classroom sessions for introduction of cell division and discussion of results combined with out of class work assigned for cell counting online.
SUPPORTING MATERIALS
- S1. A virtual laboratory on cell division - Mini-lecture, containing diagrams of the stages of cell division, notes, and representative images of real cells at each stage from the Allen Institute for Cell Science web site.
- S2. A virtual laboratory on cell division - Handout, assignment and instructions.
- S3. A virtual laboratory on cell division - Example student Excel file, containing links to images on the Allen Institute for Cell Science web site.
- S4. A virtual laboratory on cell division - Complete HTML link list, containing 927 HTML links to all currently available images of cells labeled for DNA and tubulin on the Allen Institute for Cell Science web site.
- S5. A virtual laboratory on cell division - Example images of cells, containing images and notes for cells at each stage of cell division.
- S6. A virtual laboratory on cell division - Example tallied results
ACKNOWLEDGMENTS
This work was generously supported by the National Science Foundation (IOS 1457368 to EAS). We also thank the students in MBIOS401 at Washington State University for allowing us to use their responses to this exercise, and the Allen Institute for Cell Science founder, Paul G. Allen, for his vision and support.
References
- Riemeier T, Gropengiesser H. 2008. On the Roots of Difficulties in Learning about Cell Division: Process-based analysis of students' conceptual development in teaching experiments. International Journal of Science Education 30:923-939.
- Van Hoewyk D. 2007. Using a case-study article to effectively introduce mitosis. Journal of College Science Teaching 36:12.
- Dikmenli M. 2010. Misconceptions of cell division held by student teachers in biology: A drawing analysis, vol 5.
- ?ztap H, ?zay E, ?ztap F. 2003. Teaching cell division to secondary school students: an investigation of difficulties experienced by Turkish teachers. Journal of Biological Education 38:13-15.
- Metcalf ZP. 1924. A Conventional Scheme for Teaching Cell Division (Mitosis). Science 59:165-166.
- Lawson AE. 1991. Exploring Growth (& Mitosis) through a Learning Cycle. The American Biology Teacher 53:107-110.
- Mickle JE. 1990. A Model for Teaching Mitosis & Meiosis. The American Biology Teacher 52:500-503.
- Clark DC, Mathis PM. 2000. Modeling Mitosis & Meiosis. The American Biology Teacher 62:204-206.
- Coleman D. 1986. A simple model for use in teaching cell division and genetics. Journal of Biological Education 20:158-159.
- Cordero RE, Cynthia AS. 1994. The Developmental Importance of Cell Division. The American Biology Teacher 56:176-179.
- Kenneth C, Lawson AE. 1986. Why Isn't Inquiry Used in More Classrooms? The American Biology Teacher 48:150-158.
- Borgman CL. 2010. Scholarship in the digital age: Information, infrastructure, and the Internet. MIT press.
- ST?PHAN V-L. 2006. What is Changing in Academic Research? Trends and Futures Scenarios. European Journal of Education 41:169-202.
- Gilman SL. 2006. Do Online Labs Work? An Assessment of an Online Lab on Cell Division. American Biology Teacher 68:131-134.
- Prinou L, Halkia K. 2018. IMAGES OF 'CELL DIVISION' ON THE INTERNET.
- Horwitz R, Johnson GT. 2017. Whole cell maps chart a course for 21st-century cell biology. Science 356:806-807.
- Aneja R, Rida PC. 2018. Compositions and methods for prognosis and treatment of neoplasm. Google Patents.
- Baru A, Dogra N, Mukhopadhyay T. 2016. Methyl N-(6-Phenylsulfanyl-1h-Benzimidazol-2-Yl) Carbamate: A Non-Toxic Substitute of Colchicine for Quality Metaphase Chromosome Preparation from Normal and Tumor Cells for Cytogenetic Analysis. Biol Syst Open Access 5:2.
- Alberts B. Molecular biology of the cell, Sixth edition. ed.
- Lodish HF. Molecular cell biology, Eighth edition. ed.
- Pollard TD, Lippincott-Schwartz J, Earnshaw WC, Johnson G. 2017. Cell Biology, 3d ed doi:https://doi.org/10.1016/C2014-0-00272-9. Elsevier Inc.
- Various. 2018. Behavior of hiPS Cells During Mitosis. https://www.allencell.org/hips-cells-during-mitosis.html. Accessed 7/2/2018.
- Various. 2018. 3D Cell Viewer, on Allen Institute for Cell Science. https://www.allencell.org/3d-cell-viewer.html. Accessed 7/2/2018.
- Lewis WH, Lewis MR. 1917. The duration of the various phases of mitosis in the mesen chyme cells of tissue cultures. The Anatomical Record 13:359-367.
- Wright GP. 1925. XXVI.--The Relative Duration of the Various Phases of Mitosis in Chick Fibroblasts Cultivated in Vitro. Journal of the Royal Microscopical Society 45:414-417.
- Villa RA, Thousand JS, Nevin A, Liston A. 2005. Successful inclusive practices in middle and secondary schools. American Secondary Education:33-50.
Article Files
Login to access supporting documents
A virtual laboratory on cell division using a publicly-available image database(PDF | 357 KB)
S1. A virtual laboratory on cell division-Mini-lecture.pptx(PPTX | 3 MB)
S2. A virtual laboratory on cell division-Handout assignment and instructions.docx(DOCX | 4 MB)
S3. A virtual laboratory on cell division-Example student Excel file.xlsx(XLSX | 12 KB)
S4. A virtual laboratory on cell division-Complete HTML link list.xlsx(XLSX | 28 KB)
S5. A virtual laboratory on cell vision-Example images of cells.pptx(PPTX | 3 MB)
S6. A virtual laboratory on cell division-Example tallied results.xlsx(XLSX | 21 KB)
- License terms

Comments
Comments
0 Like
Kari Howey @ on
Thanks so much! I really, really, really appreciate your work! Cheers!
Copy link Report abuse
0 Like
Esther Dalfo @ on
Thank you very much for shareing this awesome tool!
Copy link Report abuse