Teaching the Biological Relevance of Chemical Kinetics Using Cold-Blooded Animal Biology
Published online:
Abstract
This lesson plan was created to support non-chemistry major students in making connections between chemical kinetics and biological systems. Chemical kinetics is often viewed by students as an abstract topic, and one that is limited only to chemical reactions that occur in a laboratory. The subtopics of chemical kinetics can be challenging for students to apply in a different context, and students often learn the topic as remote tidbits of knowledge without a clear indication of its application. The lesson involves an activity utilizing real-life case studies with biological applications of chemical kinetics to enhance student understanding and improve interest and engagement in the topic. The case studies detail the immobilization of cold-blooded animals at very low temperatures and enlightens students about the unfortunate situation that iguanas and sea turtles faced as consequences of a particularly cold spell in in Florida during winter 2018. This activity allows students to view chemical kinetics in a new light and to brainstorm on methods to solve such problems faced in real-life.
Citation
Ramachandran, R. 2019. Teaching the biological relevance of chemical kinetics using cold-blooded animal biology. CourseSource. https://doi.org/10.24918/cs.2019.24Lesson Learning Goals
- Students will understand the effect of temperature on chemical kinetics.
- Students will appreciate the importance of chemical kinetics in living creatures.
Lesson Learning Objectives
Students will be able to:- Predict the effect of reaction temperature on the rate of a chemical reaction
- Interpret a graph plotted between rate of a chemical reaction and temperature
- Discuss chemical kinetics utilizing case studies of cold-blooded animals
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
This lesson plan was created to direct non-chemistry major students to make connections between chemical kinetics and biological systems. The topic of chemical kinetics primarily deals with the rates of chemical reactions and processes. Knowledge of chemical kinetics can assist in determining the various factors that influence the rate of a chemical reaction such as reactant concentration, temperature, catalyst or surface area, as well as provide insight into the reaction mechanism, which is especially important for multi-step reactions (1). Chemical kinetics is often viewed by students as an abstract topic, one that is limited only to chemical reactions that occur in a laboratory. The subtopics of chemical kinetics can be challenging for students to apply in a different context, and students often learn the topic as isolated tidbits of knowledge without a clear indication of its application (2-6). Contrary to student belief, chemical kinetics is relevant in several biological applications, ranging from enzyme binding, drug metabolism to medicinal therapy (7-8). Prior studies have shown that these underlying issues in understanding chemistry stem from students' inability to relate the subject to daily life occurrences, and thus their inability to make appropriate associations (9-14). Several strategies have been employed to address this deficit, such as creating integrated chemistry-biology courses (15-16), performing in-class demonstrations and activities (17-18) or including relevant biological examples in lecture (19). Keeping these in mind, I sought to develop an in-class activity that encouraged students to think about chemical kinetics in a biological context and use the principles of chemical kinetics to address problems that are faced in real life. My goal was to point out the bigger-picture examples involving chemical kinetics and elucidate to students how chemistry is present in other aspects of their life.
While teaching this topic previously in my general chemistry courses, I utilized in-class experimental demonstrations involving dye and bleach to illustrate the everyday applications of chemical kinetics (20). However, considering that a large number of my students were interested in health professions and biologically-related careers, I switched to the interesting example of geckos immobilized by ice water to explain the biological impact of chemical kinetics (1). My students enjoyed this application more than my chemical demonstrations, and thus began my search for biological examples involving chemical kinetics. In winter 2018, I was both shocked and fascinated to read about the environmental and biological repercussions aspects of a particularly cold winter spell on Florida (https://www.cbsnews.com/news/florida-frozen-iguanas-falling-from-trees-during-cold-snap-bomb-cyclone-storm-east-coast/). I wanted the information to be provided to my students in a way that promoted critical thinking, as opposed to be a purely entertaining anecdote. Using active-learning strategies in STEM courses create a larger impact on students by increasing student engagement, improving self-reflection and compelling students to think outside the context of their textbooks (21-23). Instead of informing my students about the biological relevance of chemical kinetics, how could I prompt my students to draw these conclusions? How could I initiate a discussion that would encourage my students to delve into their existing knowledge from previous courses, and apply it to what they were currently learning in my course? These questions formed the basis of my lesson plan.
INTENDED AUDIENCE
This lesson has been used for the past three academic quarters in an introductory general chemistry course for life science majors. Students are first-year or second-year college students pursuing a major in life sciences, with the intention of applying for medical school or becoming a health professional. The class size is approximately 200 students; however, this lesson plan is appropriate for small class sizes as well.
REQUIRED LEARNING TIME
This lesson plan has been designed for a 50 minutes class duration and can be inserted in a longer class period with an extended post-activity lecture part. The activity portion takes 25-30 minutes including a clicker question and small groups discussion.
PREREQUISITE STUDENT KNOWLEDGE
Students should have prior knowledge of the concepts of reaction rates, rate constants, order of reactions, activation energy, and catalysts. Students have also been introduced to the various factors affecting rates of chemical reactions. These topics have been covered by a combination of instructor lectures and pre-assigned textbook readings in the weeks prior to this activity. This activity was implemented in the middle of the chapter on chemical kinetics. Students should also be familiar with the basic concept of cold-blooded animals and their body temperature regulation, which is material that is covered in high school or introductory biology classes. The topics of Chemical Kinetics and cold-blooded animals and temperature regulation are available at the OpenSource resources listed below.
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_A_Molecular_Approach_(Tro)/14%3A_Chemical_Kinetics
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/14%3A_Chemical_Kinetics
- https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology_(Boundless)/29%3A_Vertebrates/29.4%3A_Reptiles/29.4B%3A_Characteristics_of_Reptiles
- https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_General_Biology_(Boundless)/33%3A_The_Animal_Body%3A_Basic_Form_and_Function/33.3%3A_Homeostasis/33.3C%3A_Homeostasis_-_Thermoregulation
PREREQUISITE TEACHER KNOWLEDGE
The instructor should be familiar with the basic principles of chemical kinetics, the effect of temperature on the rate of a chemical reaction, and the body temperature regulation of cold-blooded animals (poikilotherms). Instructors can receive more information about poikilotherms at the websites listed below.
SCIENTIFIC TEACHING THEMES
ACTIVE LEARNING
During the beginning of class, a clicker question is used before the activity to gauge student learning. The activity involves a small groups discussion/think-pair-share (depending on class size), and students are provided with worksheets to guide them in the discussion. After the think-pair-share activity, certain groups share their insights with the rest of the class. Students also brainstorm solutions for certain statements provided by the instructor that tie together the conclusions of the activity. These types of collaborative peer-learning activities can foster team problem solving skills, promote critical thinking and improve student attitudes towards the course material (24-26).
ASSESSMENT
In class, student learning is assessed using a clicker question before the activity. During the activity, students use worksheets to write down their insights; the instructor provides questions on the worksheets for students to answer. The activity is followed by a large group discussion where students are asked to reflect on their findings by sharing their answers with the group. The instructor evaluates student learning over the course of the activity by monitoring the small groups discussion and hearing shared student insights during the combined classroom discussion. Students have opportunities to self-evaluate their learning during the clicker question, small groups discussion, and in the large classroom discussion post-activity. At the end of the activity, the students are asked to find a solution to an existing problem which prompts them to critically analyze their group's conclusions.
INCLUSIVE TEACHING
The activity includes text and pictorial representations, thus catering to different student preferences. Using a think-pair-share/small groups discussion format encourages all participants to contribute their thoughts without fear of speaking out in a large class. The worksheets with pointed questions aids students in organizing their thoughts before exchanging views with their partner/group. This strategy helps to build confidence for students who require more time to process a concept. The method of voting silently on statements made by the instructor allows students to reflect on their learning without scrutiny and creates a safe space for learning. Furthermore, the post-activity brainstorm promotes an inclusive environment where all student opinions are equally valuable.
LESSON PLAN
PRE-ACTIVITY
I begin my class with a brief recap of how changing the reactant concentration affects the reaction rate (Supporting File S1: Temperature & Reaction Kinetics: Lecture Presentation Slides, slide 4), and the influence of reactant concentration on the frequency of molecular collisions (5 minutes), which is a topic that was covered in the previous lecture. I then show all students a graph of Reaction Rate vs Temperature (Figure 1) for a simple chemical reaction and ask them to write down their interpretation and conclusions on whether temperature affects the rate of a chemical reaction. The students write these conclusions down on a piece of paper/in their notebook and is not shared with the class. Allowing time for independent thought serves as an engagement tool and self-evaluation method for the students (27).
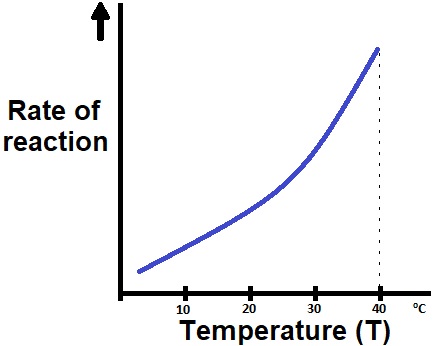
Figure 1: Graph of Reaction Rate vs Temperature is displayed to the students, and they are asked to write down their interpretation without consulting anyone else.
After 3-4 minutes, I ask my students to silently vote on the following question using their clickers:
Increasing the temperature of a reaction increases its reaction rate
A) Yes
B) No
C) Cannot say
IN-CLASS ACTIVITY
Once the students vote on the clicker question, I ask them to pair up with the student next to them or form groups of three students. I allow the students 2-3 minutes to finalize their groups, and I recommend that the instructor/teaching assistant can guide students with this if required for a large class to save time.
Next, I provide each student group with a copy of the case study activity worksheet (Supporting File S2: Temperature & Cold-blooded Animals: Case Study Activity Worksheet). Depending on the size of the class, an instructor can either distribute copies of only the first case study example or evenly divide both case studies among the various groups present. It is important to note that each group is only provided one case study example, keeping in mind the duration of the activity and the similar nature of both the case studies. The instructor has to be prepared beforehand to ensure that all students are provided with a copy of the case study. For example, a group consisting of three students is provided three copies of the same case study. Since I have a large class, I used both case study examples to avoid cross talk between groups during the activity. I tried my best to ensure that no two groups that were seated next to each other were provided with the same case study activity worksheet.
Once I hand out the worksheet, I instruct the students to spend a few minutes reading the case study on their own. The introductory paragraph details the case scenario and it is important for students to reflect on it by themselves before feeling confident enough to share their views with the other members of their group. I announce to the class that students should read the questions given under the paragraph, and jot down their ideas before sharing them with the group. In the worksheet, space is provided below each question to assist students with critical analysis and argumentation. I allow the students to take 5 minutes to reflect on the scenario by themselves. This time is important for slow readers to process the material and also for shy students to collect their thoughts.
I then instruct the students to share their views with their groups, specifically addressing the questions provided. I walk around the class observing the discussion and ask some pointed questions to groups that are stuck, or groups that are not as active. Typically, I provide another 5 minutes for groups to discuss their insights on the questions, however, more time can be provided if needed. After the students exchange their conclusions, I ask a few students from different groups to share their group's insights with the rest of the class. I prompt the students to elaborate on their conclusions by using general questions such as "Can you elaborate on that a bit?" or "Does any group have a different viewpoint?" and especially by utilizing pointed content questions like "Why does the animal get immobilized/stunned?," "Why does the rate of the reaction affect its movement?," "What kind of reactions in the body get slowed down?," and "Where do you think these reactions are specifically taking place in the body?." Detailed answers to all the questions are provided in the supplementary materials (Supporting File S3: Temperature & Cold-blooded Animals: Worksheet Answer Key).
POST-ACTIVITY
After the small groups discussion and the combined classroom sharing, I use the student insights to segue into my lecture slides summarizing the concept behind the case studies (Supporting File S1: Temperature & Reaction Kinetics: Lecture Presentation Slides, slides 7-8). I utilize visuals such as thermal images of reptiles to elucidate the dependence of body temperature on the surroundings, and I explain that the chemical reactions within the muscles of the animals get slowed down greatly. I elaborate that the reason for reptiles basking in the sun is to increase their metabolism and easily escape from predators, and that reptile exhibits at zoos are designed to mimic this and maintain reptile body temperatures within a narrow window.
I then pose another question to my students: "Identify one potential method to save these animals." I provide the students with 3-4 minutes to reflect on this question individually or with their groups (as per their choice). At this point in the class, students are quick to make the connections between rate and temperature and identify that warming the reptiles' bodies back to room temperature is sufficient to bring these animals back to mobility.
Finally, I bring the concept back to a chemical context by clarifying the dependence of temperature on rate and rate constant, tying the phenomena to activation energy and the Arrhenius equation (k = Ae-Ea/RT) which were learned in a prior lecture. This is an effective way to proceed into the topics of effective collision and the Boltzmann distribution of molecular energies, and further use those topics to introduce enzymes and reveal how enzymes are affected differently by temperature compared to simple chemical reactions (Supporting File S1: Temperature & Reaction Kinetics: Lecture Presentation Slides, slides 10-14).
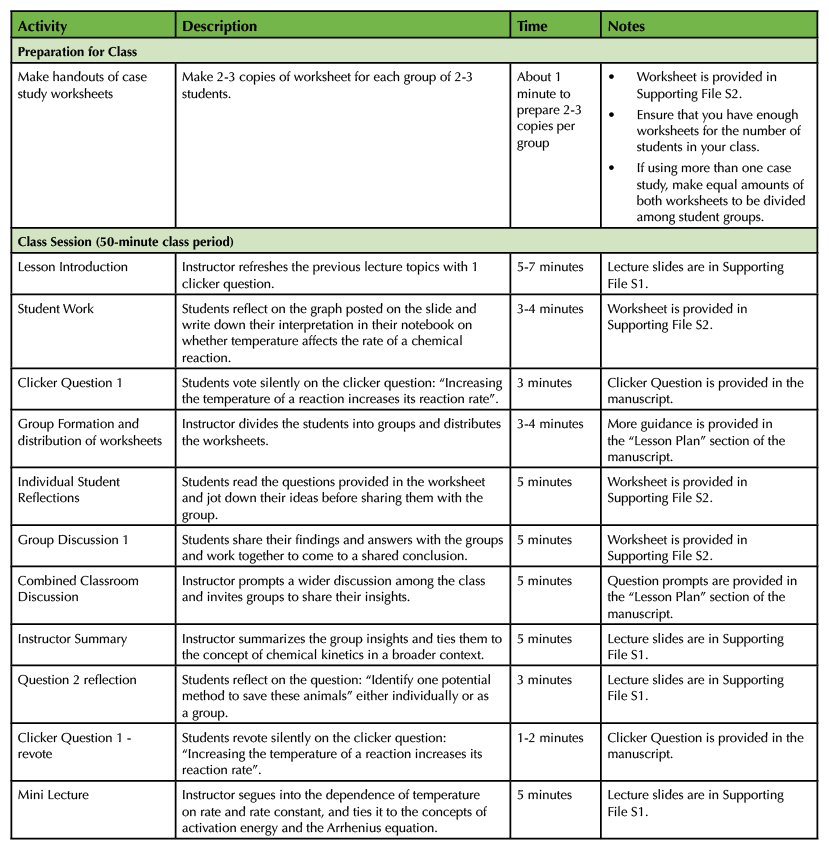
Table 1. Chemical Kinetics and Cold-Blooded Animals - Teaching Timeline
TEACHING DISCUSSION
ACTIVITY PROGRESS
After the first clicker question is posed to the students, even though a majority of the students (~70%) answered the question correctly, it was revealed during the activity that there are several students who have trouble probing the concept on a deeper level. While transitioning into the activity portion of the lesson, students are provided time to answer the questions individually before sharing their ideas with their group. While I walked around the class to monitor student progress, I was surprised at how many students were unsure about extending connecting the conclusions they made from the graph in the clicker question to the case study given in the worksheet. While the clicker question talked about the increase of reaction rate with increasing temperature, the case study implied conditions at lower temperatures. The connection between these two questions seems straightforward from an instructor's perspective, but students were initially hesitant to extend the known principles of chemical kinetics at higher temperatures to scenarios at lower temperatures. Students readily answered parts a) and b) on the worksheet (Supporting File S2: Temperature & Cold-blooded Animals: Case Study Activity Worksheet) but spent more time poring over part c). I spoke to students after the activity to understand their thinking process during the activity, and especially on their initial reactions to the worksheet questions. Several students stated that interpreting the graph coupled with clicker question before the activity was useful in directing them towards applying the concepts to the case study provided, and particularly to address part c) on the worksheet. The use of clickers over notecards or a show of hands promotes a more positive learning environment in large classrooms, particularly in addressing questions that are prone to student misconceptions or uncertainty (28-29).
Drawing connections between chemical reactions and immobilizations took time; students did grasp that the immobilization was due to the decreased rate of chemical reactions in the body, but it was only during the small groups discussion that the students had to think more deeply about what kinds of chemical reactions were being slowed down in the body of the animal. Instructor questions (see above section) are essential to guide the large classroom discussion and facilitate scientific argumentation. The final aspect of the activity where students are asked to recommend a method to save the immobilized reptiles is an effective way to tie together the rate vs temperature graph and the case studies worksheet. I noticed that students feel very confident at this time and are not shy to provide recommendations. Almost all the recommendations I receive from the students are based on altering the body temperature of the reptiles using various means, and students now understand that the principles of chemical kinetics do not suddenly change for different temperatures or different reaction systems. The active-learning-based model that this lesson plan incorporates encourages independent thinking and self-directed learning, while also imparting critical reasoning skills to the students.
IMPLICATIONS
Students were tested on the various subtopics of chemical kinetics through multiple-choice questions over the course of the quarter. Looking at the performance of the students on a five-question in-class graded quiz conducted one week after the activity, I observed that 93.8% of students correctly answered the question pertaining to the effect of temperature on reaction rate (given below). Furthermore, this question had the highest score when compared to the other questions which were also based on chemical kinetics but not covered through the activity. The question focused on the conceptual understanding that a small increase in temperature through regular body activity such as exercise increases the reaction rate; this was demonstrated by the students.
Question: Carbonic anhydrase is an important enzyme that lets CO2 and H2O to be converted into H2CO3 through the reaction: CO2 (g) + H2O (l) --> H2CO3 (l). This allows CO2 to get dissolved into the blood, and the product H2CO3 additionally helps in regulating the pH of blood. If the temperature of this reaction in the body were to be increased, such as through exercise, how would the rate of reaction change?
(A)Decrease
(B)Increase
(C)No change
Similarly, in the midterm exam conducted approximately two weeks after the activity, 82.3% of students correctly answered the question (option 'e') pertaining to the effect of temperature on reaction rate (given below). It is interesting to note that the remaining 17.7% of students picked option 'c' which is a partially correct answer; this may suggest that all students have the conceptual understanding that temperature increases the percentage of high-energy collisions in a reaction.
Question: Most reactions proceed faster at high temperatures than at low temperatures. Which of the following statements support this observation?
I. An increase in the activation energy with increasing temperature
II. An increase in the rate constant with increasing temperatures.
III. An increase in the percentage of high-energy collisions with increasing temperature
Answer:
(a) Only I
(b) Only II
(c) Only III
(d) Only I and II
(e) Only II and III
SUGGESTED MODIFICATIONS
In the future, I would like to modify this activity to include a pre-class activity such as online homework or watching an assigned video to replace the pre-activity lecture component of the lesson. This strategy can provide more time for students to do a small groups discussion for the last portion of the activity i.e. having students brainstorm solutions to address the immobilization of the reptiles. Oftentimes, the small groups discussion and combined classroom discussion can tend to go on longer than expected in larger classrooms as students are very animated and enthusiastic to share their thoughts. To combat that, I ask my students to spend a few minutes individually brainstorming solutions for the last portion instead of doing a small groups discussion or think-pair-format.
SUPPORTING MATERIALS
- S1. Temperature & Cold-blooded Animals - Lecture Presentation Slides
- S2. Temperature & Cold-blooded Animals - Case Study Activity Worksheet
- S3. Temperature & Cold-blooded Animals - Worksheet Answer Key
ACKNOWLEDGMENTS
I would like to thank my students in CHEM 14B for their enthusiasm and participation. I am grateful to the Center for Education Innovation and Learning in the Sciences (UCLA CEILS) for their workshop on scientific teaching.
References
- Tro NJ, Fridgen T, Shaw L. 2010. Chemistry: A Molecular Approach, 2(nd) US edition. Pearson.
- Nakhleh MB. 1992. Why some students don't learn chemistry: Chemical misconceptions. J. Chem. Ed. 69:191-196.
- BouJaoude S. 1993. Students' Systematic Errors When Solving Kinetic and Chemical Equilibrium Problems. Presented at the Annual Meeting of the National Association for Research in Science Teaching, Atlanta, USA. April 16-19.
- Cakmakci G. 2010. Identifying alternative conceptions of chemical kinetics among secondary school and undergraduate students in Turkey. J. Chem. Ed. 87:449-455.
- Treagust D, Duit R, Nieswandt M. 2000. Sources of students' difficulties in learning chemistry. Educacio'N Qui'Mica. 11:228-235.
- Wu HK. 2003. Linking the microscopic view of chemistry to real life experiences: intertextuality in a high school science classroom. Sci. Educ. 87:868-891.
- Le Novere N, Endler L. 2013. Using chemical kinetics to model biochemical pathways. In In Silico Systems Biology (pp. 147-167). Humana Press, Totowa, NJ.
- Balaban AT, Seitz W. 2003. Relevance of chemical kinetics for medicine: the case of nitric oxide. J. Chem. Educ. 80:662-664.
- Songer NB, Linn MC. 1991. How the students' view of science influence knowledge integration? J. Res. Sci. Teach. 28:761-784.
- Nieswandt M. 2001. Problems and possibilities for learning in an introductory chemistry course from a conceptual change perspective. Sci. Educ. 85:158-179.
- Ben-Zvi N, Gai R. 1994. Macro and micro chemical comprehension of real world phenomena. J. Chem. Educ. 71:730-732.
- Ng W, Nguyen VT. 2006. Investigating the integration of everyday phenomena and practical work in physics teaching in vietnamese high schools. Int. Educ. J. 7:36-50.
- Ceyhun I. 2011. Evaluation of chemistry teaching in terms of teachers and students. Energy Educ. Sci. Technol. Part B. 3:469-486.
- Secken N, Yilmaz A, Morgil IF. 1998. Investigating of students' relating the environment and the life about the chemical events. HU J. Educ. 8:14:37-44.
- Abdella BR, Walczak MM, Kandl KA, Schwinefus JJ. 2011. Integrated chemistry and biology for first-year college students. J. Chem. Educ. 88:1257-1263.
- Schwartz AT, Serie J. 2001. General chemistry and cell biology: An experiment in curricular symbiosis. J. Chem. Educ. 78:1490-1494.
- Pederson DM. 1974. Lecture demonstrations in kinetics relevant to the biology student. J. Chem. Educ. 51:268.
- Steinhart C. 2001. Biology of the blues: The snails behind the ancient dyes. J. Chem. Educ. 78:1444.
- Sousa AM, Waldman WR. 2013. Antimicrobial properties of spices: an activity for high school or introductory chemistry or biology. J. Chem. Educ. 91:103-106.
- Henary MM, Russell AA. 2007. An inexpensive kinetic study: The reaction of FD&C red #3 (erythrosin B) with hypochlorite. J. Chem. Ed. 84:480-482.
- Freeman S, Eddy SL, McDonough M, Smith MK, Okoroafor N, Jordt H, Wenderoth MP. 2014. Active learning increases student performance in science, engineering, and mathematics. P.N.A.S. 111:8410-8415.
- Choi Y, Jakob S, Anderson WJ. 2017. Active Learning: Developing Self-Directed Learners Through Strong Intellectual Engagement. CourseSource. https://doi.org/10.24918/cs.2017.20
- Ramachandran R, Sparck EM, Levis-Fitzgerald M. 2019. Investigating the Effectiveness of Using Application-Based Science Education Videos in a General Chemistry Lecture Course. J. Chem. Educ. 96: 479-485.
- Shibley IA Jr, Zimmaro DM. 2002. The Influence of Collaborative Learning on Student Attitudes and Performance in an Introductory Chemistry Laboratory. J. Chem. Educ. 76:745-748.
- Cooper JL, Robinson P. 2000. Getting started: informal small-group strategies in large classes. NDTL 81: 17-24.
- Bamiro AO. 2015. Effects of guided discovery and think-pair-share strategies on secondary school students' achievement in chemistry. SAGE Open 5:1-7.
- Rosenberg MJ, Abel E, Garver WS, Osgood MP. 2016. Taking the Hassle out of Hasselbalch. CourseSource. https://doi.org/10.24918/cs.2016.17
- Vital F. 2011. Creating a positive learning environment with the use of clickers in a high school chemistry classroom. J. Chem. Educ. 89:470-473.
- King DB. 2011. Using clickers to identify the muddiest points in large chemistry classes. J. Chem. Educ. 88:1485-1488.
Article Files
Login to access supporting documents
Teaching the Biological Relevance of Chemical Kinetics Using Cold-Blooded Animal Biology(PDF | 177 KB)
S1.Temperature and Cold-blooded Animals - Lecture Presentation Slides.pdf(PDF | 561 KB)
S2. Temperature and Cold-blooded Animals - Case Study Activity Worksheet.pdf(PDF | 87 KB)
S3. Temperature and Cold-blooded Animals - Worksheet Answer Key.pdf(PDF | 86 KB)
- License terms

Comments
Comments
There are no comments on this resource.