The Science Behind the ACTN3 Polymorphism
Published online:
Abstract
A common polymorphism in the alpha-actinin-3 (ACTN3) gene results in the lack of ACTN3 protein expression in fast twitch muscle fibers in ~16% of the human population (1). This genetic change has been linked with muscle performance in humans (2) but does not cause any known muscle disease (1). We have developed a series of laboratory modules that provide an authentic classroom research experience and which address the connection between science and society by examining the implications of ACTN3 genetic testing to improve sports training and performance. This article accompanies the lesson "The ACTN3 Polymorphism: Applications in Genetics and Physiology Teaching Laboratories," and summarizes background information that an instructor would need to implement the project in class.
Citation
Somers DJ, Frey TA, Lehman HL. 2019. The science behind the ACTN3 polymorphism. CourseSource. https://doi.org/10.24918/cs.2019.31Lesson Learning Goals
This article accompanies the lesson "The ACTN3 Polymorphism: Applications in Genetics and Physiology Teaching Laboratories." Learning goals for the lesson include:- Understand how a polymorphism in the ACTN3 gene affects protein structure and function (Core Concept in Genetics: How do different types of mutations affect genes and the corresponding mRNAs and proteins? (1); Vision and Change Core Concept: Information Flow, Exchange, and Storage (2)).
- Understand that skeletal muscle function is a complex phenotype that is the result of multiple genes and environmental factors (Core Concept in Genetics: How can one deduce information about genes, alleles, and gene functions from analysis of genetic crosses and patterns of inheritance? (1)).
- Understand how the ACTN3 protein impacts skeletal muscle function (Core Principle of Physiology: Structure-function relationships (3); Vision and Change Core Concept: Structure and Function (2)).
- Apply the scientific method to evaluate the claim that ACTN3 genetic variants affect skeletal muscle function (Vision and ChangeCore Competency: Ability to apply the process of science (2)).
- Apply quantitative reasoning skills to analyze the relationship between ACTN3 genotype and skeletal muscle phenotypes (Vision and ChangeCore Competency: Ability to use quantitative reasoning (2)).
- Enhance independent thinking skills and promote feelings of ownership and engagement in the scientific process (Vision and Change Core Competency: Ability to apply the process of science (2)).
Lesson Learning Objectives
This article accompanies the lesson "The ACTN3 Polymorphism: Applications in Genetics and Physiology Teaching Laboratories." Learning objectives for the lesson include:- Test hypotheses related to the role of ACTN3 in skeletal muscle function.
- Explain how polymorphic variants of the ACTN3 gene affect protein structure and function.
- List and explain the differences between fast twitch and slow twitch muscle fibers.
- List and explain possible roles of the ACTN3 protein in skeletal muscle function.
- Find and analyze relevant scientific publications about the relationship between ACTN3 genotype and muscle function.
- Formulate hypotheses related to the relationship between ACTN3 genotype and skeletal muscle function.
- Design experiments to test hypotheses about the role of ACTN3 in skeletal muscle function.
- Statistically analyze experimental results using relevant software.
- Present experimental results in writing.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
In humans, athletic performance is a complex trait influenced both by environmental factors, such as diet and training, as well as heritable genetic factors. However, the relative contribution of environment and genetics, as well as the interaction between the two, is not entirely understood. Many genes have been associated with human athletic performance in various studies; this review focuses on the human alpha-actinin-3 (ACTN3) gene, which encodes the protein alpha-actinin-3 (ACTN3). The ACTN3 protein is expressed in fast-twitch muscle fibers, which are primarily responsible for rapid and forceful contractions during sprint and power activities (1,3). A common genetic variant in the gene, in which the ancestral C nucleotide is replaced by T, results in a change from arginine (R) to a stop codon (X) at amino acid position 577 (R577X). The R577X allele prevents the production of functional ACTN3 protein (1,4,5).
GENETICS OF ACTN3
Alleles and dominance
Both variants of ACTN3 are common in the general population, with approximately 60% of the global population carrying the C allele (which encodes functional ACTN3) and 40% carrying the T allele (which encodes non-functional ACTN3) (Table 1). Every human inherits two copies of the ACTN3 gene, one maternal and one paternal. Therefore, there are three possible combinations, or genotypes, at this locus: the C/C or RR genotype, in which both copies of the ACTN3 protein are functional; the C/T or RX genotype, in which only one copy of the ACTN3 protein is functional; and the T/T or XX genotype, in which both copies of the ACTN3 are non-functional. Throughout this review article, we follow the convention in the scientific literature of designating the functional ACTN3 genotype as RR or RX, and the non-functional ACTN3 genotype as XX. It should be noted that XX refers to two premature stop codons in the ACTN3 protein, not genetic female sex chromosomes.
The dominance relationship among the R allele and the X allele is not fully understood, as the gene affects many different phenotypes related to athletic function. However, several human association studies of muscle performance support an additive, or incompletely dominant, model in which heterozygous individuals exhibit intermediate phenotypes for the traits studied (2,6). Similar trends have also been observed in studies of mouse models: heterozygous RX mice showed intermediate muscle mass and endurance capacity relative to RR and XX mice (7).
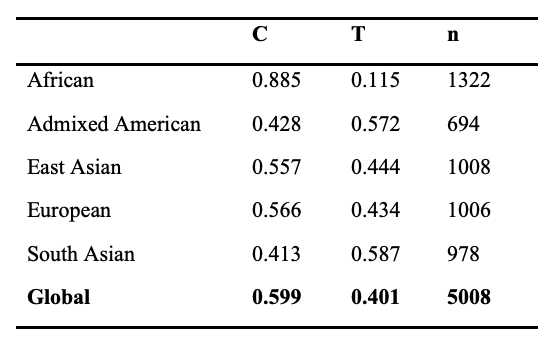
Table 1. Allele Frequency by Population, 1000 Genomes Project
Population genetics
Among different regional populations, the frequency distribution of alleles and genotypes is variable at the ACTN3 locus. For example, over 85% of individuals in African populations harbor the ancestral C allele, whereas nearly 60% of South Asian individuals carry the non-functional T allele (Table 1). Remarkably, approximately ~16% of the global population carries two non-functional alleles of ACTN3 (XX genotype), which results in a complete deficiency of ACTN3 protein in skeletal muscle (8). However, this frequency is as high as 28% in individuals of South Asian descent (Table 2). The majority of individuals in African populations (~75%) have two copies of the functional R allele (RR genotype) whereas most other populations have 15-30% of individuals with this genotype (Table 2). More information on the ACTN3 polymorphism can be found in the National Center for Biotechnology Information Single Nucleotide Polymorphism Database (NCBI dbSNP, https://www.ncbi.nlm.nih.gov/snp/) under SNP identification number rs1815739.
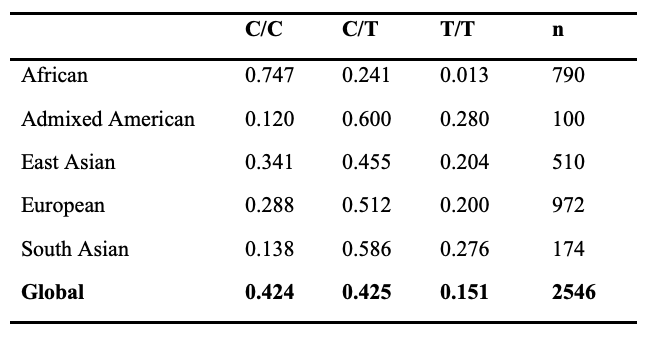
Table 2. Genotype Frequency by Population, HapMap Project
The fact that nearly ~16% of the world's population (over one billion humans) lacks a functional skeletal muscle ACTN3 protein but does not experience any obvious muscle disease suggests that other proteins can compensate for the loss of ACTN3. This functional redundancy is most likely accomplished by the closely related protein α-actinin-2 (ACTN2) (9).
ACTN3 IMPLICATIONS
FOR A ROLE IN ATHLETIC PERFORMANCE
Because of its role in skeletal muscle function and its frequency variation among different populations, many studies have indicated that ACTN3 may contribute to variation in human muscle performance. In particular, some researchers have hypothesized that ACTN3 deficiency primarily affects the function of fast twitch muscle fibers, and thus would have a greater influence in athletes competing in sprint or power activities, rather than endurance athletes whose performance relies more heavily on slow twitch muscle fibers (2,4,5).
To investigate this hypothesis, the association between ACTN3 genotype and athletic performance has been examined in many different populations. In one study, the frequencies of R577X alleles in elite athletes were compared to those of a group of non-athlete (sedentary) controls. Yang et al. identified a significant association between ACTN3 genotype and athletic performance: elite sprint athletes had significantly higher frequencies of the R allele (functional ACTN3) than a control population (5). Most other studies of elite athletes versus controls support the correlation between RR genotype and elite sprint or power athlete status (2). Although there has been some suggestion that the XX genotype is associated with endurance performance, data on this aspect are less robust (10-16).
Several other studies have examined the relationship between ACTN3 genotype and a variety of muscle functions in non-athlete populations (8,17,18), which are described in more detail below. Interestingly, across many of these studies the strength of association between ACTN3 genotype and muscle performance is different between genetic females and genetic males, further emphasizing the complex nature of this trait.
MUSCLE PHYSIOLOGY
WHAT YOU NEED TO KNOW TO UNDERSTAND THE ROLE OF ACTN3
Cellular organization
Muscle fibers consist of single muscle cells or myocytes. Myocytes are multinucleated, surrounded by the sarcolemma, and fuse with a tendon at each end of the fiber. Myofibrils make up the muscle fibers and are contractile elements surrounded by the sarcoplasm. Cellular organelles such as mitochondria and the sarcoplasmic reticulum lie between myofibrils (Figures 10.3 and 10.4 at https://cnx.org/contents/FPtK1zmh@15.1:bfiqsxdB@6/10-2-Skeletal-Muscle).
Molecular organization
The myofibril contractile elements are composed of myofilaments, which are primarily made up of the proteins actin and myosin. The sarcomere is the functional unit of the myofibril and is composed of thick and thin filaments (myosin and actin, respectively) and the filamentous structural protein titin (connectin). The sarcomeres are what give skeletal muscle its striated appearance due to the banding pattern visible by light microscopy. The sarcomere is made up of a complete A-band (myosin thick filaments) and the two halves of the I-bands (actin thin filaments) adjacent to it. The Z line (Z disc) demarcates the borders of the sarcomere and is in the middle of an I-band (Figures 10.4 and 10.5 at https://cnx.org/contents/FPtK1zmh@15.1:bfiqsxdB@6/10-2-Skeletal-Muscle).
Structure and function
Skeletal muscle contraction results from the sliding action of interdigitating actin and myosin filaments. During contraction every sarcomere shortens, as do the myofibrils and the entire muscle cell. The myofilaments do not shorten; rather they slide by each other, overlapping as the I-bands and the H-zone (within the A-band) shorten, causing the Z lines to come closer together. The ACTN3 protein is a structural component of the Z line, therefore connecting with actin filaments and playing a role in coordinating myofilament contraction (Figure 10.5 at https://cnx.org/contents/FPtK1zmh@15.1:bfiqsxdB@6/10-2-Skeletal-Muscle).
Muscle fiber types and ACTN3 expression and function
Skeletal muscle consists of two major types of fibers: Type I and Type II. Type I, or slow twitch fibers, have a high resistance to fatigue, increased oxidative metabolism, and are better for endurance activities. Type II, or fast twitch fibers, have low resistance to fatigue, primarily anaerobic metabolism, and an increased ability to generate speed and force. The α-actinins (ACTN2 and ACTN3) are structural components of the Z-line in skeletal muscle. The expression of the ACTN2 gene is ubiquitous in skeletal muscle while the ACTN3 gene is expressed only in Type II (fast twitch) muscle fibers where it has been shown to play roles in structural, metabolic, signaling, and calcium handling properties. While no skeletal muscle phenotype has been documented in relation to ACTN2 mutation, ACTN3 deficiency disrupts the aforementioned aspects of fast twitch fiber function, causing these fibers to behave more like slow twitch fibers (19).
MUSCLE FUNCTIONS
POTENTIALLY AFFECTED BY ACTN3 GENOTYPE
A number of muscle-related phenotypes have been studied in relation to ACTN3 genotype including maximal oxygen consumption (VO2max), jumping ability, grip strength, and muscle fatigue.
VO2max
Slow twitch muscle fibers are red due to a high capillary density, have high myoglobin levels resulting in increased oxygen binding capacity, contain high numbers of mitochondria, and show increased levels of oxidative metabolic enzymes (20). Therefore, individuals with a higher number of slow twitch fibers have improved endurance and higher VO2max (21). VO2max is defined as the maximal volume of oxygen consumed by the body during incremental exercise. VO2max is a complex phenotype dependent on both oxygen delivery (lung & heart function, blood composition, circulation) and utilization (metabolism in target cells, primarily muscle during exercise).
Based on the evidence that fast twitch muscle fibers behave more like slow twitch muscle fibers in ACTN3-deficient individuals, some researchers have hypothesized that ACTN3-deficient (XX) individuals have higher VO2max than individuals who express functional ACTN3 (RX or RR). A number of published studies have tested this hypothesis with inconsistent results. A study done on male professional soccer players from Brazil indicated that VO2max was higher in XX individuals than in RR individuals (22). In addition, a study on non-professional athletes had similar results. In a population of male police recruits, XX individuals had higher VO2max levels than RR individuals both before and after endurance training (23). Additional studies on male endurance cyclers and runners, elderly women, and male and female university students did not indicate any significant differences in VO2max levels by ACTN3 genotype (24-26), but one study on male and female university students indicated a higher VO2max in RR individuals compared to XX individuals (27). Among the studies that did report significantly increased VO2max in XX individuals, a small effect was observed. For example, untrained male police recruits with the RR genotype had an average VO2max of 47.2 +/- 7.5 mL/kg/min as compared to 52.8 +/- 6.2 mL/kg/min for the XX genotype. After training, RR male police recruits had an average VO2max of 52.6 +/- 5.9 mL/kg/min., compared to 56.1 +/- 5.6 mL/kg/min. for XX individuals (23), similar to the values reported for the male professional soccer players (22). Therefore, it is likely that ACTN3 genotype contributes only a small amount to oxygen consumption, if at all.
Jump height
It has been demonstrated in previous studies that individuals are able to jump higher in a countermovement jump (CMJ) than in other types of jumps (28). During a CMJ, the individual starts from an upright standing position, makes a preliminary downward movement by flexing at the knees and hips, and then immediately extends the knees and hips again to jump vertically off the ground. The greater jump height associated with the CMJ has been attributed to the idea that the countermovement during flexion of the knees and hips allows individuals to achieve greater joint movements at the start of the push-off (28). Specifically, Bobbert et al. (28) demonstrated that the preparatory countermovement allows muscles to build up a high level of active-state myosin-actin cross-bridges, and therefore the muscles are able to produce more force.
Jumping performance has been studied previously in relation to the ACTN3 R577X polymorphism. Orysiak, et al. (14) studied Polish male athletes and used the CMJ to indirectly measure lower limb power. In this study it was demonstrated that subjects with the RR genotype had significantly higher jump heights than subjects with the XX genotype. Further studies of Polish athletes of various sports also demonstrated RR genotype subjects had significantly greater power output during a spike jump test compared to XX individuals (29). An additional study performed in non-athletic young men showed similar results, where men with the RR genotype jumped 5% higher during CMJ tests (30).
Grip strength
Grip strength is defined as the force applied by the hand to pull on or suspend from objects and can be used as an estimator for whole body strength. Grip strength requires the use of fast-twitch (type II) muscle fibers responsible for generating rapid and powerful contractions that are less resistant to fatigue. Broos et al. (30) demonstrated a significant difference in grip strength between individuals with the RR genotype and those with the XX genotype (RR genotype had 6% improved grip strength). These findings also confirm what has been demonstrated in mouse models. Previous studies investigating the forelimb grip strength of Actn3 knockout (Actn3 -/- (KO)) mice, Actn3 heterozygous (Actn3 +/- (HET)) mice, and Actn3 homozygous (Actn3 +/+ (WT)) mice (note: mouse gene symbols only capitalize the first letter as compared to human gene symbols, which use all upper-case letters. Therefore, in humans the gene symbol is ACTN3, whereas in mice it is Actn3), showed that the Actn3 genotype significantly affected grip strength (7). Specifically, Actn3 KO mice displayed significantly reduced grip strength compared with HET or WT mice (7).
Muscle fatigue
The ACTN3 R577X polymorphism may negatively impact the performance of type II muscle fibers that are attributable to muscle mass, force generation, and resistance to fatigue (5). Additionally, activities that require endurance and have been more closely associated with ACTN3 deficiency require more fatigue-resistant slow-twitch muscle fiber activity (31). While fewer studies have examined resistance to fatigue and its association with ACTN3 genotype, Broos et al. (30) studied the fatigue index of a group of healthy young men. The fatigue index is a measure of endurance and defined as the rate at which power declines. This study demonstrated that individuals with the XX genotype had a significantly lower fatigue index, meaning these individuals took a longer time to fatigue and lose power/strength (30). These observations suggest that the improved fatigue index of XX individuals may be beneficial for endurance activities, such as rowing, swimming or long distance (10,11).
CONCLUSION
The R577X variant of ACTN3 prevents the production of functional ACTN3 protein in fast twitch muscle fibers. In humans who do not express ACTN3 (XX), fast twitch muscle fibers behave more like slow twitch muscle fibers, and many studies have tested the effect of ACTN3 deficiency on various muscle-related functions including maximal oxygen consumption (VO2max), jumping ability, grip strength and muscle fatigue. Other studies have also examined the correlation of ACTN3 genotype and athletic performance in sprint or endurance activities. The findings in these studies are frequently heterogeneous, emphasizing the complex nature of this trait.
In this article, we have provided instructors with information and references related to ACTN3 genetics, muscle physiology, and muscle functions potentially affected by ACTN3 genotype. These topics encompass background information to accompany the lesson "The ACTN3 Polymorphism: Applications in Genetics and Physiology Teaching Laboratories."
References
- North KN, Yang N, Wattanasirichaigoon D, Mills M, Easteal S, Beggs AH. 1999. A common nonsense mutation results in alpha-actinin-3 deficiency in the general population. Nat Genet 21:353-354.
- Ma F, Yang Y, Li X, Zhou F, Gao C, Li M, Gao L. 2013. The association of sport performance with ACE and ACTN3 genetic polymorphisms: a systematic review and meta-analysis. PLoS One 8:e54685.
- North KN, Beggs AH. 1996. Deficiency of a skeletal muscle isoform of alpha-actinin (alpha-actinin-3) in merosin-positive congenital muscular dystrophy. Neuromuscul Disord 6:229-235.
- MacArthur DG, North KN. 2007. ACTN3: A genetic influence on muscle function and athletic performance. Exerc Sport Sci Rev 35:30-34.
- Yang N, MacArthur DG, Gulbin JP, Hahn AG, Beggs AH, Easteal S, North K. 2003. ACTN3 genotype is associated with human elite athletic performance. Am J Hum Genet 73:627-631.
- Garton FC, North KN. 2016. The effect of heterozygosity for the ACTN3 null allele on human muscle performance. Med Sci Sports Exerc 48:509-520.
- Hogarth MW, Garton FC, Houweling PJ, Tukiainen T, Lek M, Macarthur DG, Seto JT, Quinlan KG, Yang N, Head SI, North KN. 2016. Analysis of the ACTN3 heterozygous genotype suggests that alpha-actinin-3 controls sarcomeric composition and muscle function in a dose-dependent fashion. Hum Mol Genet 25:866-877.
- Moran CN, Vassilopoulos C, Tsiokanos A, Jamurtas AZ, Bailey ME, Montgomery HE, Wilson RH, Pitsiladis YP. 2006. The associations of ACE polymorphisms with physical, physiological and skill parameters in adolescents. Eur J Hum Genet 14:332-339.
- Mills M, Yang N, Weinberger R, Vander Woude DL, Beggs AH, Easteal S, North K. 2001. Differential expression of the actin-binding proteins, alpha-actinin-2 and -3, in different species: Implications for the evolution of functional redundancy. Hum Mol Genet 10:1335-1346.
- Ahmetov, II, Druzhevskaya AM, Astratenkova IV, Popov DV, Vinogradova OL, Rogozkin VA. 2010. The ACTN3 R577X polymorphism in Russian endurance athletes. Br J Sports Med 44:649-652.
- Ben-Zaken S, Eliakim A, Nemet D, Rabinovich M, Kassem E, Meckel Y. 2015. ACTN3 polymorphism: Comparison between elite swimmers and runners. Sports Med Open 1:13.
- Doring FE, Onur S, Geisen U, Boulay MR, Perusse L, Rankinen T, Rauramaa R, Wolfahrt B, Bouchard C. 2010. ACTN3 R577X and other polymorphisms are not associated with elite endurance athlete status in the Genathlete study. J Sports Sci 28:1355-1359.
- Kikuchi N, Miyamoto-Mikami E, Murakami H, Nakamura T, Min SK, Mizuno M, Naito H, Miyachi M, Nakazato K, Fuku N. 2016. ACTN3 R577X genotype and athletic performance in a large cohort of Japanese athletes. Eur J Sport Sci 16:694-701.
- Orysiak J, Busko K, Michalski R, Mazur-Rozycka J, Gajewski J, Malczewska-Lenczowska J, Sitkowski D, Pokrywka A. 2014. Relationship between ACTN3 R577X polymorphism and maximal power output in elite Polish athletes. Medicina 50:303-308.
- Papadimitriou ID, Lockey SJ, Voisin S, Herbert AJ, Garton F, Houweling PJ, Cieszczyk P, Maciejewska-Skrendo A, Sawczuk M, Massidda M, Calo CM, Astratenkova IV, Kouvatsi A, Druzhevskaya AM, Jacques M, Ahmetov, II, Stebbings GK, Heffernan S, Day SH, Erskine R, Pedlar C, Kipps C, North KN, Williams AG, Eynon N. 2018. No association between ACTN3 R577X and ACE I/D polymorphisms and endurance running times in 698 Caucasian athletes. BMC Genomics 19:13.
- Yang N, MacArthur DG, Wolde B, Onywera VO, Boit MK, Lau SY, Wilson RH, Scott RA, Pitsiladis YP, North K. 2007. The ACTN3 R577X polymorphism in East and West African athletes. Med Sci Sports Exerc 39:1985-1988.
- Clarkson PM, Devaney JM, Gordish-Dressman H, Thompson PD, Hubal MJ, Urso M, Price TB, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Hoffman EP. 2005. ACTN3 genotype is associated with increases in muscle strength in response to resistance training in women. J Appl Physiol (1985) 99:154-163.
- Clarkson PM, Hoffman EP, Zambraski E, Gordish-Dressman H, Kearns A, Hubal M, Harmon B, Devaney JM. 2005. ACTN3 and MLCK genotype associations with exertional muscle damage. J Appl Physiol (1985) 99:564-569.
- Lee FX, Houweling PJ, North KN, Quinlan KG. 2016. How does alpha-actinin-3 deficiency alter muscle function? Mechanistic insights into ACTN3, the 'gene for speed'. Biochim Biophys Acta 1863:686-693.
- Burton DA, Stokes, K., Hall, G.M. 2004. Physiological effects of exercise. Continuing Education in Anaesthesia, Critical Care & Pain 4:185-188.
- Bergh U, Thorstensson A, Sjodin B, Hulten B, Piehl K, Karlsson J. 1978. Maximal oxygen uptake and muscle fiber types in trained and untrained humans. Med Sci Sports 10:151-154.
- Pimenta EM, Coelho DB, Veneroso CE, Barros Coelho EJ, Cruz IR, Morandi RF, De APG, Carvalho MR, Garcia ES, De Paz Fernandez JA. 2013. Effect of ACTN3 gene on strength and endurance in soccer players. J Strength Cond Res 27:3286-3292.
- Silva MSM, Bolani W, Alves CR, Biagi DG, Lemos Jr JR, Silva JLd, de Oliveira PA, Alves GB, de Oliveira EM, Negr?o CE, Krieger JE, Dias RG, Pereira AC. 2015. Elimination of influences of the ACTN3 R577X variant on oxygen uptake by endurance training in healthy individuals. Int J of Sports Phys & Perform 10:636-641.
- Holdys J, Krysciak J, Stanislawski D, Gronek P. 2011. Polymorphism of the ?-ACTN3 gene in individuals practising different sports disciplines. Biol Sport 28:101-106.
- Lucia A, Gomez-Gallego F, Santiago C, Bandres F, Earnest C, Rabadan M, Alonso JM, Hoyos J, Cordova A, Villa G, Foster C. 2006. ACTN3 genotype in professional endurance cyclists. Int J Sports Med 27:880-884.
- Min SK, Lim ST, Kim CS. 2016. Association of ACTN3 polymorphisms with BMD, and physical fitness of elderly women. J Phys Ther Sci 28:2731-2736.
- Deschamps CL, Connors KE, Klein MS, Johnsen VL, Shearer J, Vogel HJ, Devaney JM, Gordish-Dressman H, Many GM, Barfield W, Hoffman EP, Kraus WE, Hittel DS. 2015. The ACTN3 R577X polymorphism is associated with cardiometabolic fitness in healthy young adults. PLoS One 10:e0130644.
- Bobbert MF, Gerritsen KG, Litjens MC, Van Soest AJ. 1996. Why is countermovement jump height greater than squat jump height? Med Sci Sports Exerc 28:1402-1412.
- Orysiak J, Busko K, Mazur-RoZycka J, Michalski R, Gajewski J, Malczewska-Lenczowska J, Sitkowski D. 2015. Relationship between ACTN3 R577X polymorphism and physical abilities in Polish athletes. J Strength Cond Res 29:2333-2339.
- Broos S, Van Leemputte M, Deldicque L, Thomis MA. 2015. History-dependent force, angular velocity and muscular endurance in ACTN3 genotypes. Eur J of Appl Phys 115:1637-1643.
- Van Damme R, Wilson RS, Vanhooydonck B, Aerts P. 2002. Performance constraints in decathletes. Nature 415:755-756.
Article Files
Login to access supporting documents
The Science Behind the ACTN3 Polymorphism(PDF | 165 KB)
- License terms

Comments
Comments
There are no comments on this resource.