Fruit Fly Genetics in a Day: A Guided Exploration to Help Many Large Sections of Beginning Students Uncover the Secrets of Sex-linked Inheritance
Published online:
Abstract
Moving beyond the basic concepts of autosomal Mendelian inheritance to sex-linkage can be difficult for introductory biology students. Although crosses with Drosophila fruit flies have long been a mainstay of genetics units that teach these concepts, they can be unwieldy for large numbers of students, take much of a semester to complete, and require substantial preparation time. We developed a guided exploration laboratory activity that illustrates the contrasts between sex-linked and autosomal inheritance mechanisms in one class period and can be applied easily in multiple sections of a large course. The activity sets up the background information of Thomas Hunt Morgan's famous crossing experiments that demonstrated sex linkage of the white eye color gene in Drosophila and asks students to apply skills learned in the previous autosomal inheritance unit to predict key aspects of his results (i.e., sex and phenotypic ratios of the F2 offspring). They then do a hands-on genetics laboratory activity by anesthetizing and sexing flies before analyzing provided data from Morgan's cross. Students interpret these results, pose an inheritance hypothesis, and revisit their original ratio predictions. Finally, review questions guide students toward recognizing and applying the contrasts between autosomal and sex-linked inheritance. We have tested this activity in over twenty of our own sections, assessed student performance with pre- and post-tests concerning sex-linked inheritance, and surveyed student opinion of the activity. Student response has been positive, both in terms of learning and enthusiasm. Our lesson can be modified and adapted easily to different classroom environments and course contexts.
Citation
Croshaw DA, Palmtag MR. 2019. Fruit Fly Genetics in a Day: A Guided Exploration to Help Many Large Sections of Beginning Students Uncover the Secrets of Sex-linked Inheritance. CourseSource. https://doi.org/10.24918/cs.2019.39
Society Learning Goals
Genetics
- Transmission - Patterns of Inheritance
- How can one deduce information about genes, alleles, and gene functions from analysis of genetic crosses and patterns of inheritance?
- What are the mechanisms by which an organism’s genome is passed on to the next generation?
Lesson Learning Goals
- Students will recognize the difference between autosomal and sex-linked inheritance.
- Students will develop skills in genetics problem-solving.
Lesson Learning Objectives
- Students will be able to handle and anesthetize Drosophila fruit flies.
- Students will be able to use a dissecting microscope to sex Drosophila fruit flies.
- Students will implement some steps of the scientific method.
- Students will successfully predict the results of sex-linked genetics crosses.
- Students will interpret genetic data.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
Many studies on the scholarship of teaching and learning have demonstrated the value of active over passive forms of learning (1-5). Genetics, in particular, is an area of biology that requires students to actively engage with information and data to understand the discipline. Practice in genetics problem solving may be one of the best ways to reinforce ideas from lectures and readings. Moreover, hands-on, process-oriented laboratories demonstrate key concepts, provide memorable case studies and context for advanced problem-solving, and offer opportunities for students with different learning preferences to engage the topic successfully (5-6). Active learning can be especially effective in science disciplines, such as biology and genetics, in which problem solving, case studies, and complex synthesis activities can be combined with labs to foster critical thinking and quantitative skills. Moreover, laboratories with wet-lab experimentation and handling of preserved and living organisms can be utilized as inquiry-based or guided exploration approaches in which students must implement portions of the scientific process (7-11).
In accordance with these active learning principles, we aimed to combine our knowledge of Drosophila fruit fly culture with genetics problem-solving to create a guided exploration of Mendelian and sex-linked genetics for many large sections of students enrolled in an introductory General Biology course. Drosophila fruit flies are well understood biologically and genetically, convenient for use in experimentation, and, therefore, used widely in laboratory teaching activities (12). The life science fields addressed with the Drosophila system as a teaching tool vary widely from evolutionary biology (13) to biochemistry (14) and from behavior (12) to bioinformatics (15) and genetics (16). Amongst the resources commonly accessed by faculty, peer-reviewed pedagogical publications describing basic genetics crossing activities for students in biology courses are limited because they use simulation data instead of real flies (17), demonstrate advanced applications such as linkage mapping (16), recommend weeks-long multigenerational projects (18), and/or use traits that are not widely discussed in general biology textbooks (15,18).
Herein, we describe a new hands-on laboratory lesson that easily corresponds to most introductory textbooks and provides experience working with the fruit fly system that can be completed in a single lab period by many large sections of students. The premise of the activity is that it places students in the position of Thomas Hunt Morgan (and his undergraduates, some of whom were authors on the papers), one of the most influential early geneticists who brought fruit flies to prominence in the field and was the first to demonstrate that genes are present on chromosomes (19). Students, having already learned about Mendel’s laws and autosomal inheritance, are first presented with a traditional lecture on sex-liked inheritance that does not include the white eye phenotype example, though some instructors may prefer to start with the discovery activity. Students are challenged to predict the sex and eye color ratios of the F1 and F2 generations in Morgan’s famous sex-linked white eye mutation cross (20), assuming that the gene for eye color exists on an autosome because they are already proficient at autosomal inheritance. Students then work with live fruit flies to gain skills required for research with this model system. They learn several techniques with the animals: anesthesia, handling and transfer, observing under a dissecting microscope, distinguishing the sexes, and disposing of the flies properly. Students are then provided with raw data from the F2 generation of Morgan's actual experiment. The biggest critical thinking challenge for students is to analyze and understand why Morgan's data are different from their original predictions (i.e., autosomal inheritance). With the help of hints, they should recognize that the eye color gene in question exhibits sex-linked rather than autosomal inheritance. In part because students are not taught the true inheritance mechanism and instead must "discover" it on their own, the activity reinforces the contrast between autosomal and sex-linked patterns. Our lesson achieves active learning, use of the scientific method with guided exploration, hands-on experience working with an influential model organism, and data analysis followed by interpretation. Finally, although this particular fruit fly cross is used widely in teaching, it typically takes up to a month of a semester to complete, is difficult to implement with large numbers of students, and requires intensive laboratory preparation. Our version solves these problems.
Intended Audience
We have taught this lesson at a large public, regional university in an introductory course (General Biology I) intended for biology majors that is also highly populated by non-biology majors. Many other programs at our university require this course and it is our experience that biology major students account for ~20% of the population. The course is taught in the Scale-Up format, meaning that content delivery involves lecture and lab combined on a large scale, typically 72-81 students. The department administers this course as its largest collective effort, sometimes with as many as 15 sections and six instructors involved in a single semester. We have implemented this lesson successfully, with help from laboratory preparation staff, across all sections. The student body is extremely diverse in level of interest, motivation, and scientific background. Most are first-year college students, and we have geared the level of difficulty and expected achievement appropriately. This lesson also has been expanded and modified for effectiveness in a mid-level course in Genetics.
Required Learning Time
This activity can be completed in a 75-minute time block allocated to laboratory work, but we also precede that with a standard 60-minute lecture. If time is tight, data analysis and interpretation can be assigned as homework or revisited during the next class. Students will vary in how much time they take to become comfortable using the dissecting microscope and to make careful observations of their fruit flies.
Prerequisite Student Knowledge
Prior to the lesson, students have already experienced a lecture and problem-solving session on basic Mendelian inheritance. They have learned about Mendel's two laws, practiced using genetics notation, and solved problems with Punnett squares. They can express their solutions in terms of genotypic and phenotypic ratios, discuss the importance of dominance and recessiveness, and use basic probability. In the laboratory, they have been exposed to dissecting microscopes, but have little experience in using them effectively to observe biological specimens and no experience in using them to collect scientific data. We recommend that students have all this background before doing the activity.
Prerequisite Teacher Knowledge
Instructors should be extremely proficient in working with and observing Drosophila fruit flies. This includes transfer between vials, anesthesia with Fly Nap, and observing traits that distinguish the sexes. Instructors must be well-trained in use of the dissecting microscopes available for students. They also should understand Mendel's laws of inheritance and the contrast between autosomal and sex-linked patterns, allowing them to use the proper notation to predict the results of genetics crosses and solve genetics problems. For example, the expected results of Morgan's cross that is used in this lesson are extremely important. A true-breeding homozygous wild-type female fly was crossed with a true-breeding white-eyed mutant male fly in the parental generation. F1 offspring were entirely wild-type (both male and female). These flies were then mated to produce the F2 generation in which all female flies were wild-type but the male flies were roughly 50% mutant white-eyed and 50% wild-type.
For further information, interested instructors may refer to the following websites about fruit fly biology and teaching ideas:
SCIENTIFIC TEACHING THEMES
Active Learning
This lesson is almost entirely active learning for students because they develop hands-on skills working with the model organism and evaluate different genetic inheritance hypotheses themselves by making predictions for comparisons to provided data. Though we recommend that instructors address the entire class for a few minutes with an overview of the protocol and logistical aspects, students should do most of the laboratory and conceptual work in small groups. At the end of the activity, or during the next class, if time is constrained, instructors should either discuss the results with the class or lead a group discussion about the activity, data, and what was learned.
Assessment
The lesson (Supporting File S1. Sex-linked Inheritance - Student Handout) contains six conceptual review questions (pp. 7-8) that can be used to assess student learning. We often use these as a capstone assignment for the activity to focus the thoughts and data interpretation of students. The questions force students to consider and understand the major issues addressed in the lesson (including learning goals 1 and 2 outlined above). An answer key to the entire laboratory handout is provided as Supporting File S2 (Sex-linked Inheritance - Answer Key). Additionally, we developed multiple choice questions that may be used both before and after the activity to measure its effectiveness in teaching students about sex-linked inheritance (Supporting File S3. Sex-linked Inheritance - Assessment Questions). These also may be used in graded quizzes and exams.
Inclusive Teaching
This lesson is designed to engage a number of different learning preferences (visual, physical, social, logical) and therefore material and ideas are considered in multiple ways, though we acknowledge that there is not strong evidence linking student learning gains to pedagogy focused on the learning preference. For example, visual learners are accommodated with PowerPoint lectures, physical learners by hands-on activity with the living organisms, logical learners through conceptual problem-solving with the associated worksheet, and social learners by peer group discussions engaging the lesson. All students are challenged with different approaches to learning. Furthermore, it is a group activity in which at least 2-3 students are expected to work together, facilitating contributions from diverse perspectives that can be enlightening, refreshing, and inspiring. Although we typically allow students to self-select into groups, in our experience, individuals in such lab groups tend to take on unique roles that are in line with their diverse academic strengths and personalities. For example, one student may have solid skills working with data and numbers but lack experience with live subjects. Another might learn better by hands-on activities and already have some good experience in field biology. This would be a naturally successful pair. Encouraging students to approach the work in the way that is most helpful to them is beneficial. Finally, the topic inherently involves questions of inheritance and family, which are subjects of natural interest to a variety of identities.
LESSON PLAN
Pre-Class Preparation
This is a laboratory activity and therefore requires some time-consuming preparation. First, the equipment and supplies must be available and organized. Each student group needs a dissecting microscope and a fly observation supply kit. Our kits consist of small food storage containers stocked with paint brushes, index cards, a funnel, empty vials and plugs for anesthesia, and wands for administering FlyNap (purchased from Carolina Biological, Burlington, North Carolina, USA). The anesthetic is normally placed in small containers at a few stations around the classroom. A disposal morgue for flies when students are finished is also necessary. We recommend a large container with ethanol or mineral oil and a funnel to direct sleeping flies.
We usually order flightless strains that look like the wild-type from Carolina Biological and allocate them in enough food vials for each student group to observe. On some occasions in the past, we have ordered F2 offspring (also from Carolina) for this specific cross so that students are able to collect their own data rather than working with provided data. Vials are typically shipped during development, so flies must be monitored after arrival and before emergence. Once these F2 adult flies have emerged, it is helpful to place them in new food vials. We use a pre-mixed food recipe from Genesee Scientific (San Diego, California, USA) that is easy to prepare.
Order of Events
We start with a standard lecture on sex-linked inheritance (Table 1; e.g., sex determination, X and Y chromosomes, genetics notation, patterns of transmission, etc.). To make sure that the discovery aspect of the activity is not compromised, we use other examples in the discussion, such as the X-linked body color gene in fruit flies (yellow recessive to gray) or the X-linked fur color gene in domestic cats (orange recessive to black). Then we introduce the procedure and logistics of the laboratory activity, making sure especially that students understand how to work with live fruit flies. Typically, we include some images about traits to observe for sexing flies. We emphasize the abdominal plumpness and overall larger size of females along with their lack of uniform black coloration at the abdominal tip. We also point out the difference in shape of the genitalia and the presence of sex combs on the forelimbs of males.
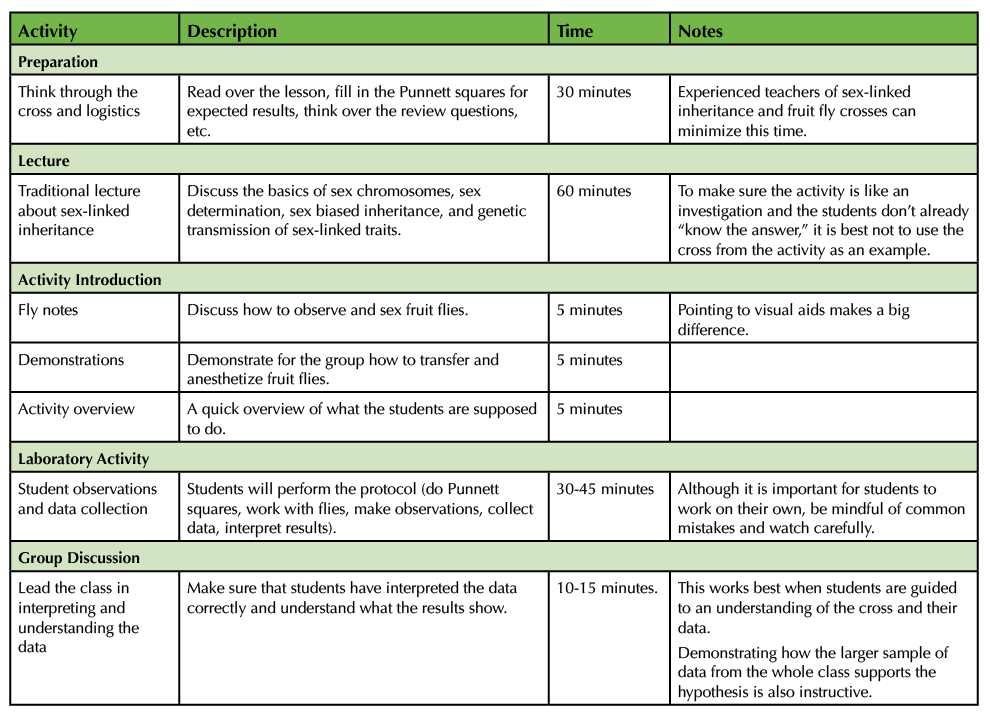
Table 1. Uncovering the Secrets of Sex-linked Inheritance - Teaching Timeline
We do demonstrations to show how flies are transferred from food vials to anesthesia vials before administering FlyNap. The major points of emphasis are tapping flies off the vial plug, quick plug removal followed by placing two fingers over the vial and more tapping, and finally inverting the vial over a funnel that has been placed in an empty anesthesia vial. Holding the two vials together with the thumb and forefinger makes a sealed system from which flies cannot escape. They now just need to be tapped down on the benchtop to be removed from the original food vial. Once all flies are in the anesthesia vial, the funnel must be quickly removed and the vial plugged.
At this point, flies are ready to be anesthetized. We demonstrate this by dipping a small cleaner brush into the anesthetic, tapping flies off the plug and quickly inserting the brush end into the vial. We then watch flies for a few minutes as they begin to fall onto their backs. When about 80-90% of them are down, the anesthetic should be removed. Failure to do this may result in fly death, although that outcome would be compatible with our purposes in this lesson because they are only needed for observation. Each student group is provided with an instruction manual from Carolina Biological that explains the protocol for using FlyNap along with the supplies delineated in the pre-class preparation section. In brief, students receive a flightless fly strain (looks like wild-type) ordered from Carolina Biological, anesthesia vials, paintbrushes, funnels, and index cards. Enough vials of live flies to accommodate one per lab group are placed in a cardboard tray for students to pick up at the same place where they receive the supply kits.
In our experience, students at this level need a guided overview about what to do. We follow these demonstrations with a quick rundown of the activity, what predictions to make, what to do with the data, and what data to collect (if any). We go over Figures 13.1 and 13.3 and Tables 13.1 and 13.3 (see Supporting File S1. Sex-linked Inheritance - Student Handout, Unlocking the Secrets of Sex Biased Inheritance Patterns: the laboratory activity that we provide to students as part of a manual for the course) on which students will enumerate their genetics hypotheses and manipulate phenotypic and sex ratio data. Before students start working with the flies, we emphasize that they should complete their first critical thinking challenge in which they are asked to predict the sex and eye color ratios of Morgan's F2 generation assuming autosomal inheritance.
Once these introductory pieces are completed, we turn the students loose to gather their supplies, work through the paper portions of the activity, and prepare to observe their flies. First students read through the introductory information in the laboratory handout which sets up the context for Morgan's famous cross of a mutant white-eyed male to wild-type females. Next, they use Punnett squares to allow predictions of phenotypic and sex ratios of offspring in the F1 and F2 generations. They then put flies to sleep using Fly Nap and observe them under the dissecting microscope, practicing how to distinguish the sexes and the phenotypes. Morgan's original data are provided, and students use them to make some basic calculations for comparison of their predictions (which assumed autosomal inheritance) to the observed data. Students are asked whether the data are consistent with their prediction and, if not, to pose a new inheritance hypothesis. Under sex linkage, they generate new ratio predictions and see that these are consistent with Morgan's data.
As the students start work with their flies and the paper portion of the lesson, we circulate around the room and carefully watch what students are doing so that we can correct mistakes and answer questions. Even after students have transferred and anesthetized their flies successfully, errors are still likely, especially when sexing. It is also important to make sure that each student has carefully considered and completed the paper portion of the activity. The most common questions students have when working with the flies is how long they will stay asleep and whether they are correctly distinguishing the sexes. We find it useful to check the sex assignment of students who are unsure.
We monitor student progress until all groups have finished, which is not true until they have put back all supplies, cleaned up their work areas, answered all review questions, and completed the data tables and figures. We engage in conversations with students to evaluate their conclusions from the second critical thinking challenge: addressing the disparity between predictions that considered the eye color as either autosomal or sex-linked.
One of the major goals for the activity is to make sure that all students learn by doing. Although division of labor is fine and encouraged, we want everyone to participate in anesthetizing flies, using the dissecting microscope and making observations. Moreover, we do not want to give too much away about the inheritance of the trait or the expected results beforehand. It would be easy to spend too much time going over details in the lecture and activity introduction so that most students know what to expect. Be aware that to keep the focus on the guided exploration and scientific method, we recommend leaving a lot unsaid for the students to learn on their own.
Assessment
In the fall semester of 2017, we assessed student learning from our lesson in two ways (N = 51 students in one section). First, we administered a short multiple-choice quiz (Supporting File S3. Sex-linked Inheritance - Assessment Questions, eight questions) that was given after a traditional lecture on the topic of sex-linked genetics, but before the activity. After performing the activity, we again administered a second version of the quiz (see Supporting File S3. Sex-linked Inheritance - Assessment Questions). Thus, the timing of events was as follows: 1) traditional lecture on sex-linked inheritance, 2) pre-activity assessment quiz, 3) hands-on activity described here, and finally 4) post-activity assessment quiz. Students were given the option to participate in the study and remained anonymous to their instructor because they were assigned random numbers by an assistant. After confirming that the data conformed to the expectations of normality (Kolmogorov-Smirnov test: p > 0.09 in both pre- and post-lesson scores), quiz scores were analyzed statistically with a paired t-test to test the hypothesis that students scored higher after doing the lesson. Pre- and post-activity scores were paired by the randomly assigned student identification number. We found that student scores were significantly higher following the lesson, indicating substantial learning (Figure 1, pre-activity mean = 61.25%, post-activity mean = 68.38%, t50 = 2.33, p = 0.02). Twenty-nine students had improved quiz scores after doing the activity, 14 scored worse, and eight had the same score.
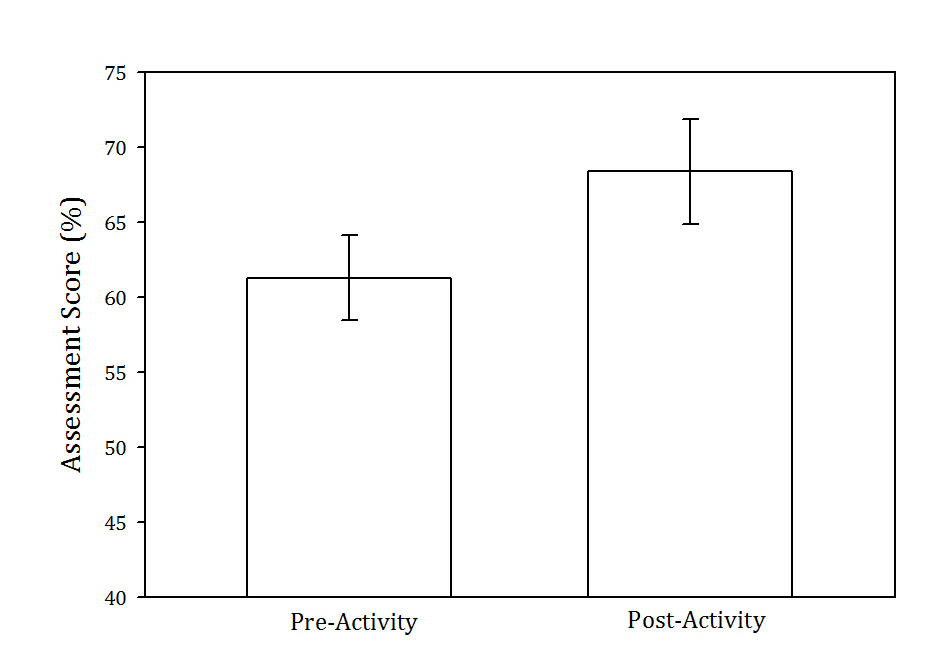
Figure 1. Mean pre- and post-activity assessment scores for the fruit fly lesson (Supporting File S3. Sex-linked Inheritance - Assessment Questions). Error bars represent ± standard error. The difference is statistically significant (paired t-test: t50 = 2.33, p = 0.02).
For assessments of student satisfaction with the learning experience, we appended five questions to the end of the post-activity quiz (Supporting File S3. Sex-linked Inheritance - Assessment Questions) that asked for student opinion of the lesson. An overwhelming majority (95.8%) recommended that the activity be continued in future semesters, and most (81.6%) felt that the activity would allow them to perform better on the upcoming exam. As an overall rating of the activity's quality, 75% of students rated it either 4 or 5 on a five-point scale (5 being positive, mean = 3.91). Students also felt that the activity stimulated their interest in the topic (mean = 3.88) and helped them to understand it (mean = 3.90).
TEACHING DISCUSSION
The assessment results show that the lesson increases student achievement on both learning goals and the fourth and fifth learning objectives. Our quiz questions were designed specifically to measure these outcomes and the significant difference between scores before and after the activity confirm its efficacy. We believe this positive result occurred because students must actively engage with a genetics problem and think about how to use their skills and experimental results to solve it. They actually experience the process of discovery rather than just passively listening to the instructor talk about the genetics mechanisms. This active learning and the exploration aspect may serve to solidify the concept for many students. Although the experiential nature of the first three learning objectives makes them more challenging to assess, we are extremely confident that mere participation in the lesson will provide students with a certain level of achievement in performing some steps of the scientific method, working with fruit flies, and using a dissecting microscope.
In our experience, students very much enjoy this particular lesson and are excited about it. We presented assessment results showing that they mostly rated the activity positively. One likely reason is that it is interesting for them to explore and interpret real scientific data instead of a standard lab in which they merely follow a protocol for demonstration of some result or process. Students also enjoy working with the animals, even though some are shy about these particular organisms. In this General Biology 1 course there are not many opportunities to interact with live animals, something that students often find compelling.
We have noticed that the two most likely things to go wrong are that students do not sex the flies correctly or they lose them by being careless during transfer. It would be good for the instructor to verify that all students have learned how to distinguish male from female without mistakes. Although this would add more time to the activity, it would increase the quality of the data if students are collecting their own. During demonstrations, we encourage instructors to emphasize the importance of being careful during the fly transfers. No matter how much we make of that, though, it is inevitable that some students will drop the vials and let flies loose. We have found that it is good to plan for this and always have back-up vials at the ready. Additionally, it is prudent to have some baited fly traps available to capture the rogue pests.
As mentioned previously, we have done more complex and costly versions of this laboratory activity. Notably, it is possible to order the F2 offspring from an actual cross exactly like Morgan's. Although this version of the lesson is probably too costly and logistically challenging for large courses with many sections, it achieves an additional objective and can be implemented in smaller courses. We also have provided the two different fruit fly strains, wild-type and white-eyed mutant, for the students to anesthetize and observe. This also is much less costly than purchasing the F2 cross vials from Carolina Biological. We have found that student achievement is robust to these logistical variations. Our informal assessment data show that in any of these situations, with the cross or not, students experience significant learning and increased post-activity quiz scores. The results reported here were gathered from a section in which students were provided Morgan's original data as in the appended student laboratory protocol (Supporting File S1. Sex-linked Inheritance - Student Handout, Unlocking the Secrets of Sex Biased Inheritance Patterns).
Although we have only used this particular lesson in General Biology I, it has potential for use in an intermediate level Genetics course as well. One of us (DAC) has developed a much more involved inquiry-based laboratory project that lasts most of a month and uses the same cross (Supporting File S4. Sex-linked inheritance - Fruit Fly Laboratory Project in Genetics). The goal is for students to use their knowledge of transmission genetics to pose multiple hypotheses for the inheritance mechanism of the eye-color gene, devise expected phenotypic ratios, work with fly crosses to collect data, and do statistical analysis to test their preferred hypothesis. Students start with vials of F1 offspring because the instructor and teaching assistants collect virgin flies and set up the parental generation crosses (each reciprocal cross: white-eyed male x wild-type female and white-eyed female x wild-type male). They practice observing and working with the flies, while waiting for the F1 adults to emerge. They collect sex and phenotypic data from these and place them into new vials for production of the F2 generation. While waiting for the F2 generation, students work on their inheritance hypotheses and expected data. Once F2 adults emerge, students collect as much F2 sex and phenotype data as possible before finally testing multiple hypotheses with Chi-square statistical analysis and interpreting the results. This same model for a project has been used for Genetics by DAC with other fruit fly genes (e.g., vestigial, apterous), although care must be taken to select one that is easy for students to score. The most common problem is students making errors while distinguishing the sexes and phenotypes. Enhanced student learning is provided by this extension activity because students must understand, apply, and interpret statistical hypothesis testing. They also gain more experience in data compilation, data organization, and potentially also in hypothesis modification than is possible with the single-class lesson described here.
Our activity achieves significant learning on the topic of sex-linked inheritance, is compatible with multiple learning preferences, allows active and experiential learning, engenders critical thinking and data interpretation, teaches practical skills in laboratory genetics, and does all of this in a single class period. It offers considerable feasibility and versatility that allow adaptation for students at multiple levels and in different classroom environments. The perfect correspondence to a prominent example discussed in most general biology textbooks allows an easy way for struggling and unprepared students to understand and synthesize the connection between classroom activities and science content. Furthermore, most surveyed students enjoy it and assessments suggest substantial learning outcomes. This activity will help instructors of introductory biology teach sex linkage, reinforce autosomal inheritance, and offer experiential learning opportunities in the field of genetics.
SUPPORTING MATERIALS
- S1. Sex-linked Inheritance - Student Handout. The laboratory manual copy used for the students as reference during the lesson.
- S2. Sex-linked Inheritance - Answer Key. For items in the paper portion of the lesson.
- S3. Sex-linked Inheritance - Assessment Questions. Used in pre-activity and post-activity quizzes.
- S4. Sex-linked inheritance - Fruit Fly Laboratory Project in Genetics. A more advanced versionh of the lesson that we have used in Genetics.
ACKNOWLEDGMENTS
DAC and MRP contributed equally to this work. Special thanks to Seeka Agama, Savannah Berger, Luke Lamos, and Samantha Strohl for assistance with photographs and administration of the assessment. We also thank members of the Florida Gulf Coast University (FGCU) Biology teaching cell for offering ideas and feedback on this lesson at all stages, from the initial idea through to long-term and large-scale implementation of the lesson. This research was conducted under permit #2017-57 from the FGCU Institutional Review Board.
References
- Prince M. 2004. Does active learning work? A review of the research. J. Eng. Educ. 93:223-231.
- Favero TG. 2011. Active review sessions can advance student learning. Adv. Phys. Educ. 35:247-248.
- Gleason BL, Peeters MJ, Resman-Targoff BH, Karr S, McBane S, Kelley K, Thomas T, Denetclaw TH. 2011. An active-learning strategies primer for achieving ability-based educational outcomes. Am. J. Pharm. Educ. 75.
- Freeman S, Eddy SL, McDonough M, Smith MK, Okoroafor N, Jordt H, Wenderoth MP. 2014. Active learning increases student performance in science, engineering, and mathematics. Proc. Nat. Acad. Sci. USA 111:8410-8415.
- Jeffery E, Nomme K, Deane T, Pollock C, Birol G. 2016. Investigating the role of an inquiry-based biology lab course on student attitudes and views toward science. CBE Life Sci. Educ. 15:ar61.
- Soltis, R, Verlinden N, Kruger N, Carroll A, Trumbo T. 2015. Process-oriented guided inquiry learning strategy enhances students' higher level thinking skills in a pharmaceutical sciences course. Am. J. Pharm. Educ. 79:11.
- Myers MJ, Burgess AB. 2003. Inquiry-based laboratory course improves students' ability to design experiments and interpret data. Adv. Phys. Educ. 27:26-33.
- Casotti G, Rieser-Danner L, Knabb MT. 2008. Successful implementation of inquiry-based physiology laboratories in undergraduate major and nonmajor courses. Adv. Phys. Educ. 32:286-296.
- Metz AM. 2008. Teaching statistics in biology: using inquiry-based learning to strengthen understanding of statistical analysis in biology laboratory courses. CBE Life Sci. Educ. 7:317-326.
- Vanags T, Pammer K, Brinker J. 2013. Process-oriented guided-inquiry learning improves long-term retention of information. Adv. Phys. Educ. 37:233-241.
- Beck C, Butler A, da Silva KB. 2014. Promoting inquiry-based teaching in laboratory courses: are we meeting the grade? CBE Life Sci. Educ. 13:444-452.
- Patel S, DeMaine S, Heafield J, Bianchi L, Prokop A. 2017. The droso4schools project: long-term scientist-teacher collaborations to promote science communication and education in schools. Sem. Cell Dev. Biol. 70:73-84.
- Coleman SW, Jensen JS. 2007. Male mating success: Preference or prowess? Investigating sexual selection in the laboratory using Drosophila melanogaster. Amer. Biol. Teacher 69:351-358.
- Sofer W, Tompkins L. 1994. Drosophila genetics in the classroom. Genetics 136:417-422.
- Calie PJ, Lee S, Hicks EM. 2007. The bioinformatic enhancement of exercises in Drosophila genetics. Amer. Biol. Teacher 69:482-487.
- Marshall PA. 2008. Mapping linked genes in Drosophila melanogaster using data from the F2 generation of a dihybrid cross. Amer. Biol. Teacher 70:554-556.
- Bierema A, Schwartz R. 2016. Learning from the fruit fly. A card game for teaching Mendel's laws, meiosis and Punnett squares. The Science Teacher 83:39-47.
- Chinnici JM, Farland AM, Kent JW. 2005. An inquiry-based investigation of modes of inheritance using "flightless" fruit flies. Amer. Biol. Teacher 67:38-44.
- Morgan TH. 1910. Sex limited inheritance in Drosophila. Science 32:120-122.
- Hyde DR. 2009. Introduction to Genetic Principles. McGraw-Hill, New York, pp. 71-75.
Article Files
Login to access supporting documents
Fruit Fly Genetics in a Day: A Guided Exploration to Help Students Uncover the Secrets of Sex-linked Inheritance(PDF | 181 KB)
S1. Sex-linked Inheritance - Student Handout.pdf(PDF | 917 KB)
S2. Sex-linked Inheritance - Answer Key.pdf(PDF | 70 KB)
S3. Sex-linked Inheritance - Assessment Questions.pdf(PDF | 77 KB)
S4. Sex-linked Inheritance - Fruit Fly Laboratory Project in Genetics.pdf(PDF | 189 KB)
- License terms

Comments
Comments
Comment removed by administrator @ on (Edited: @ on )