From Cre/LoxP to Fate Maps: Inclusive and Equitable Approaches for Engaging Developmental Biology Students in Experimental Design
Editor: Yolanda Cruz
Published online:
Abstract
Engaging first generation underrepresented minority students in the process of inquiry in developmental biology is important to increase diversity in future graduate STEM education. One important challenge is how we design curricula to foster critical thinking skills in designing experiments in developmental biology to foster self-confidence, resiliency, and persistence in STEM fields. As a step to address this challenge, I describe an inquiry-based semester-long developmental biology class and lab embedded within a culturally-responsive mentoring and teaching framework. After describing the curriculum design of the developmental biology course, I illustrate a specific lesson on how to engage students in designing a fate map experiment using Cre/LoxP technology for mouse and zebrafish. In summary, this innovative framework may be adapted into undergraduate class and lab settings to help all undergraduate students prepare for future developmental biology graduate research settings.
Citation
Kao RM. 2020. From Cre/LoxP to fate maps: Approaches for engaging developmental biology students from diverse backgrounds in experimental design. CourseSource. https://doi.org/10.24918/cs.2020.23
Lesson Learning Goals
By the end of the lesson, students will achieve the following learning goals that are aligned with the Developmental Biology Learning Framework on Experimental Approaches:
- Clarify concepts and terms on heart development and regeneration.
- Design genetic Cre/LoxP approaches to determine the origins of cardiac muscle cells.
- Interpret and evaluate data from fate mapping experiments in cell and developmental genetics.
Lesson Learning Objectives
- Create genetic methods to determine the fate map of cardiac muscle cells during mouse and zebrafish development.
- Distinguish between gene knockout and fate mapping experimental approaches using Cre/LoxP technology.
- Grasp the significance of how fate mapping methods are applied to answer important questions in developmental biology.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
Fate mapping is one of the important experimental tools in developmental biology research. The fate map approach is like a Google map device that allows scientists to pinpoint which mature cell type arose from an earlier progenitor cell type over time and space. For example, Drs. Hilde Mangold and Nicole Le Douarin both developed elegant fate mapping experiments (1,2); each of their classic experiments led to important insights into the origin of cell fates during embryo and organ development. Furthermore, genetic methods, such Cre/LoxP technology, have provided important insights into cell and developmental processes. The Cre/LoxP technology is like the molecular programming device to generate the fate map made up of two parts: 1.) the tissue-specific expression of the bacteriophage enzyme called Cre recombinase; and 2.) the special genetic arrangement of specific genetic barcode sequence called LoxP that is only recognized by the enzyme Cre recombinase. Cre/LoxP technology in fate mapping studies have yielded important discoveries in developmental biology. For instance, Cre/LoxP approaches have been used to gain further insights into the origin of cell fates during organ formation, as well as tissue-specific transgenic approaches, gene knockout, and gene inactivation studies at the cellular level (3-11). As seen in recent CourseSource Lesson on hands-on demonstrations in homologous recombination in gene targeting approaches (12), Cre/LoxP technology may also be integrated within modern developmental biology undergraduate classroom setting to engage undergraduates in critical thinking, making predictions, and experimental design.
Inclusive and equitable teaching and mentoring approaches can be used along with cutting-edge experimental tools, such as Cre/LoxP technology in developmental biology, to help all students engage in both critical thinking and the process of science. For instance, establishing inclusive and equitable approaches for culturally-responsive teaching and mentoring is vital for student learning and their participation as a community of scholars (13-23). In order to engage students from diverse backgrounds, a special framework called SOAR was developed over the past three years. The flexible SOAR framework is a culturally responsive mentoring approach that stands for the following: Spiral curricula and process of inquiry (spiralquiry); Observations from experiments to evaluation of data; Affective learning in active learning settings; and Research year-round across the undergraduate curricula (19). Within the SOAR framework, the instructor is part of the inclusive learning community in the classroom, and is used to engage a diverse background of learners in both class and lab settings. Once a community of scholars has been established, active learning tools can be used in class and lab settings—such as think-pair-share, gallery walks, encouraging students’ metacognition, and course-based authentic undergraduate research settings or CUREs (24-33). In addition, students are also engaged in the analysis and evaluation of data from developmental biology research articles. The Cre/LoxP Lesson is specifically designed to help biology majors take observations from a developmental process and further refine their critical thinking skills through experimental design and making predictions.
In addition, hands-on demonstration approaches have been used to engage diverse background of learners in developmental biology. For instance, the creation of the origami embryo (34-36) and the origami heart development (37) have been provided for the teaching community. However, one important challenge for instructors is how to integrate both hands-on demonstrations–especially for Cre/LoxP–and its applications in cell fate and lineage tracing within the active learning classroom setting. As a step towards addressing this question, this article highlights an example of how Cre/LoxP technology is used as a tool for engaging students to make predictions and utilize critical thinking skills through the process of experimental design in developmental biology (38,39). The Cre/LoxP Lesson is aligned with two topics outlined by the Society for Developmental Biology: 1.) experimental approaches; and 2.) application of methods and tools using model organisms.
Intended Audience
The lesson plan is intended for biology majors at a liberal arts college or university setting. Students must have general biology from either community college or university setting, and may be taking genetics concurrently with developmental biology. A semester or quarter of molecular cell biology is highly encouraged. Students may have or may not yet have prior summer research experiences. If students have not taken genetics and/or molecular cell biology yet, primers containing instructor-designed questions and presentation of developmental process to complement chapter readings was included at the start of the start of class for students. The following are three primers instructors may wish to use with the Cre/LoxP lesson:
- Supporting File S6. From Cre/LoxP to Fate Maps – Teaching Slides on Cell Fate Decisions During Heart Development Primer clarifies concepts about cell fate decisions during heart development.
- Supporting File S7. From Cre/LoxP to Fate Maps – Teaching Slides on Genetics and Molecular Cell Biology Primer Handout clarifies concepts on cell receptor signaling.
- Supporting File S8. From Cre/LoxP to Fate Maps – Teaching Slides on Cre LoxP Class Discussions Primer Handout clarifies themes from Cre/LoxP technology in developmental biology.
Below is the step-by-step process I used to help prepare for potentially difficult developmental genetics topics for students during the Cre/LoxP lesson.
- Reflect on the difficulties that students may have as they learn developmental biology concepts. These difficulties may arise from trying to link phases during cell fate decisions of heart development. For example, students may have difficulties as they learn about the following: molecular and cellular mechanisms in heart tube formation; spatial structure of the heart cone; and cell specification versus cell differentiation. For example, one of the conceptual difficulties identified by Hiatt and colleagues is "a single gene affects a single trait" (40). To help clarify that one single gene may affect multiple aspects during heart development, I use an example of the role of pbx4 during zebrafish heart development. Here, the loss-of-function of pbx4 during zebrafish heart development results in not only changes in heart cone formation, but also changes in the number of atrial and ventricular cardiomyocytes at different points during heart development (41).
- I then wrote down in my teaching journal the Cre/LoxP and fate mapping topics covered from the class, and then compared notes I had written the previous year. I adopted Saldaña’s continuous reflection (refraction) approach to help me synthesize students' difficulties (42). Peter O'Connor first described the process of refraction in the context of process drama (43). Here, O'Connor defined refraction as "the ability of process drama to bend light into places that have been well hidden to reveal new and often quite startling discoveries." In the context of classroom teaching, refraction is the continuous reflection of the instructor to deeply listen to the way students use words and phrases to describe a biological mechanism or definition. For example, I used refraction to help me revisit my instructor reflection notes I took during the Cre/LoxP lesson plan over the past two years. I carefully paid attention to words and phrases students use to describe the developmental process during in-class discussions. In addition, refraction helped me adjust to specific students' thought process and informed me on how I will teach for the next class.
- Synthesize the terms and concepts from this activity into themes of the course.
- Explore terms or concepts along with students, to create an inclusive community of scholars environment. Affirm individual students' voices, and connect it back to themes related to students' semester research or topics that are relevant.
These four steps will be revisited again below in the teaching tips.
Required Learning Time
As shown in Table 1, the Cre/LoxP in-class discussion lesson lasts 150 minutes. The in-class discussions and experimental design are done on Monday, Wednesday, and Friday. Each in-class discussion lasts for 50 minutes. The Cre/LoxP lesson in embedded within 15-week semester class and lab schedule. Course learning outcomes linked to the Society for Developmental Biology Learning Framework are provided for instructors (Table 1).
Prerequisite Student Knowledge
Students should have had one year of general biology, and may either be concurrently taking genetics or may have molecular and cellular biology courses with their respective labs. Students are expected to be familiar with the following genetic and molecular cell biology concepts: genetic inheritance; dominant and recessive alleles; transcription; promoter function; translation; and receptor signaling.
Prerequisite Teacher Knowledge
In terms of content knowledge, instructors should have good working knowledge of genetics and developmental biology. For Cre/LoxP lesson plan, instructors should be familiar with the following articles:
- Patricia Jensen and Susan Dymecki's Essentials of Recombinase-Based Genetic Fate Mapping in Mice (44)
- Slack's Essential Developmental Biology on fate mapping and Cre/LoxP technology (45)
- Hayashi and McMahon's Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse (3).
In addition, Hilde Mangold's tissue graft experiments (2) and Nicole Le Dourian's quail-chick chimera experiments (1,46) may also be reviewed and shared with students in preparation for student discussions (1-3,44-46). In addition, two open source links are also provided on Cre/LoxP from Jackson Labs:
- https://www.jax.org/explore-by-topic/genetic-tools/cre-lox-system
- https://jackson.jax.org/rs/444-BUH-304/images/Cre-lox_Basics_Webinar_17Mar2016.pdf
Finally, textbook readings on fate map and Cre/LoxP technology and research video on heart regeneration from HHMI (47) are also used for Cre/LoxP lesson using the following website: https://www.biointeractive.org/classroom-resources/zebrafish-heart-regeneration.
SCIENTIFIC TEACHING THEMES
Active Learning
Inclusive active teaching approaches were used to engage students during Cre/LoxP lesson (48-50). For example, group peer-discussions were used during instructor-led discussions on questions from Cre/LoxP in-class worksheet. In addition, to help clarify difficulties in understanding heart development, students participated in think-pair-share to identify terms or concepts that were unclear or confusing, and I clarified the terms and concepts using Supporting File S6. From Cre/LoxP to Fate Maps – Teaching Slides on Cell Fate Decisions During Heart Development Primer and Dr. Kimberly Tanner's articles on promoting metacognition and classroom equity (48,49). For instance, I invited our diverse background of learners that includes first-generation Native American, Latinx, and non-traditional undergraduates to share topics from the chapter readings in developmental genetics that are confusing while also maintaining a culturally-responsive and inclusive learning environment. Culturally-responsive teaching will be defined in Inclusive Teaching section of my article. In addition, after completing a cumulative exam, I encouraged undergraduates to reflect on what did not work well, and what they could change to better prepare for future exams. As I listened to each student's thought process, I thought intentionally about teaching approaches that could help each student learn the developmental genetics of Cre/LoxP, and how it can be applied to the student's research proposal. Furthermore, students applied Cre/LoxP technology into the process of designing an experimental plan on fate mapping experiment and also made predictions using Supporting File S8. From Cre/LoxP to Fate Maps – Teaching Slides on Cre LoxP Class Discussions Primer Handout.
Assessments
In order to measure student gains in attitudes related to thinking like a scientist and self-confidence in scientific concepts from developmental biology, I implemented key sections of Weston and colleagues' validated Undergraduate Research Student Self-Assessment (URSSA) survey (51). To shorten the amount of time it took to complete the survey, each student completed key sections of the Likert scale URSSA survey both midterm and at the end of the semester (Table 2A). Finally, in addition to formative assessment during in-class discussions, weekly homework and student portfolio assignments were also included to monitor student learning and to identify concepts that were unclear or confusing (52). For instance, I would quickly scan through students' responses before in-class discussions to identify unclear or confusing concepts. I then clarified these unclear or confusing concepts or terms at the start of in class discussions and linked them to learning objectives and outcomes of the class and lab. The weekly assigned homework guided students to identify unclear or confusing concepts and was used to help encourage and promote student metacognition (29,30,48).
Inclusive Teaching
In order to engage students in experimental approaches within Developmental Biology Learning Framework, a set of active learning methods was used during the Cre/LoxP Lesson: inclusive and equitable in-class group discussions, random calling, and think-pair-share (49,50). During our first in-class discussion, I use a set of integrated culturally-responsive mentoring and teaching strategies for student-centered and place-based teaching (16,18,19,21). Dr. Geneva Gay defines culturally-responsive teaching as "...using cultural knowledge, prior experiences, frames of references, and performance styles of ethnically diverse students to make learning encounters more relevant and effective for them. It teaches to and through the strengths of these students" (18). The culturally-responsive mentoring and teaching approach applies Dr. Gregory Cajete's concept of the creative process instructional model (16). Dr. Cajete mentions that "In Native American cultures, science and art are complementary dimensions of the community mind. This subtle yet profound relationship becomes apparent only when one focuses upon the processes of thought as opposed to its end products. It is, therefore, my contention that one can use art to teach science, and science to teach art, and cultural philosophy to teach both" (16). The creative cycle contains three steps: 1.) provide frame of reference for students; 2.) utilize principles, themes, and classification approaches in both Native American and Western cultural science; and 3.) integrate both Native American and Western scientific approaches. The cyclical flow allows for deeper understanding of a scientific process and broader perspective on a specific concept in science. Here, the creative process cycle starts by linking cultural relevance of a developmental process. As we explore and discuss the cross-cultural dimensions and ethics on congenital heart defects in the greater Yakima Valley, we link the affective learning dimensions and explore in-depth the process of the molecular and cellular process of developmental biology. For example, before I began discussions on Cre/LoxP and fate mapping experiments, I engaged students in a community classroom discussion on articles that highlight emerging incidences of congenital heart diseases in the homeland of Yakama Nation, as well as the greater Yakima Valley of Washington State. Upon reading these articles, students explored how topics discussed in heart development and Cre/LoxP research in zebrafish heart cell lineages are linked to providing future insights into congenital heart diseases. Once the importance of how investigating heart development is linked to the health of our community, we then began to discuss the molecular and cellular aspects of heart development and the function of Cre/LoxP technology in fate mapping experiments on the in-class worksheet.
LESSON PLAN
In order to implement the lesson plan on heart development and designing a fate map experiment using Cre/LoxP technology, I have put together the following sections: 1) Pre-class Preparation, and 2) In-Class Discussion. Table 1 provides a timeline for each section of the Cre/LoxP lesson plan.
Pre-class Preparation
Instructor Preparations Prior to In Class Discussions
To prepare for Cre/LoxP lesson, there are several aspects to consider (Table 1). First, print out in-class worksheet for Cre/LoxP in Supporting File S1. From Cre/LoxP to Fate Maps – In-Class Worksheet for Cre/LoxP Design and Fate Mapping, and review the instructor guide to in-class worksheet in Supporting File S2. From Cre/LoxP to Fate Maps – In-Class Worksheet for Cre/LoxP Design and Fate Mapping Instructor Guide and Key. Two open source links are also provided on Cre/LoxP from Jackson Labs:
- https://www.jax.org/explore-by-topic/genetic-tools/cre-lox-system
- https://jackson.jax.org/rs/444-BUH-304/images/Cre-lox_Basics_Webinar_17Mar2016.pdf
Second, I also assign homework prior to class discussions as shown in Supporting File S3. From Cre/LoxP to Fate Maps – Homework Example Assigned Prior to First Class.
Third, I also prepare Cre/LoxP model demonstration as shown in Supporting File S4. From Cre/LoxP to Fate Maps – Cre/LoxP Catalysis Hands-on Demonstration and Animation. Use Supporting File S5. From Cre/LoxP to Fate Maps – Defining "floxed" and Direction of LoxP Sites Handout, to clarify the term "floxed" that may come up during in-class discussions. These PowerPoint slides may also be shown and used as handouts during class discussions.
Finally, in the spirit of being a reflective practitioner with the mindset of a diagnostician (53), I recommend instructors to be mindful and actively listen to students' responses during in-class worksheet questions on Cre/LoxP technology and experimental design. For example, I provide clarification on molecular action of Cre recombinase. In addition, I also help students integrate molecular and cellular principles of heart development. I emphasize that students and instructor are part of a community of researchers and scholars. I recommend that instructors read Harold Modell's articles on establishing community of learners in the classroom and helping learners to learn (29,30,54).
To help instructors adapt Cre/LoxP approaches into their curricula, I have provided learning outcomes and how they are linked to themes in developmental biology (Table 1).
Student Handout Preparations
First, complete the Cre/LoxP models and printouts of in-class worksheet and homework in Supporting File S1. From Cre/LoxP to Fate Maps – In-Class Worksheet for Cre/LoxP Design and Fate Mapping and Supporting File S3. From Cre/LoxP to Fate Maps – Homework Example Assigned Prior to First Class. Next, review Supporting File S2. From Cre/LoxP to Fate Maps – In-Class Worksheet for Cre/LoxP Design and Fate Mapping Instructor Guide and Key, instructor guide to rehearse and write down key notes on "teaching scripts" prior to giving the in-class discussions. In some cases, students will send their homework via email the night before giving class, and paying attention to terms/concepts students felt were unclear or confusing can help the instructor to re-adapt the lesson plan. Finally, prepare Cre/LoxP pipe cleaner and Post-It note model for demonstrating Cre recombinase molecular action to clarify function of this key enzyme from bacteriophage in class from Supporting File S4. From Cre/LoxP to Fate Maps – Cre/LoxP Catalysis Hands-on Demonstration and Animation. Instructors may also want to include handout on defining "floxed" concept using Supporting File S5. From Cre/LoxP to Fate Maps – Defining "floxed" and Direction of LoxP Sites Handout. Examples of primer discussion PowerPoints on cell fate decisions and dominant negative approach are provided in Supporting File S6. From Cre/LoxP to Fate Maps – Teaching Slides on Cell Fate Decisions During Heart Development Primer and Supporting File S7. From Cre/LoxP to Fate Maps – Teaching Slides on Genetics and Molecular Cell Biology Primer Handout, respectively. Finally, instructors may also wish to prepare cardiac heart origami models (37). I have also provided a PowerPoint presentation for class discussions to clarify the function of Cre recombinase in Cre/LoxP applications in fluorescently labeled early progenitor cell and its descendants in Supporting File S8. From Cre/LoxP to Fate Maps – Teaching Slides on Cre LoxP Class Discussions Primer Handout.
In Class Discussions: Tips for Instructors
An integrated set of culturally-responsive teaching and mentoring approaches is used alongside Tanner's active learning approaches to provide inclusive and equitable class discussion settings (49). For instance, as mentioned in the Inclusive Teaching section, I link concepts in developmental biology with cultural relevance in the health and environment in a think-pair-share class discussion. I encourage instructors to utilize both the in-class worksheet in Supporting File S1. From Cre/LoxP to Fate Maps – In-Class Worksheet for Cre/LoxP Design and Fate Mapping and the primer Supporting File S8. From Cre/LoxP to Fate Maps – Teaching Slides on Cre LoxP Class Discussions Primer Handout to guide students not only critical thinking, but also in designing Cre/LoxP approaches for fate map experiments.
Students are engaged in think-pair-share to generate terms or concepts that were unclear or confusing in class (55-61). During these five minutes, I quickly glance through my teaching script I had jotted down the night before, and explain vague or misunderstood terms during class discussions. Alternatively, if student misconceptions are rampant, I give a short five-minute primer to provide immediate clarification of the notion of fate maps. There are two examples of conceptual difficulties. First, one conceptual difficulty is linking the cellular changes during heart development with the function of transcription factors. Another conceptual difficulty is how cell potency informs key steps in cell specification and differentiation during heart development. I use the approach for creating the five-minute primer as outlined under "Intended Audience" section. For instance, if I hear the term "cell potency" mentioned as an unclear term from the textbook readings, I integrate students' responses and link them into the theme of the developmental cell history and cell decision-making over the course of embryonic development (Table 1, Teaching Timeline). In addition, I also discuss the importance of cell fate decisions during heart development in Supporting File S6. From Cre/LoxP to Fate Maps – Teaching Slides on Cell Fate Decisions During Heart Development Primer.
Another example from molecular cell biology includes concepts of signal transduction and the role of cell receptors. To help clarify topics on genetics and cell signaling, I use an analogy of "input" and "output" cell computational device of how cells integrate ligand binding receptor and how it can affect gene expression of a cell type (e.g., atrial cardiomyocyte or ventricular cardiomyocyte). In Supporting File S7. From Cre/LoxP to Fate Maps – Teaching Slides on Genetics and Molecular Cell Biology Primer Handout, the genetics concept of dominant negative approach to inhibit cell signaling is included in the primer (62). In addition, a concept map diagram on theme of how cells "compute" information for organ function can be diagrammed from the easel board, or provided as a handout in Supporting File S7. From Cre/LoxP to Fate Maps – Teaching Slides on Genetics and Molecular Cell Biology Primer Handout. In the next section, I provide the following teaching tips for instructors to engage students during in-class discussions:
1.) Ask a question to highlight the importance of the direction and location of tissue specific promoters in Question 1 from Supporting File S1. From Cre/LoxP to Fate Maps – In-Class Worksheet for Cre/LoxP Design and Fate Mapping. Students participate in think-pair-share to answer the sharing part of this strategy. I check-in with every student, including students who do not like to speak to the whole class. During the community class discussion, I use different kinds of random calling approaches (63). For example, I randomly draw student's name from a hat. I then ask the student and their team members if they would like to contribute their team responses to our community class discussion. Another example of random calling is I close my eyes and spin my arm around like a pinwheel and then I open my eyes and ask if a randomly selected student and their team would like to contribute their response. The first question is designed to invite students to consider how location of enhancer regions of regulatory promoters is crucial for the design of Cre/LoxP genetic cassette. When fielding response, I first listen and then restate student's response. I then mention "This is what I am hearing... is that correct?" to confirm student's response. Then, after completing and writing down students' response, and debriefing best response to the question, I ask, "Does it make sense?"
On occasion, students may propose another version of tissue specific promoters for question one. For instance, students may wonder if different combinations of silencers and enhancer sequences within regulatory promoters may be required for appropriate tissue-specific expression of Cre recombinase. When students ask if a set of silencer and enhancer sequences are required for tissue-specific expression, I illustrate how there can be different types of arrangements (either enhancers or silencer regulatory promoters) for appropriate tissue-specific expression. These themes will also be important in question three and four in the in-class discussion questions.
2.) After debriefing question one, prompt students to begin question two. Use Supporting File 7 to further clarify question two. In addition, students may benefit from opportunities to engage in tactile and auditory learning. I utilize the Cre/LoxP model to act out Cre recombinase catalysis in Supporting File S4. From Cre/LoxP to Fate Maps – Cre/LoxP Catalysis Hands-on Demonstration and Animation. I design LoxP sites with smooth post-it note paper, and represent paper clip as "floxed" stop codon. I also record a podcast of our community in-class discussions. During the podcast, I use specific phrases to describe the specific location of each LoxP site relative to the "floxed" stop codon. In addition, I describe what I see for the Cre/LoxP model during each step of the Cre recombinase catalysis. For instance, I describe the representation of LoxP sites by saying "two yellow triangle-folded post-its represent the LoxP sites in a 5' to 3' direction." During the in-class discussions, there will be a series of clarification of terms or concepts from Cre/LoxP technology and in heart development. In order to clarify key steps during zebrafish heart development, use the step-by-step instructor guide to clarifying heart development and also available on "Heart Origami" at the online website http://www.robertkaoscied.com/resources/ (37).
Another highlighted example in Supporting File S8. From Cre/LoxP to Fate Maps – Teaching Slides on Cre LoxP Class Discussions Primer Handout is a student-centered discussion to encourage students to predict what happens before and after Cre recombination. During this time, I would also clarify the function of Cre recombinase using the pipe cleaner/post-it model of Cre/LoxP mechanism made during pre-class preparation. Alternatively, I encourage instructors to give out these models to have student groups develop their models of Cre recombinase function and how to design Cre/LoxP cassettes used to address key questions in cell lineage studies. Project an appropriate figure from the textbook and use a dry-erase board and the in-class worksheet to make this discussion interactive. For the next two class periods during the week, students work in their groups and complete all questions in the in-class worksheet related to experimental design. Use Supporting File S2. From Cre/LoxP to Fate Maps – In-Class Worksheet for Cre/LoxP Design and Fate Mapping Instructor Guide and Key as an instructor guide.
3.) Upon completion of discussion questions one and two, I utilize supplemental figure seven handout to clarify key function of LoxP sites, Cre recombinase catalysis, Rosa26 (mouse) or ubiquitin (zebrafish) promoter, and tissue specific promoter. The handout helps engage students in critical thinking and making predictions. The next set of questions focus on how Cre/LoxP approaches are applied into experimental design in fate mapping experiments.
4.) The last discussion question helps students design and revise experimental approaches in fate mapping experiments. One misconception that typically comes up during discussions is students may switch the wild-type control group and the experimental group. I use this as an opportunity to clarify the purpose of the controls, and return back to the research question at hand. I emphasize how the control and experimental groups are used to address the specific research question of the fate mapping experiment. In addition, a helpful resource from Journal of Visualized Experiments (JoVE) on wholemount RNA in situ hybridization may also be used to supplement textbook readings on how RNA in situ hybridizations are utilized in experimental design and to help verify specificity of Cre/LoxP approach in fate mapping experiments (64). A link to the RNA in situ hybridization resource can be found here and requires library access:
https://www.jove.com/science-education/5330/whole-mount-in-situ-hybridization
The Whole-Mount In Situ Hybridization link helps add another resource to compliment in-class discussions on experimental design in developmental biology.
In summary, these inclusive in-class discussions and primers help students to think like scientists through a guided approach in fate map experimental design using Cre/LoxP technology.
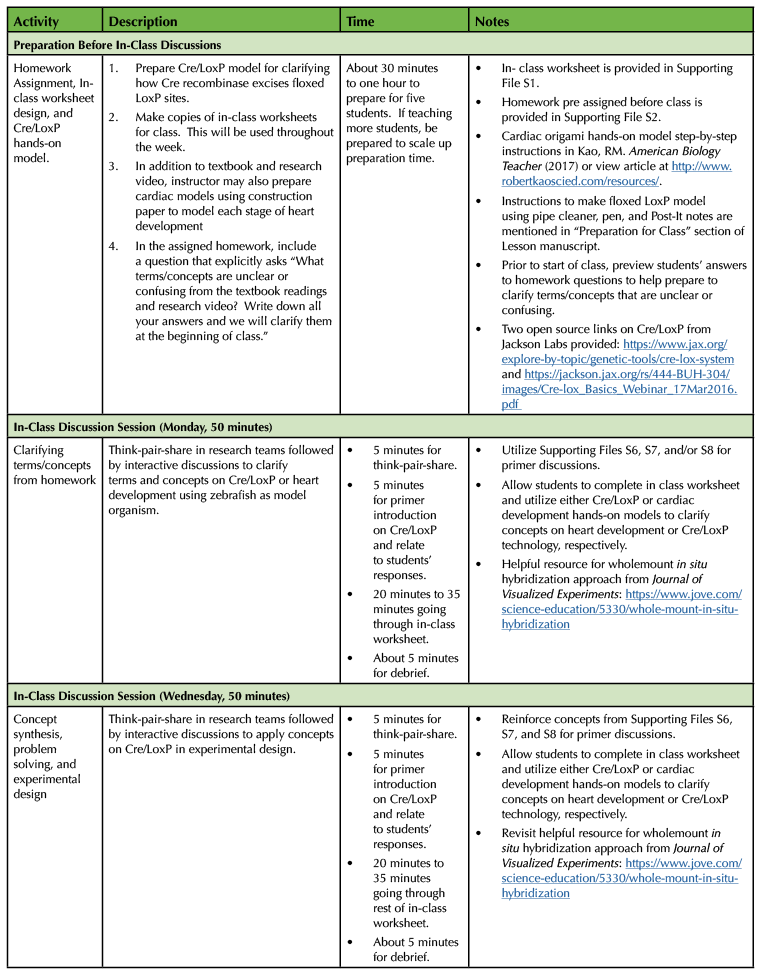
Table 1. Lesson plan timeline for Designing Fate Map Experiment using Cre/LoxP technology.
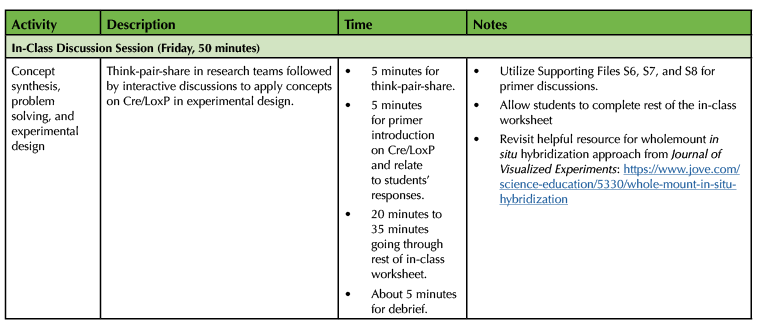
Table 1. Lesson plan timeline for Designing Fate Map Experiment using Cre/LoxP technology (continued).
TEACHING DISCUSSION
Student Reaction to Lesson
Over the past two years, undergraduate students have provided formative feedback and found the Cre/LoxP demonstration approach a useful learning tool to help clarify the molecular action of Cre recombinase from Essential Developmental Biology textbook (45). Specifically, in the fall 2017 semester, students reported significant gains in thinking like a scientist, as well as gains in attitudes related to being a scientist and being part of a community (Table 2). For example, undergraduates felt that they gained problem-solving skills, confidence in their ability to contribute to science, and confidence in their ability to do well in future science courses. Due to time restrictions during the semester, a subset of dimensions from the URSSA survey was used to monitor students' attitudes in thinking like a scientist and self-confidence in developmental biology concepts (Table 2). Furthermore, first-generation Native American, Latinx, and non-traditional undergraduates were taught in the developmental biology class and lab settings. Eight of the eleven total undergraduates have had at least one summer research experience before taking developmental biology. For larger classroom sizes, I recommend using culturally-responsive teaching and mentoring approaches discussed in earlier Active Learning section by Drs. Megan Bang and Gregory Cajete and for instructors and teaching assistants to help facilitate culturally-responsive teaching along with active learning. In addition, students described integration of core concepts in developmental biology along with how to think like a scientist (Table 3). The question that was asked in final reflection at the end of the course was the following: After reading the learner outcomes for this course, how do they connect with your life, other coursework, and your planned long-term career goal(s)? Write down all your answers. The Developmental Biology Course and Lab learner outcomes are listed in Table 1 and are linked to Developmental Biology Topics in the Society for Developmental Biology. Over the course of the semester, students applied Cre/LoxP approach for fate mapping into their own original research proposals. The infusion of the short primer combined with in-class discussions based on clarifying terms and concepts from homework on heart development or Cre/LoxP approach has proven to be useful for student-centered classroom discussions in my classroom. Finally, as shown in Table 2, I used a subset of dimensions on thinking like a scientist from the validated URSSA survey (49) to monitor students' attitudes in thinking like a scientist after they were engaged in designing Cre/LoxP approaches in fate mapping experiments.
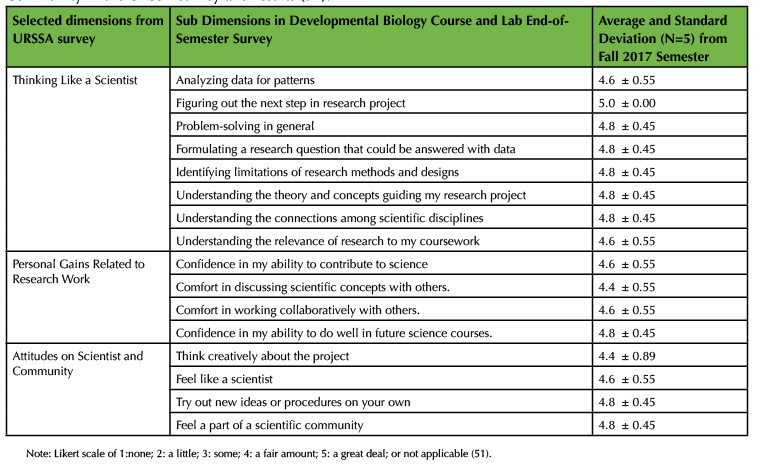
Table 2. Selected dimensions on Thinking Like a Scientist, Personal Gains, and Attitudes on Scientist and Community in the URSSA survey and results (51).

Table 3. Example of end-of-semester anonymous student reflections in developmental biology.
Suggestions for Improvements and Adaptations
The Cre/LoxP technology can be adapted to genetics class to illustrate how positioning the LoxP sites can lead to translocation, deletion, or inversion of the flanked DNA strands with LoxP recombination sites. This in-class discussion approach can also be reemphasized in other contexts later in the course. For instance, the Cre/LoxP approach can used to overexpress a dominant negative construct to test if Fibroblast growth factor (FGF) signaling is required for a specific cellular process during lung or kidney development. In addition, Cre/LoxP genetic approaches may also be extended to invertebrates, such as C. elegans (65). The in-class worksheet discussion format may be adjusted into a journal-club-style format to engage students in analyzing and evaluating an original research paper. For instance, Rinkevich and colleagues used the Cre/LoxP technology as a fate mapping tool to identify the Engrailed-1 dermal fibroblast lineage to probe the cellular origins of scar tissue formation (66). In the future, I will plan to incorporate Round and Campbell's Figure Facts approach to engage students in an original Cre/LoxP fate mapping research paper discussions in development and regenerative medicine (67). Finally, combining Cre/LoxP genetic approaches with CRISPR-Cas9 genome editing methods could be used within the lab setting as a semester or year-long team research experience. In addition, the Cre/LoxP approach can be incorporated with fluorescent reporters of the cell cycle or for labeling individual neurons using Brainbow (6,8-11) . The in-class worksheet may be adapted into figure facts analysis of a cutting-edge research paper using genetic approach, such as Cre/LoxP, to unravel the cellular histories each cell type during development and disease states. Finally, future courses may also adopt usage of tamoxifen-inducible recombinase approaches into an internship or capstone class.
SUPPORTING MATERIALS
- Supporting File S1. From Cre/LoxP to Fate Maps – In-Class Worksheet for Cre/LoxP Design and Fate Mapping.
- Supporting File S2. From Cre/LoxP to Fate Maps – In-Class Worksheet for Cre/LoxP Design and Fate Mapping Instructor Guide and Key.
- Supporting File S3. From Cre/LoxP to Fate Maps – Homework Example Assigned Prior to First Class.
- Supporting File S4. From Cre/LoxP to Fate Maps – Cre/LoxP Catalysis Hands-on Demonstration and Animation.
- Supporting File S5. From Cre/LoxP to Fate Maps – Defining "floxed" and Direction of LoxP Sites Handout.
- Supporting File S6. From Cre/LoxP to Fate Maps – Teaching Slides on Cell Fate Decisions During Heart Development Primer.
- Supporting File S7. From Cre/LoxP to Fate Maps – Teaching Slides on Genetics and Molecular Cell Biology Primer Handout.
- Supporting File S8. From Cre/LoxP to Fate Maps – Teaching Slides on Cre LoxP Class Discussions Primer Handout.
ACKNOWLEDGMENTS
I wish to thank anonymous reviewers and colleague Dr. Rebecca M. Price for their input and suggestions, as well as the Native Education Certification program at University of Washington, support from Society for Developmental Biology education grant, and Heritage University's Center for Intercultural Learning and Teaching. I also want to thank Drs. Jihong Bai, Suzanne Lee, Emily Wiley, and Lina Dahlberg for discussions on authentic course-based undergraduate research ideas. Anonymous students' reflections and self-assessment modified URSSA survey used in this study were approved by the Institutional Review Board of Heritage University.
References
- Le Douarin NM. 1988. The Claude Bernard Lecture, 1987 - Embryonic chimeras: a tool for studying the development of the nervous and immune systems. Proc R Soc London B 235:1-17.
- Spemann H. 1938. Embryonic Development and Induction. Yale University Press, New Haven.
- Hayashi S, McMahon, A. P. 2002. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244:305-18.
- Kao RM, Vasilyev A, Miyawaki A, Drummond IA, McMahon AP. 2012. Invasion of distal nephron precursors associates with tubular interconnection during nephrogenesis. J Am Soc Nephrol 23:1682-90.
- Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. 2008. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3:169-81.
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. 2007. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450:56-62.
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. 2007. A global double-fluorescent Cre reporter mouse. Genesis 45:593-605.
- Pan YA, Freundlich T, Weissman TA, Schoppik D, Wang XC, Zimmerman S, Ciruna B, Sanes JR, Lichtman JW, Schier AF. 2013. Zebrabow: multispectral cell labeling for cell tracing and lineage analysis in zebrafish. Development 140:2835-46.
- Pan YA, Livet J, Sanes JR, Lichtman JW, Schier AF. 2011. Multicolor Brainbow imaging in zebrafish. Cold Spring Harb Protoc 2011:pdb prot5546.
- Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, Imamura T, Ogawa M, Masai H, Miyawaki A. 2008. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132:487-98.
- Sakaue-Sawano A, Miyawaki A. 2014. Visualizing spatiotemporal dynamics of multicellular cell-cycle progressions with fucci technology. Cold Spring Harb Protoc 2014.
- Jakob SA, W. J. 2019. Make It Stick: Teaching Gene Targeting with Ribbons and Fastners. CourseSource doi:https://doi.org/10.24918/cs.2019.9.
- Aikenhead GS. 1997. Toward a First Nations Cross-Cultural Science and Technology Curriculum. Science Education 81.
- Bang M. 2015. Culture, Learning, and Development and the Natural World: The Influences of Situative Perspectives. Educational Psychologist 50:220-233.
- Bangera G, and Brownell, Sara E. 2017. Course-Based Undergraduate Research Experiences Can Make Scientific Research More Inclusive. CBE Life Sciences Education 13:602-606.
- Cajete GA. 1999. Ingniting the Sparkle: An Indigenous Science Education Model. Kivai Press, Skyand, NC.
- Clutterbuck DR, Belle Rose. 2002. Mentoring and Diversity. .
- Gay G. 2010. Culturally Responsive Teaching: Theory, Research, and Practice. Teachers College Press, New York and London.
- Kao RM. 2018 Helping Students SOAR: Quizfolio Tips to Engauge First Generation Underrepresented Minority Undergraduates in Scientific Inquiry. The American Biology Teacher 80:228-234.
- Rendon LI. 1994. Validating Culturally Diverse Students: Toward a New Model of Learning and Student Development. Innovative Higher Education 19:33-51.
- Ross KA. 2016. Breakthrough Strategies: Classroom-Based Practices to Support New Majority College Students. Harvard Education Press, Cambridge.
- Tanner KA, Deborah. 2007. Cultural Competence in the College Biology Classroom. CBE Life Sciences Education 6:251-258.
- Valdez R. 2016. Relationships between First Generation College Students and Faculty: A Case Study of a Small Rural Private University. Doctor of Education. University of Washington.
- Auchincloss LC, Laursen SL, Branchaw JL, Eagan K, Graham M, Hanauer DI, Lawrie G, McLinn CM, Pelaez N, Rowland S, Towns M, Trautmann NM, Varma-Nelson P, Weston TJ, Dolan EL. 2014. Assessment of course-based undergraduate research experiences: a meeting report. CBE Life Sci Educ 13:29-40.
- Bozzone DM. 2000. Investigating Phagocytosis in Tetrahymena: An Experimental System Suitable for Introductory & Advanced Instruction. The American Biology Teacher 62:136-139.
- Chiappetta EA, April. 2004. Inquiry-based Instruction. The Science Teacher 71:46-50.
- Deffit SN, Neff, Cori, Kowalski, Jennifer R. . 2017. Exploring Caenorhabditis elegans Behavior: An Inquiry-Based Laboratory Module for Middle or High School Students. The American Biology Teacher 79:661-667.
- McLaughlin JS, Patel, Mit A. . 2017. An Authentic Research Experience for Undergraduates in the Developmental Biology and Physiology Laboratory Using the Chick Embryonic Heart. The American Biology Teacher 79:645-653.
- Michael JA, and Modell, H.I. 2003. Active learning in secondary and college science classrooms: a working model for helping the learner to learn. Lawrence Erlbaum Associates, Mahwah, NJ.
- Modell H, Michael, J., and Wenderoth, M.P. 2005. Helping the learner to learn: the role of uncovering misconceptions. The American Biology Teacher 67:20-26.
- Quitadamo IJ, Kurtz, Martha J. 2007. Learning to Improve: Using Writing to Increase Critical Thinking Performance in General Education Biology. CBE - Life Sciences Education 6:140-154.
- Stone EM. 2014. Guiding Students to Develop an Understanding of Scientific Inquiry: A Science Skills Approach to Instruction and Assessment CBE - Life Sciences Education 13.
- Accorsi A, Williams, Monique M. , Ross, Eric J., Robb, Sofia M. C., Elliott, Sarah A., Tu, Kimberly C. , Sánchez Alvarado, Alejandro 2017. Hands-On Classroom Activities for Exploring Regeneration and Stem Cell Biology with Planarians. The American Biology Teacher 79:208-223.
- Darnell D. 2008-2016. The Origami Embryo.
- Tosney KW. 2004. Origami Embryo.
- Tosney KW. 2008-2016. The Origami Embryo: A Model of Early Organogenesis.
- Kao RM. 2017. Heart Origami: Student Activities for Exploring Principles of Cardiac Development. The American Biology Teacher 79:417-420.
- Handelsman J, Miller, S., and Pfund, C. 2007. Scientific Teaching. W. H. Freeman and Company, New York, NY.
- 2011. Vision and Change in Undergraduate Biology Education: A Call to Action. 1-100.
- Hiatt A, Davis GK, Trujillo C, Terry M, French DP, Price RM, Perez KE. 2013. Getting to evo-devo: concepts and challenges for students learning evolutionary developmental biology. CBE Life Sci Educ 12:494-508.
- Kao RM, Rurik JG, Farr GH, 3rd, Dong XR, Majesky MW, Maves L. 2015. Pbx4 is Required for the Temporal Onset of Zebrafish Myocardial Differentiation. J Dev Biol 3:93-111.
- Saldaña J. 2016. The Coding Manual for Qualitative Researchers. Ashford Colour Press Ltd., Great Britain.
- O'Connor P. 2007. Reflection and Refraction--The Dimpled Mirror of Process Drama: How Process Drama Assists People to Reflect on Their Attitudes and Behaviors Associated With Mental Illness. Youth Theatre Journal 21:1-11.
- Jensen P, Dymecki SM. 2014. Essentials of recombinase-based genetic fate mapping in mice. Methods Mol Biol 1092:437-54.
- Slack J, M.W. 2014. Essential Developmental Biology, 3rd edition ed. John Wiley & Sons, Ltd.
- Biology SfD. Quail-Chick Chimeras.
- Porch B, Liu, Dennis, Poss, Ken, Amagai, Satoshi. 2006. Zebrafish Heart Regeneration.
- Tanner KD. 2012. Promoting student metacognition. CBE Life Sci Educ 11:113-20.
- Tanner KD. 2013. Structure Matters: Twenty-One Teaching Strategies to Promote Student Engagement and Cultivate Classroom Equity. CBE Life Sciences Education 12:322-331.
- Dewsbury B, Brame CJ. 2019. Inclusive Teaching. CBE Life Sci Educ 18:fe2.
- Weston TJ, and Laursen, S. L. 2015. The Undergraduate Research Student Self-Assessment (URSSA): Validation for use in program evaluation. CBE-Life Sciences Education 14.
- Chappuis J. 2014. Seven strategies of assessment for learning, 2nd edition ed. Pearson Education, Upper Saddle River, NJ.
- Schon DA. 1984. The Reflective Practitioner: How Professionals Think in Action. Basic Books.
- Modell H. 2017. What They Neglected to Tell You About Classroom Practice in Graduate School. The Physiologist 60:137, 144-147.
- Allen D, and Tanner, K. 2002. Questions about questions. Cell Biol Educ 1:63-7.
- Chi M, de Leeuw, N., Chiu, MH., LaVancher, C. 1994. Eliciting self explanations improves understanding. Cogn Sci 18:439-477.
- Clarke J. 1994. Pieces of the puzzle: the jigsaw method, p 34-50. In Sharan S (ed), Handbook of Cooperative Learning Methods. Greenwood Press, Westport, CT.
- Cohen E. 1994. Designing Groupwork: Strategies for the Heterogenous Classroom. Teachers College Press, New York.
- Lyman F. 1981. The responsive classroom discussion: the inclusion of all students. In Anderson A (ed), Mainstreaming Digest. University of Maryland, College Park.
- Smith MK, Wood, W.B., Adams, W.K., Wieman, C., Knight, J.K., Guild, N., Su, T.T. 2009. Why peer discussion improves student performance on in-class concept questions. Science 323:122-124.
- Tanner KD. 2009. Talking to learn: why biology students should be talking in classrooms and how to make it happen. CBE Life Sciences Education 8:89-94.
- Herskowitz I. 1987. Functional inactivation of genes by dominant negative mutations. Nature 329:219-222.
- Tanner KD. 2011. Moving theory into practice: a reflection on teaching a large introductory biology course for majors. CBE Life Sciences Education 10:112-122.
- JoVE Science Education Database Developmental Biology. 2019. Whole-Mount In Situ Hybridization. Journal of Visualized Experiments.
- Kage-Nakadai E, Imae, Rieko, Suehiro, Yuji, Yoshina, Sawako, Hori, Sayaka, and Mitani, Shohei. 2014. A conditional knockout toolkit for Caenorhabditis elegans Based on the Cre/loxP Recombination. PLoS One 9.
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, Weissman IL, Longaker MT. 2015. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348:aaa2151.
- Round JE, Campbell AM. 2013. Figure facts: encouraging undergraduates to take a data-centered approach to reading primary literature. CBE Life Sci Educ 12:39-46.
Article Files
Login to access supporting documents
From Cre/LoxP to Fate Maps: Inclusive and Equitable Approaches for Engaging Developmental Biology Students in Experimental Desig(PDF | 205 KB)
S1. From CreLoxP to Fate Maps In-Class Worksheet for CreLoxP Design and Fate Mapping.docx(DOCX | 77 KB)
S2. From CreLoxP to Fate Maps In-Class Worksheet for CreLoxP Design and Fate Mapping Instructor Guide and Key.docx(DOCX | 77 KB)
S3. From CreLoxP to Fate Maps Homework Example Assigned Prior to First Class.docx(DOCX | 17 KB)
S4. From CreLoxP to Fate Maps CreLoxP Catalysis Hands-on Demonstration and Animation.pptx(PPTX | 1 MB)
S5. From CreLoxP to Fate Maps Defining floxed and Direction of LoxP Sites Handout.pptx(PPTX | 38 KB)
S6. From CreLoxP to Fate Maps Teaching Slides on Cell Fate Decisions During Heart Development Primer.pptx(PPTX | 416 KB)
S7. From CreLoxP to Fate Maps Teaching Slides on Genetics and Molecular Cell Biology Primer Handout.pptx(PPTX | 88 KB)
S8. From CreLoxP to Fate Maps Teaching Slides on Cre LoxP Class Discussions Primer Handout.pptx(PPTX | 66 KB)
- License terms

Comments
Comments
1 Like
Caitlin Hayes @ on (Edited: @ on )
Posting these additional resources on behalf of the author!
Two Native Indigenous education tools on culturally-responsive teaching:
Indigenous STEAM – Indigenous science education resources
Indigenous Education Tools (en-US)
Copy link Report abuse