Exploring the Lytic and Lysogenic Life Cycles of Bacteriophages
Editor: Sue Merkel
Published online:
Abstract
The goal of this lesson is to introduce students to the lytic and lysogenic cycles of T4 and lambda bacteriophages, respectively, using student-centered pedagogies. Bacteriophages are viruses that infect bacteria and are either virulent or temperate; virulent phages can only undergo the lytic cycle, which results in death of the host cell, while temperate phages can undergo either the lytic or lysogenic cycle, the latter of which results in the long-term association between host and virus. Bacteriophages significantly affect bacterial population in nature and are even attractive therapeutic interventions for some bacterial infections in humans. Therefore, this lesson was designed to educate students about the structure and function of bacteriophages and how viral infections can impact bacterial populations. The learning goals of this lesson are to understand how the lytic and lysogenic cycles of bacteriophages vary, affect bacterial growth, and are dictated by their unique genomes. To this end, the lesson incorporates a homework assignment for students before class, a series of in-class activities and critical thinking scenarios, and a homework assignment after class. This lesson was successfully employed in an upper-level undergraduate virology course for biology majors. Students were enthusiastic and expressed thoughtful and educated ideas during class discussion and through answers to open-ended questions. Taken together, this lesson provides an interactive and student-centered approach to studying the form and function of bacteriophages, the most abundant organisms in the biosphere.
Primary image: Bacteriophage invasion: Image portrays a bacteriophage infecting a host bacterium as the first step of its replication cycle.
Citation
Joy JP. 2021. Exploring the lytic and lysogenic life cycles of bacteriophages. CourseSource. https://doi.org/10.24918/cs.2021.6
Society Learning Goals
Microbiology
- Cell Structure and Function
- How are replication cycles of viruses (lytic and lysogenic) different among viruses and how are they determined by their unique structures and genomes?
Lesson Learning Goals
Students will:
- Understand the differences between a lytic and lysogenic bacteriophage life cycle.
- Understand the role of bacteriophages as therapeutic agents in humans based on a medical case of bacterial cystic fibrosis.
- Compare and contrast the different life cycles of bacteriophages.
- Understand how temperate and virulent phages affect bacterial growth.
- Predict outcomes of hypothetical laboratory experiments.
- Interpret graphical representations of results/data from hypothetical qPCR and plaque assay experiments.
- Understand how replication cycles of lytic and lysogenic viruses differ among viruses.
- Understand how replication cycles are determined based on viral structures and genomes.
Lesson Learning Objectives
Students will be able to:
- Label the key parts of a bacteriophage.
- Arrange the steps of lytic and lysogenic viral infections by bacteriophage.
- Predict the replication cycle of a virus based on the genes it carries.
- Compare and contrast the replication cycles of bacteriophages T4 and lambda.
- Compare and contrast the differences between lysogenic and latent viral infections.
- Interpret and analyze graphical representations of qPCR and plaque assay data.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
Bacteriophages (phages) are viruses that infect bacteria and have been detected in nature wherever their host microbes exist (1). In fact, phages outnumber bacteria by more than ten-fold, on the order of 1031 phage particles on the planet (2). Two types of phages exist; virulent phages are those that lead to lysis of the host cell, termed the lytic cycle, and temperate phages are those that can either lead to host cell lysis or integration of viral DNA into the bacterial host cell genome, termed the lysogenic cycle. Unlike phages that enter the lytic cycle, phages that enter the lysogenic cycle maintain a long-term association with their host, meaning that the phage genome becomes integrated into the host cell genome and remains a part of the host cell until induced to enter the lytic cycle. Integrated viral DNA, known as a prophage, can dramatically alter host cell biology. For example, prophages can modulate gene expression and function of host cells and/or change host cell physiology via novel functions, such as develop immunity to further phage infection (3). A prophage is propagated by host cell division and the lysogenic state is maintained by the repression of the phage lytic genes. A stressor, such as DNA damage or nutrient deprivation, can result in a switch from the lysogenic to lytic cycle. This switch leads to excision of the phage genome from the bacterial chromosome and the subsequent activation of phage genes that promote its replication and host cell killing (4).
Many phage related wet-lab activities have been described for undergraduate biology courses (5,6). Indeed, the importance of studying phages among undergraduates is well understood and appreciated, as demonstrated by the Phage Hunters Integrating Research and Education (PHIRE) program at the University of Pittsburgh, which was developed in 2002 by Graham Hatfull and colleagues, and has since been expanded nationwide to the SEA-PHAGES program (7,8). This program provides undergraduates and high school students with the opportunity to isolate novel bacteriophages, sequence their genomes, annotate them, and analyze them from a comparative genomics perspective. While these activities are invaluable for teaching lab techniques in the study of phages, "dry" lab lessons are an excellent alternative in cases where there are limited resources, or the course is a seminar or lecture with no accompanying lab. The current lesson was developed, in part, to provide students with the skill set to analyze lab-generated, albeit hypothetical, data and relate it to the principles of lytic and lysogenic cycles of phages.
This lesson introduces two of the most well-studied and well-characterized bacteriophages, T4 and lambda (λ), as examples of lytic and temperate phages, respectively, that infect Escherichia coli. Students will engage in active learning strategies to better understand the structure and function of phage genomes. Many students in the course are interested in a career in the biomedical sciences, so an active effort was made to relate these concepts to human health. Thus, lysogenic and latent viral infections, such as Herpes Simplex Virus (HSV), are compared in this lesson. Further, students are exposed to the use of bacteriophages as treatment against bacterial infections in humans (9) as part of a pre-classwork assignment to arouse interest in students.
The learning goals were aligned with core competencies "ability to apply the process of science" and "ability to use quantitative reasoning", as outlined in Vision and Change (10). Further, the activity addresses the core concept of "structure and function" by presenting how the structure and genome organization of a bacteriophage impact its functional outcome and ability to undergo the lytic and/or lysogenic cycle.
Active learning increases student participation, motivation, and academic performance compared to traditional lecturing (11). There are several different student-centered pedagogies used in this lesson, which is intended to foster a more inclusive environment for all students. Throughout the class period, students work independently, in small groups, and as a class, to learn and reinforce the concepts. Students work through a strip sequence, critical thinking questions, and the interpretation of graphical representations of data. This exercise strongly engages students to think critically about the replication cycles of bacteriophages and apply their knowledge towards interpreting data and making logical conclusions.
Intended Audience
This lesson was designed for upper-level undergraduates and graduate students at a mid-sized private University. Most students in the course were biological science majors and had a prerequisite of introductory biology and genetics. However, this lesson can be modified for an introductory course by removing Section 3 (Supporting File S2c. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 3) and modifying Section 4 of the in-class worksheet (S2d. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 4). Alternatively, the lesson can be modified for a more advanced course by expanding Section 3 of the in-class worksheet (S2c. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 3) to include a modeling or simulation exercise (see Teaching Discussion).
Required Learning Time
The in-class time for this activity was one 90-minute class period and approximately 1 hour outside of class in two 30-minute segments, which are completed as homework assignments.
Prerequisite Student Knowledge
Students should be comfortable with the use of technology in the classroom and be able to connect to the internet on their laptops or other electronic devices that support the use of a text editing software and access a learning management system to which the instructor uploads the assignments and submission folders. As the assignments are electronic via Microsoft Word, students should also be familiar with how to use basic features in this application. If students do not have their own internet-connected devices, a hard copy of the assignment should be provided by the instructor and subsequently collected for grading when students have completed them. In cases where students only have a smart phone, the instructor may opt to have students take a photo of the assignment and email it to the instructor. Alternatively, if a computer lab is available and accessible for the class, it is recommended that the instructor utilize it for this lesson.
This lesson is designed for science majors, so basic scientific knowledge is required. Students should have some background knowledge in general biology, genetics, and methods in virology. Concepts with which students should be familiar before this lesson: Central Dogma of Molecular Biology (DNA ➝ mRNA ➝ protein), transcription and translation, bacterial cell structure, cell division, and simple feedback pathway modeling. Students should be knowledgeable about the principles of plaque assays and quantitative polymerase chain reaction (qPCR) as well as the interpretation of data derived from these methods, though it is not required that students perform these techniques for this lesson.
The following references are helpful sources of background content knowledge for students:
McGraw Hill Prescott's Microbiology 11e. Chapter 26.2 (pp. 596-605). This reference provides an overview of the lytic and lysogenic cycles, using T4 and lambda phages as examples, respectively. It provides detailed information on the life cycles of both phages, including phage adsorption and entry, DNA replication, phage assembly, and host cell lysis. Further, this reference details the decision-making process for establishing lysogeny or the lytic cycle as well genome organization of the lambda phage and how this impacts its function.
Video tutorial: https://www.jove.com/v/10514/plaque-assay-method-to-determine-viral-titer-as-plaque-forming-units. This video tutorial provides a summary of the principle and protocol of plaque assays. This is useful for the interpretation of plaque assay data in Section 4 of the in-class worksheet (S2d. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 4).
Kralik, P. and M. Ricchi (2017). "A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything." Front Microbiol 8: 108. This article provides an introduction to qPCR and its use in detecting and quantifying microbes and viruses. This is useful for the interpretation of qPCR in Section 4 of the in-class worksheet (S2d. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 4).
Prerequisite Teacher Knowledge
Instructors should have a basic understanding of basic cell biology of bacteria, prokaryotic transcription and translation, bacteriophage structure and replication cycles, latent viral infections, and principles of virology methods.
The following references are helpful sources of background content knowledge:
Video: https://www.youtube.com/watch?v=hFwA0aBX5bE. This short video is a rudimentary and concise introduction to the lytic and lysogenic replication cycles. Though it does not provide much detail, it can be useful for instructors who would like a foundational video.
Video: https://www.youtube.com/watch?v=J4BN4dARpio. This short video provides a more in-depth look into the lytic and lysogenic replication cycles compared to the previous one. It superficially covers the steps of the replication cycles, though it does not discuss phage structure or genome organization.
McGraw Hill Prescott's Microbiology 11e. Chapter 26.2 (pp. 596-605). This reference provides an overview of the lytic and lysogenic cycles, using T4 and lambda phages as examples, respectively. It provides detailed information on the life cycles of both phages, including phage adsorption and entry, DNA replication, phage assembly, and host cell lysis. Further, this reference details the decision-making process for establishing lysogeny or the lytic cycle as well genome organization of the lambda phage and how this impacts its function.
Howard-Varona, C., et al. (2017). "Lysogeny in nature: mechanisms, impact and ecology of temperate phages." ISME J 11(7): 1511-1520. This reference highlights concepts about lysogeny, including its importance, benefits, and consequences. This is an interesting read to better understand the rationale of lysogens in the environment and how temperate phages affect complex ecological communities. Though not directly relevant to the lesson, this is valuable background knowledge that provides context for the role of lysogeny in nature.
Video: https://www.jove.com/v/10514/plaque-assay-method-to-determine-viral-titer-as-plaque-forming-units. This video tutorial provides a summary of the principle and protocol of plaque assays. This is useful for the interpretation of plaque assay data in Section 4 of the in-class worksheet (S2d. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 4).
Kralik, P. and M. Ricchi (2017). "A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything." Front Microbiol 8: 108. This article provides an introduction to qPCR and its use in detecting and quantifying microbes and viruses. This is useful for the interpretation of qPCR in Section 4 of the in-class worksheet (S2d. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 4).
SCIENTIFIC TEACHING THEMES
Active Learning
This lesson uses several strategies for students to actively engage in learning the concepts, including random call, a short interactive lecture, groupwork, think-pair-share, whole class discussion, and small group discussion.
Before class, students actively engage in a timed homework assignment to prepare for class (S1. Lytic and lysogenic – Bacteriophages in Medicine Pre-Class Homework Assignment). In class, students initially work in pairs to label the parts of a bacteriophage (S2a. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 1). Subsequently, the instructor facilitates a short and interactive lecture (S3. Lytic and lysogenic – In-Class Lecture); the interactive portion is mediated by critical thinking questions posed to the class, which should elicit whole class discussion. At the end of the lecture, students split into small groups and each group works together to complete a strip sequence activity (S4. Lytic and lysogenic – In-Class Strip Sequence). During this activity, each group competes against one another to accurately complete a puzzle that links together the lytic and lysogenic cycles. The competition is not timed, but the first group to accurately complete the puzzle could win a prize, should the instructor choose to distribute one.
The next activity in the lesson utilizes think-pair-share to solve critical thinking problems. Think-pair-share is a collaborative learning technique that requires students to individually think about a topic (think), share their ideas with a partner (pair), and finally discuss their insights with the class (share) (12,13). For the remainder of class, students work in small groups to apply their knowledge and interpret graphical representations of data (S2d. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 4). The use of random call throughout the lesson is suggested as it has been shown to improve the participation gap between genders in a classroom setting (14). To avoid feelings of anxiety among students during random call, it is recommended that the instructor lead students to the correct answer by asking a series of simpler questions. Further, if students give an inaccurate response, instructors should thank students for bringing up an important opportunity for clarification. By doing so, students are likely to be more comfortable in a learning environment that welcomes active participation, even when students give the wrong answers.
Assessment
Students complete the multiple-choice homework assignment (S1. Lytic and lysogenic – Bacteriophages in Medicine Pre-Class Homework Assignment) and receive their grades before class. In class, students have many opportunities for self-evaluation as the problems within the lesson are intermittently reviewed. Discussions within small groups provide students with opportunities for formative self-assessment. Formative assessment by the instructor occurs via interaction with each small group and the large class discussion. The instructor should circulate the room to be available for questions and to provide immediate feedback where required. Depending on class size, the instructor should ensure that sufficient time is allocated for interaction with each group. If possible, assistance from a teaching assistant would be helpful in cases of large class sizes.
For summative assessment, instructors should read the answers for all sections of the in-class and post-class worksheets to ensure that each student has met the learning objectives. Section 1 of the in-class worksheet (S2a. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 1) addresses the learning goal "label key parts of a bacteriophage". During the strip sequence activity, students will arrange steps of the lytic and lysogenic viral infections by bacteriophage. The learning goals "compare and contrast replication cycles of T4 and lambda", "predict replication cycle of virus based on the genes it carries", and "interpret and analyze graphical representations of qPCR and plaque assay data" are addressed in Sections 2, 3, and 4 of the in-class worksheet, respectively. Finally, the learning goal "compare and contrast the differences between lysogenic and latent viral infections" is addressed in the post-class worksheet (S5. Lytic and lysogenic – Post-class Homework Assignment). Sufficient feedback should be provided to students by the instructor on the post-class worksheet as the content is not discussed in a follow-up class, unless the instructor chooses to do so. In this course, students are given a participation grade, which is based on thoughtful contributions during discussions in class and active engagement in groupwork. Students who prefer to take more time to complete sections of the worksheet are welcome to submit them online up to 24 hours after the end of class.
Throughout the class period, students are prompted to submit completed sections of the assignment on the learning management system. This facilitates the grading process and is more likely to keep a uniform pace throughout the class despite different levels of student achievement. Each submitted section of the lesson is evaluated for accuracy and effort by the instructor after class.
Inclusive Teaching
This lesson was designed to involve all students in the learning process. Thus, the lesson includes several strategies, including think-pair-share, small group discussions, and a strip sequence, which allows for students who favor different learning approaches to engage with the concepts and topics. At the beginning of class, instructors can explicitly state to the class that the intention of the varied learning strategies is to promote an equitable and accessible classroom for all students (15). This promotes inclusive learning as students feel less targeted during random call or when students are asked to contribute during class discussion. I have found that a high level of transparency in my teaching approaches is well received by students and it encourages students who are more accustomed to traditional lecture classes open up to active learning styles. Though there is a short lecture within this lesson, it is designed to be concise and engaging as intellectual engagement is minimally elicited during traditional in-class lectures (16).
Students work together in small groups multiple times during this lesson as it cultivates social learning and peer education. As small groups have been shown to support learning gains (17) and support students of various disciplines and levels (18,19), they are heavily utilized in my classes to create a more inclusive and inviting environment. The groups are intentionally assigned by the instructor to promote inclusivity and allow for students of varying achievement levels to collaborate and learn from one another. This lesson employs think-pair-share, which allows for more meaningful class discussions as students take the time to critically think about a topic and exchange ideas with classmates before whole class discussion. Further, students write down their thoughts before class discussion, which eases anxieties for students who are uncomfortable with public speaking.
The use of technology is generally promoted in this course as assignments can be completed and submitted online, thus decreasing the environmental burdens associated with paper. However, as some students may not have a laptop or other classroom-friendly internet-connected device, instructors should print out a few copies of the assignments to accommodate all students. Students can submit their final work by emailing the instructor a photo of their completed assignment.
LESSON PLAN
Pre-Class Instructor Preparation
Instructors should assign homework as an electronic file to students at least 24 hours before class (S1. Lytic and lysogenic – Bacteriophages in Medicine Pre-Class Homework Assignment). The assignment is ideally presented to students as a timed multiple-choice assessment on the learning management system. If instructors prefer not to time the homework assignment, it can simply be presented as a multiple-choice assessment.
In preparation for class, download and print the puzzle activity (S4. Lytic and lysogenic – In-Class Strip Sequence) for as many groups as necessary (each group should have 3-4 students). Cut out pieces along the dotted lines and keep all pieces from the same page together. In a standard 4⅛"x 9½" envelope, insert a folded copy of the puzzle template paper and all the cutout pieces (19 in total). Securely close the envelope with 2 paperclips to ensure that all the components remain together. Ensure that there is sufficient scotch tape for each group and prizes (i.e., candy, if the instructor opts to include them) for students. Instructors should also print a few copies of the assignment for students who do not bring a laptop to class.
Download the PowerPoint presentation (S3. Lytic and lysogenic – In-Class Lecture) and ensure that the referenced figures are appropriately inserted and that the animations are properly working on the file slide. The PowerPoint slides (all except the final slide) should be made available for students to download during class. The final slide contains the answer key to the strip sequence, so instructors must ensure this is manually deleted before sharing with students. The lesson (Supporting File S2(a-d): Lytic and lysogenic: Bacteriophage Replication Cycles In-Class Worksheet) should be scheduled for student access on the learning management system 5 minutes before class time. The post-class homework assignment (S5. Lytic and lysogenic – Post-class Homework Assignment) should be made available to students at the end of the class session and due 48 hours later, or later at the discretion of the instructor.
Finally, submission folders should be set up on the learning management system or any platform of the instructor's choosing. There should be 5 folders set up in total- one for each section of the in-class activity and one for the post-class homework assignment.
Pre-Class Student Preparation
Students should complete the homework assignment (S1. Lytic and lysogenic – Bacteriophages in Medicine Pre-Class Homework Assignment) before class so that the instructor has time to review their performance before the lesson. The goal of the homework assignment is to introduce bacteriophages and to elicit curiosity and interest in students in preparation for class.
Lesson Introduction
Instructors should ensure that all students have a laptop and can connect to the learning management system to access the worksheets. Students are made aware of the learning goals by the instructor projecting them and reading them to the class (S3. Lytic and lysogenic – In-Class Lecture, slide 1).
Labeling Bacteriophages
Students partner with their neighbors and access Section 1 of the in-class worksheet (S2a. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 1). Each pair of students works together to complete the assignment using online resources. Upon completion, students should submit their files in the submission folder on the learning management system. After all students submit their assignments, the instructor uses random call to review the answers.
In-Class Lecture Script
The next exercise is a short and interactive instructor-facilitated lecture (S3. Lytic and lysogenic – In-Class Lecture). Students should take notes during this time. The prompting questions throughout the lecture ("Ask") are meant to stimulate critical thinking in students; instructors can call on volunteers to offer their responses.
Slide 2: A phage begins the host invasion process by landing on a host bacterium, as shown in figure (a). The long tail fibers are the first part of the phage to make contact with the bacterium and function to "walk" across the bacterium until the preferred surface site is found. This is a reversible process. As more long tail fibers contact the host cell surface, the short tail fibers interact with their receptors as shown in (b), which is an irreversible process. Thus, at this point, the phage has committed to infection of the host cell. Next, the baseplate (point to baseplate on the figure) settles down onto the surface of the bacterium and expands to create a pore. The tail sheath contracts, as shown in (c), which pushes the central tube through the outer membrane of the host, as shown in (d). Finally, the phage DNA is pushed into the host cell from the phage, shown in (e). It is important to note that not all bacteriophages look alike; there is tremendous diversity among them in terms of genomes and structures. The genomes can be either DNA or RNA, either single or double stranded, and range in size from a few kilobases to a few hundred kilobases. Structurally, there is high variability among phages; though the most studied phages are those with tails, not all phages have tails. "Tail-less" phages have either icosahedral or filamentous capsid shapes. Among the tailed phages, some contain contractile tails, which can be long, short, or complex, and some have non-contractile tails. I mention this to clarify that that not all phages have the same structure and mode of infection.
Slide 3: We will first discuss the lytic cycle of bacteriophages and use the example of the T4 phage, a virulent phage that infects E. coli. The first step is adsorption and DNA ejection, as we have just discussed. After this, linear viral DNA is ejected into the host cell, early mRNAs are made by the host polymerase. The reason these mRNAs are termed "early" is because they are made before viral DNA replication occurs.
Ask: What function do you think the early mRNAs serve in the life cycle of T4?
The early genes include those that encode for proteins that bind to host factors and promote degradation of host cell factors. This allows for the transcription and translation of phage genes as there is now minimal competition from that of host genes. A few minutes later, phage DNA replication begins and is mediated by virus-encoded DNA-dependent DNA polymerase. As shown in the diagram, within a few minutes, phage replication is completed.
Ask: How do you think replication occurs so quickly?
DNA replication is initiated from many origins of replication and occurs bidirectionally. After DNA replication, late mRNAs are synthesized, which encode for structural components of the final virion, or infectious viral particle, as well as proteins that are involved in cell lysis and virion release. Next, the T4 genome must be packaged into the head of the phage virion (shown on the diagram as "heads filled"). This step is accomplished by the packasome, a complex of proteins that exerts more power than an automobile motor!
Ask: Why must the packasome have so much power?
The packasome has the immense task of pushing dsDNA through a small pore in the head of the virion. As DNA carries a negative charge and can be somewhat bulky, it is not easy to push it through, so it requires a lot of power via ATP hydrolysis to accomplish this. Next, the tails are attached onto the heads of the phage and virions are finally released from the host cell. This whole process takes ~22 minutes, as shown in the diagram.
Ask: What questions do you have about what we have discussed?
Questions that students have previously asked followed by instructor response:
What directs the host polymerase to divert transcription of its own genes and transcribe the phage early genes?
Excellent question. Using the example of T4, the phage takes over E. coli RNA polymerase through the action of phage-encoded factors that interact with polymerase and change its specificity for promoter DNA. Early T4 promoters, which have -10 and -35 elements that are like that of the host, are recognized by the specificity factor, sigma70. Simply put, the host polymerase has increased specificity for phage promoters.
What is the difference between "virus" and "virion"?
Great question. A virus is a small infectious agent that remains inside the host cell and is obligatory intracellular parasite and replicates only inside the living host cells of an organism. A virion is an infectious particle that is designed for transmission of the nucleic acid genome among host cells. Virions contain nucleic acid surrounded by a capsid and are extracellular.
Can many phages infect a single bacterial cell?
Good question. The short answer is, yes, a single bacterial cell can be infected by multiple phages, but generally speaking, the ratio of phages to bacterial cells is typically not high enough to observe a bacterial cell that has been infected by many phages in nature.
Slide 4: Now, we will discuss the lysogenic cycle and use the example of lambda, a temperate phage that infects E. coli. The lysogenic cycle is very different from the lytic cycle as the viral DNA becomes incorporated into the host genome and remains "harmlessly" associated with the host (i.e., no observable detrimental effects on the host cell). When the viral DNA is incorporated into the host genetic material, it is known as the prophage. In the case of lambda, there is a specific site in the host DNA where integration occurs, but not all temperate phages integrate in a designated location.
Whenever the host cell divides, the prophage divides with the cell and is, therefore, propagated without causing any harm to the host. This relationship is maintained until there is a stressor, such as nutrient deprivation or ultraviolet (UV) light, which often, but not always, causes the viral DNA to excise from the bacterial chromosome. Once excised, the phage DNA can enter the lytic cycle, which leads to host cell death.
Slide 5: T4 gene expression is tightly regulated by two major factors - the timed synthesis of mRNAs and the structure of the genome. As we discussed earlier, there are "early," "middle or intermediate," and "late" mRNAs, so called based on their time of expression during the replication cycle. Middle mRNAs encode proteins that are important in viral replication. The timing of transcription is important as different genes and proteins are required at different stages of the virus life cycle.
Ask: What factors do you think control the timed synthesis of mRNAs?
One factor that dictates the timed synthesis of mRNAs is the regulation of host RNA polymerase. Viral enzymes modify the host polymerase by addition of chemical groups, such as ADP-ribose, which can turn off transcription of some early genes. Another factor is the viral factor gp55, which is encoded by a later gene, and directs the host polymerase to synthesize phage "late" genes. Next, let's discuss how the structure of the genome is important in T4 gene expression. As shown in the figure on the screen, genes with related functions are clustered together in the genome. For example, the genes that encode for phage head proteins are grouped together and those that encode for phage tail proteins are grouped together. In fact, the early and late genes are grouped separately in the genome and are transcribed in opposite directions.
Ask: Why is this early and late gene grouping significant?
The temporal gene grouping is significant as transcription of these genes can occur continuously at around the same time. Thus, genes that are important during early, intermediate, and late host cell infection are all synthesized and fulfill their purpose in the time frame they are required. The final point I want to make about this slide is that you should pay attention to the color coding of this image; the genes shaded in orange encode for proteins that govern whether the lysogenic or lytic cycle occurs, those shaded in yellow are required for lysogeny, and those in tan are required for the lytic cycle. This will be important for an upcoming exercise you will do later today.
Slide 6: Each group will receive an envelope with this template inside as well as pieces that fit onto the template. You will use scotch tape to adhere the pieces onto the template. The first group to correctly complete the sequence can receive a small prize, at the discretion of the instructor.
(At this point, instructor forms groups of 3-4 students and passes out one envelope per group; see "Strip Sequence Activity" below).
Slide 7: (Only present this slide after students have completed the strip sequence.) Now, let's quickly review the proper sequence of events and ensure that everyone has the correct answers. Instructor should ask for volunteers to provide the answers to each of the steps of the lytic/lysogenic cycles in the order presented on the Powerpoint slide. For example, "what is step one on the schematic? Please provide both the text label and the letter of the correct image." Instructor should choose one student to provide the correct answer, "Adsorption and the image is E." Instructor should confirm this to be true by advancing the slide and then continue going through all of the steps in a consecutive manner.
Strip Sequence Activity
Instructor forms groups of 3-4 students and distributes one envelope per group. While students work on the exercise, the instructor circulates the room to monitor student involvement and achievement. If all students are not actively participating, instructor should encourage that everyone contributes to completion of the strip sequence. When the first group completes the strip sequence, the instructor ensures the accuracy of the sequence and if all is correct, the group receives a small prize (e.g., chocolate, candy, or a non-food food item). The instructor can use slide 7 from the PowerPoint and random call to review the proper sequence of steps with the whole class.
Compare and Contrast Replication Cycles
Students should download Section 2 of the in-class worksheet (S2b. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 2) and work with a partner to fill in the Venn Diagram that compares the lytic and lysogenic cycles. Instructors circulate the room to provide feedback to students and ensure that all students are actively engaged in the exercise. The instructor can ask for students to volunteer their responses and then submit their files in the Section 2 folder on the learning management system.
Think-Pair-Share
Students should download Section 3 of the in-class worksheet (S2c. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 3) and first work on the exercise on their own for ~10 minutes. Afterwards, students share and discuss their insights and rationale with a neighbor for ~10 minutes. During this time, the instructor circulates the room and provides feedback where necessary. Finally, the instructor asks for volunteers to share their answers, which leads into a class discussion. Students submit their completed files in the Section 3 folder on the learning management system.
Small Group Activity
Students should download the fourth and final section of the lesson (S2d. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 4). In groups of 3-4, students work together to solve application and data interpretation problems. The groups may remain the same from the previous exercise or may be reshuffled, at the discretion of the instructor. The instructor should be available for questions during this time and provide immediate feedback to groups based on their discussions. As time is limited in a single class session, the instructor will likely not be able to review answers for Section 4. Students are still expected to work through the assignment and submit their completed files in the Section 4 folder on the learning management system by the end of class. The instructor can review the answers during the next class period or provide an answer key for students after class.
Post-Class Homework Assignment
After class, instructors post a homework assignment (S5. Lytic and lysogenic – Post-class Homework Assignment) for students that compares lysogenic and latent infections. Instructors should make the background reading (reference provided in the file) accessible to students as a .pdf on the learning management system. Students should complete the assignment individually within 48 hours after class and submit it online.
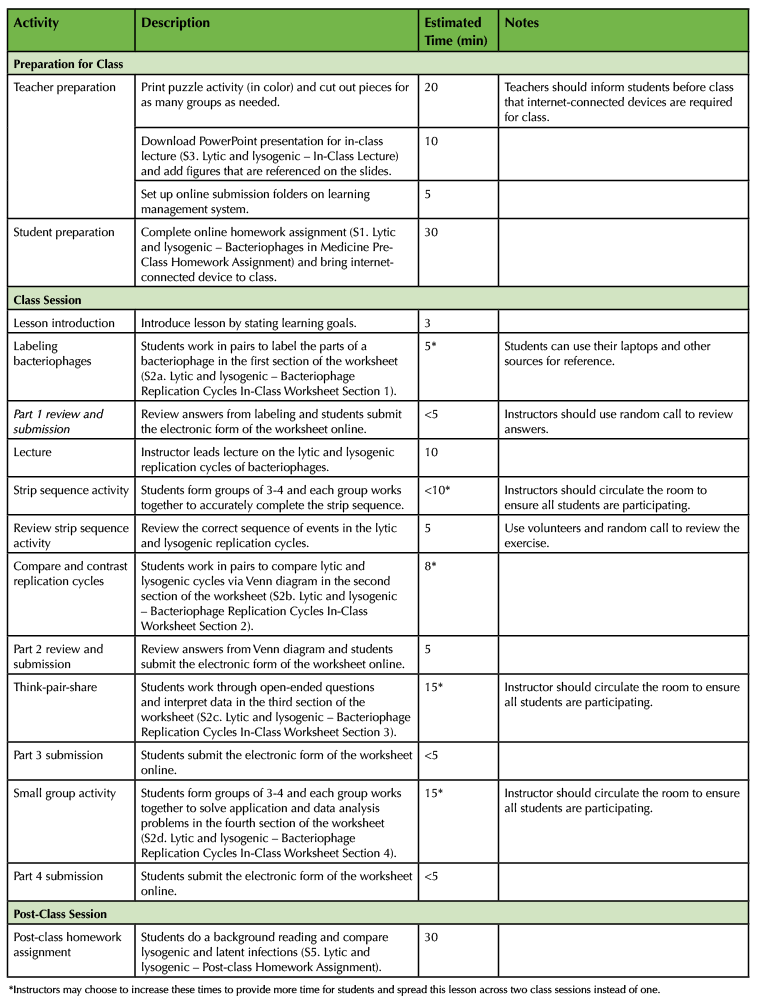
Table 1. Lesson plan timeline.
TEACHING DISCUSSION
Teaching Insights
This lesson was implemented in an upper-level virology course in a class of ~20 students. Most students were biology majors, though there were some students in other fields, such as mathematics. This lesson requires that students have some basic background knowledge, but students were encouraged to ask questions if anything was unclear throughout the class period. Some students took advantage of in-class technology and simply researched unclear concepts or asked their classmates. That students felt comfortable asking one another questions in an unsolicited manner was a sign of a positive and cooperative learning environment. Students responded favorably to the activity based on active participation in small groups and class discussions. This lesson was successful in achieving the learning goals and outcomes based on students' written and verbal responses in class. Before the lesson, students were informally asked about their familiarity with bacteriophages and the lytic and lysogenic cycles and most students were not aware of the lytic and lysogenic cycles of phages, though they reported being superficially familiar with the concept of phages.
Students were visibly invigorated and motivated during the strip sequence, which required collaboration, cooperation, and knowledge of the material. Students were driven by a healthy level of competition and an incentive (i.e., candy), which promoted the learning process and reinforced concepts. During the exercise, students were focused and engrossed in ordering the steps of the lytic and lysogenic replication cycles. The use of notes and technology was discouraged as students worked through the activity to challenge students' recall and knowledge of the material. There were productive discussions during the activity about the content and students asked one another questions such as "How does excision occur when the phage breaks out of the lysogenic cycle?" and "Do all cells with prophages undergo the lytic cycle upon induction?"
In Section 4 of the lesson, students discussed the importance of keeping a detailed notebook, which was not an original learning goal of this exercise. Students shared their stories about good and bad note-keeping in a laboratory setting and its benefits and consequences. Those who worked in labs were especially vocal about the significance of maintaining a well-organized and descriptive notebook. Students discussed whether unknown samples in a lab are feasible for use and how to handle a situation in which specimens and/or samples are questionable.
Lesson Limitations and Suggested Adaptations
Though this lesson was designed for an upper-level biology course, it can be modified for an introductory course. A suggested modification is to remove Section 2 of the in-class worksheet (S2b. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 2) and eliminate question 7 from Section 4 (S2d. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 4). With these modifications, questions 5 and 6 (Is phage #1/2 temperate or virulent?) from Section 4 should be a think-pair-share exercise instead of a small group activity. The modified lesson will allow students to still achieve most of the learning goals and objectives outlined in the original lesson, but will omit "compare and contrast the differences between lysogenic and latent viral infections" and "interpret and analyze graphical representations of qPCR and plaque assay data".
The activities within this lesson were designed to provide an engaging and advanced approach to educating students about the replication cycles of bacteriophages. An attempt was made to align the learning goals with core competencies outlined in Vision and Change (10). Specifically, I sought to incorporate the "ability to apply the process of science" and "ability to use quantitative reasoning" in the lesson. Suggestions to expand the learning goals to integrate more core competencies are provided below.
Models are an especially useful pedagogical tool to represent a complex and detailed reality that can be used to predict outcomes (20), in keeping with a core competency defined in Vision and Change, "ability to use modeling and simulation." To adapt this lesson for a more advanced course, I suggest expanding section 3 of the in-class worksheet (S2c. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 3) to include modeling. This lesson can integrate modeling by asking students to draw a model of the lytic/lysogenic pathway based on the text. Follow-up questions, such as "Based on your model, what do you expect will happen if Cro protein levels are elevated?" will bolster the significance of the model and allow students to better appreciate its purpose.
This lesson was intended to be ~90 minutes long, but students felt that they needed more time to complete the final section. Thus, I recommend that instructors assign Section 2 of the in-class worksheet (S2b. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 2) as a homework assignment instead of an in-class exercise. This will allow students to focus on the critical thinking sections of the lesson and feel less pressured by time constraints. Another potential time saving modification to the lesson is to have students watch a video or animation of the lytic and lysogenic cycles (https://www.youtube.com/watch?v=hFwA0aBX5bE; see " Prerequisite Teacher Knowledge") before class so that they have a stronger basis for the lecture, which will likely save time by reinforcing concepts instead of introducing them. Instructors can even assign comprehension questions for homework to ensure that students completed the assignment.
SUPPORTING MATERIALS
- Supporting File S1. Lytic and lysogenic – Bacteriophages in Medicine Pre-Class Homework Assignment
- Supporting File S2a. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 1
- Supporting File S2b. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 2
- Supporting File S2c. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 3
- Supporting File S2d. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Section 4
- Supporting File S3. Lytic and lysogenic – In-Class Lecture
- Supporting File S4. Lytic and lysogenic – In-Class Strip Sequence
- Supporting File S5. Lytic and lysogenic – Post-class Homework Assignment
- Supporting File S6. Lytic and lysogenic – Bacteriophages in Medicine Pre-Class Homework Assignment Answer Key
- Supporting File S7. Lytic and lysogenic – Bacteriophage Replication Cycles In-Class Worksheet Answer Key
- Supporting File S8. Lytic and lysogenic – Post-class Homework Assignment Answer Key
ACKNOWLEDGMENTS
I would like to thank Dr. John Olson, Dr. Benson George, the students who participated in this lesson and provided feedback, and the anonymous reviewers.
References
- Weinbauer MG. 2004. Ecology of prokaryotic viruses. FEMS Microbiol Rev 28:127-81. doi:10.1016/j.femsre.2003.08.001.
- Breitbart M, Rohwer F. 2005. Here a virus, there a virus, everywhere the same virus? Trends Microbiol 13:278-84. doi:10.1016/j.tim.2005.04.003.
- Hargreaves KR, Kropinski AM, Clokie MR. 2014. Bacteriophage behavioral ecology: How phages alter their bacterial host's habits. Bacteriophage 4:e29866. doi:10.4161/bact.29866.
- Clokie MR, Millard AD, Letarov AV, Heaphy S. 2011. Phages in nature. Bacteriophage 1:31-45. doi:10.4161/bact.1.1.14942.
- Khan LB, Read HM. 2018. A Simple Exercise for Teaching Bacteriophage Concepts in the Undergraduate Laboratory Using Commercially Available Disinfectant. J Microbiol Biol Educ 19. doi:10.1128/jmbe.v19i2.1527.
- Allen ME, Gyure RA. 2013. An undergraduate laboratory activity demonstrating bacteriophage specificity. J Microbiol Biol Educ 14:84-92. doi:10.1128/jmbe.v14i1.534.
- Jordan TC, Burnett SH, Carson S, Caruso SM, Clase K, DeJong RJ, Dennehy JJ, Denver DR, Dunbar D, Elgin SC, Findley AM, Gissendanner CR, Golebiewska UP, Guild N, Hartzog GA, Grillo WH, Hollowell GP, Hughes LE, Johnson A, King RA, Lewis LO, Li W, Rosenzweig F, Rubin MR, Saha MS, Sandoz J, Shaffer CD, Taylor B, Temple L, Vazquez E, Ware VC, Barker LP, Bradley KW, Jacobs-Sera D, Pope WH, Russell DA, Cresawn SG, Lopatto D, Bailey CP, Hatfull GF. 2014. A broadly implementable research course in phage discovery and genomics for first-year undergraduate students. mBio 5:e01051-13. doi:10.1128/mBio.01051-13.
- Hanauer DI, Jacobs-Sera D, Pedulla ML, Cresawn SG, Hendrix RW, Hatfull GF. 2006. Inquiry learning. Teaching scientific inquiry. Science 314:1880-1. doi:10.1126/science.1136796.
- Dedrick RM, Guerrero-Bustamante CA, Garlena RA, Russell DA, Ford K, Harris K, Gilmour KC, Soothill J, Jacobs-Sera D, Schooley RT, Hatfull GF, Spencer H. 2019. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 25:730-733. doi:10.1038/s41591-019-0437-z.
- AAAS. 2011. Vision and Change in Undergraduate Biology Education: A Call to Action, abstr Washington, DC.
- Freeman S, Eddy SL, McDonough M, Smith MK, Okoroafor N, Jordt H, Wenderoth MP. 2014. Active learning increases student performance in science, engineering, and mathematics. Proc Natl Acad Sci U S A 111:8410-5. doi:10.1073/pnas.1319030111.
- Lyman F. 1981. The responsive classroom discussion: the inclusion of all students, University of Maryland, College Park, MD.
- Angelo TAaC, K. Patricia. 1993. Classroom Assessment Techniques: A Handbook for College Teachers. Jossey-Bass, San Francisco, CA.
- Eddy SL, Brownell SE, Wenderoth MP. 2014. Gender gaps in achievement and participation in multiple introductory biology classrooms. CBE Life Sci Educ 13:478-92. doi:10.1187/cbe.13-10-0204.
- Tanner KD. 2013. Structure matters: twenty-one teaching strategies to promote student engagement and cultivate classroom equity. CBE Life Sci Educ 12:322-31. doi:10.1187/cbe.13-06-0115.
- Smith KA, Sheppard SD, Johnson DW, Johnson RT. 2005. Pedagogies of Engagement: Classroom-Based Practices. Journal of Engineering Education 94:87-101. doi:10.1002/j.2168-9830.2005.tb00831.x.
- Marbach-Ad G, Rietschel CH, Saluja N, Carleton KL, Haag ES. 2016. The Use of Group Activities in Introductory Biology Supports Learning Gains and Uniquely Benefits High-Achieving Students. J Microbiol Biol Educ 17:360-369. doi:10.1128/jmbe.v17i3.1071.
- Smith MK, Wood WB, Adams WK, Wieman C, Knight JK, Guild N, Su TT. 2009. Why peer discussion improves student performance on in-class concept questions. Science 323:122-4. doi:10.1126/science.1165919.
- Smith MK, Wood WB, Krauter K, Knight JK. 2011. Combining peer discussion with instructor explanation increases student learning from in-class concept questions. CBE Life Sci Educ 10:55-63. doi:10.1187/cbe.10-08-0101.
- Fischer HP. 2008. Mathematical modeling of complex biological systems: from parts lists to understanding systems behavior. Alcohol Res Health 31:49-59.
Article Files
Login to access supporting documents
Exploring the Lytic and Lysogenic Life Cycles of Bacteriophages(PDF | 189 KB)
S1. Lytic and lysogenic-Bacteriophages in Medicine Pre-Class Homework Assignment.docx(DOCX | 15 KB)
S2a. Lytic and lysogenic-Bacteriophage Replication Cycles In-class workheet Section 1.docx(DOCX | 159 KB)
S2b. Lytic and lysogenic-Bacteriophage Replication Cycles In-class workheet Section 2.docx(DOCX | 36 KB)
S2c. Lytic and lysogenic-Bacteriophage Replication Cycles In-class workheet Section 3.docx(DOCX | 156 KB)
S2d. Lytic and lysogenic-Bacteriophage Replication Cycles In-class workheet Section 4.docx(DOCX | 1 MB)
S3. Lytic and lysogenic- In-Class Lecture.pptx(PPTX | 1 MB)
S4. Lytic and lysogenic- In-Class Strip Sequence.pptx(PPTX | 1 MB)
S5. Lytic and lysogenic- Post-class Homework Assignment.docx(DOCX | 24 KB)
S6. Lytic and lysogenic- Bacteriophages in Medicine Pre-Class Homework Assignment Answer Key.docx(DOCX | 15 KB)
S7. Lytic and lysogenic- Bacteriophage Replication Cycles In-Class Worksheet Answer Key.docx(DOCX | 1 MB)
S8. Lytic and lysogenic- Post-class Homework Assignment Answer Key.docx(DOCX | 33 KB)
- License terms

Comments
Comments
There are no comments on this resource.