Investigating Enzyme Structure and Function Through Model-Building and Peer Teaching in an Introductory Biology Course
Editor: Leocadia V. Paliulis
Published online:
Abstract
A foundational knowledge of the relationship between structure and function is critical to understanding how enzymes work. The seemingly invisible nature of these molecular interactions makes it challenging for undergraduate students to conceptualize the dynamic changes that occur during a catalytic cycle. In this Lesson, we describe an interactive, collaborative modeling activity that we use in introductory biology courses to teach students how enzymes catalyze chemical reactions. First, the students imagine a fictitious enzyme and its associated reaction, and use modeling compound to demonstrate the progression of the reaction while focusing on the three-dimensional shape of active site and substrate in facilitating this catalysis. Second, they then select one of four types of enzymatic regulation (competitive inhibition, allosteric inhibition, allosteric activation, or feedback inhibitions) to incorporate into their model. They then demonstrate these reactions to groups of peers. This student-centered approach uses active learning and peer instruction to provide students with prompt feedback to strengthen their understanding of the inter-relatedness of structure and function. This modeling activity concludes with student reflection and discussion, and student learning is assessed with exam questions.
Citation
Friedman EJ, Terry CH. 2020. Investigating enzyme structure and function through model-building and peer teaching in an introductory biology course. CourseSource. https://doi.org/10.24918/cs.2020.4
Society Learning Goals
Biochemistry and Molecular Biology
- Energy is required and transformed in biological systems
- How do enzymes catalyze biological reactions?
- Macromolecular Structure Determines Function and Regulation
- How are structure and function related?
- How is macromolecular structure dynamic?
- How is the biological activity of macromolecules regulated?
- How is structure (and hence function) of macromolecules governed by foundational principles of chemistry and physics?
Lesson Learning Goals
Student will:
- know the stages and components of an enzymatic reaction.
- understand enzyme structure and function.
- appreciate the dynamic nature of protein catalysts.
- understand that regulatory factors can alter enzyme structure and therefore function.
- be able to describe the different types of inhibition and regulation of enzymatic reactions.
Lesson Learning Objectives
Students will be able to:
- explain how enzymes catalyze chemical reactions.
- describe interactions between enzymes, substrates, and products.
- evaluate the effects of different types of enzyme inhibitors and regulators.
- demonstrate the relationship between structure and function of enzymes.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
The relationship between structure and function is a foundational principle in biology, and an understanding of this relationship with respect to proteins is a critical aspect of molecular and cell biology courses (1). Enzymes are introduced early in the undergraduate curriculum and are one of the first examples in which students learn how structure impacts function (2). However, the molecular nature of enzymes prevents students from visualizing the interaction between the active site and substrate(s). Further, enzymes are dynamic protein machines with structures that oscillate between multiple forms based on many conditions, which include the presence of regulators and abiotic conditions like cofactor availability, temperature, and pH. This complex suite of factors complicates student comprehension of enzyme structure and function (3). These issues can be further compounded by traditional instructional delivery, which commonly includes textbook reading, instructor lectures, and the use of 2-D textbook images. Presentation of material in this manner is inconsistent with the expectation that students move beyond the memorization of key concepts to synthesize and apply their knowledge to big-picture scenarios in biology (4,5).
Scientific models are simplified representations of complex biological concepts that explain how or why phenomena exist or processes occur (6). Enzymatic reactions involve the interaction of a number of different components (enzyme, substrate, and a host of regulatory factors) resulting in product formation. The three-dimensional structure of each component is critical to the efficiency and outcome of the reaction. Although students can glean the basics of enzyme function through traditional instructional delivery, teaching students to translate these concepts into their own simple models allows them to learn science by "doing" (7). Through building their own mental models, students can better understand these complex processes and describe them to others (8).
Creating a mental model is helpful, but building a dynamic, three-dimensional, physical representation of this mental model provides a concrete entry point to scaffold abstract concepts (9). To understand the relationship between enzyme structure and function, students need to think in three dimensions; thus, the tools they use should also be three-dimensional. Although the use of static physical models has been shown to increase student understanding of a variety of topics, including meiosis (10) and protein structure-function (11), the dynamic nature of enzyme-catalyzed reactions requires that the models depicting these reactions are also dynamic.
This Lesson describes an interactive, collaborative modeling activity that teaches students how an enzyme's structure is critical for its role in catalyzing chemical reactions. It specifically focuses on i.) the three-dimensional nature of active site-substrate interactions, ii.) the physical similarity between competitive inhibitors and the active site, and iii.) how allosteric regulators affect the shape of the active site from afar. The Lesson is intended to be used after students have been introduced to the basics of enzyme function. Working in teams, the students imagine a hypothetical enzyme and the reaction it catalyzes. They additionally choose one of four possible regulatory scenarios (competitive inhibition, allosteric activation or inhibition, or feedback inhibition) and create an interactive model that demonstrates its effects using modeling compound. They then present that model to a group of peers, and receive both instructor and peer feedback in real time. Presenting to and learning from the other groups allows for a rich comparison of the different models. Multiple rounds of revision and presentation are completed as time allows. The Lesson includes instructional pedagogies previously shown to increase student-learning outcomes, such as active learning (12), prompt feedback (13), and peer instruction (14). These techniques have the added benefit of allowing us to implement this activity in a relatively large class (48 students) with one instructor during a single 50-minute session.
Intended Audience
This Lesson is intended for either major or non-major introductory biology students. The Lesson has been successfully implemented in a 48-student introductory biology course at a small liberal arts university. The course serves biology and biomedical science majors and non-majors in the health sciences or students using the course to fulfill a general education requirement. The students in this course are primarily first-year undergraduates.
Required Learning Time
This Lesson can be implemented in a single 50-minute class period. The allotted time includes group and topic selection, model creation, and teaching/revision. Content delivery about enzyme structure and function is not included in this Lesson time and should be conducted during a prior class or as pre-class homework.
Prerequisite Student Knowledge
Because this Lesson focuses on application of concepts, students should have prior knowledge of the structures and processes that they will be asked to model, including enzyme function, regulation, and the associated terminology: enzyme, substrate, active site, product, activation energy, competitive inhibition, allosteric inhibition, allosteric activation, and feedback inhibition.
This necessary material may be delivered by lecture (in a prior class meeting), online interactive homework, and/or flipped classroom videos. The content is available in standard introductory biology textbooks, e.g., section 6.5 of OpenStax Biology 2e (15) or concepts 8.4 and 8.5 of Campbell Biology (16).
In short, biochemical reactions are typically catalyzed by macromolecules known as enzymes. These enzyme catalysts speed up reactions without being irreversibly altered; this allows them to be used repeatedly to convert one or more substrates (i.e., starting molecules or reactants) into one or more products. The location within the enzyme where a substrate binds is known as the active site. The interplay between an active site and its substrate is very specific due to temporary molecular interactions that occur between the substrate and the amino acids in the active site.
When a substrate binds the active site, an enzyme-substrate (E-S) complex is formed. In this E-S complex, the enzyme and substrate both change shape slightly so that their mutual interactions are enhanced. These three-dimensional changes brought on by E-S complex formation are referred to as induced fit. This helps the enzyme catalyze the reaction by contorting the substrate so that breaking bonds or forming new bonds is energetically favorable. The E-S complex then becomes the enzyme-product (E-P) complex, and the product is released from the enzyme's active site. The enzyme is now available to catalyze another round of the reaction (Figure 1).
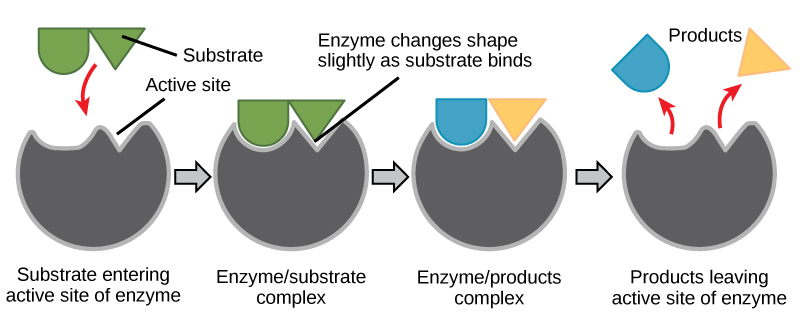
Figure 1. Once the substrate has bound to the active site, the enzyme contorts the substrate into its transition state, thereby increasing the reaction rate, allowing products to form and be released (Image source: Biology 2e by OpenStax, under CC BY 4.0 license).
Activation energy (Ea) is required to manipulate chemical bonds within the substrate in order for the reaction to proceed. Enzymes facilitate bond manipulation in the transition state (ES) and in doing so, lower the Ea to make the reaction more favorable. This is done without changing the free energy (ΔG) of the reaction.
Regulating enzyme activity is an important means to control metabolic reactions in the cell. The activity of enzymes can either be increased or decreased. In the case of competitive inhibition, an inhibitor molecule (which is structurally similar but not identical to the substrate) binds in the active site of the enzyme and prevents the normal substrate from binding. During allosteric inhibition, an inhibitor binds to an allosteric site (which is located away from the active site) and propagates structural changes through the enzyme, such that the active site no longer "matches" the substrate. Allosteric activators also influence substrate-active site interactions, although their binding to the allosteric site improves interactions between the substrate and the active site. Oftentimes, these inhibitory molecules are related to the metabolic pathways that they regulate. With feedback inhibition, the end product of a series of reactions acts as an allosteric inhibitor for the enzyme that catalyzes an earlier reaction in the pathway.
If the optional extension homework activity is utilized (Supporting File S3: Enzyme Structure Function - Optional Extension Homework Activity and Rubric), students will additionally need to be familiar with the following additional concepts and terminology as they relate to enzyme structure and function:
- Changes in pH affect the structure of the active site by altering chemical interactions between amino acid side chains. Although individually small, these changes collectively change the three-dimensional shape of the enzyme, especially as it relates to its ability to interact with the substrate. Each enzyme operates optimally within a narrow pH range, and deviations from this range reduce functionality. Extreme changes in pH can cause protein denaturation.
- Temperature changes also affect enzyme function. Although temperature affects kinetics (i.e., increased temperature results in increased molecular motion and therefore collisions between enzyme and substrate), we ask our students to focus primarily on the effects of temperature on the enzyme's structure. Similar to pH, changes in temperature impact chemical interactions between amino acids, which together impact the shape of the enzyme and active site. Each enzyme operates optimally within a specific range of temperatures, and deviations above or below the optimal range can decrease enzyme efficiency. Extreme changes may even denature the enzyme.
- Some enzymes require the presence of a cofactor (typically metal ions) or coenzyme (organic molecules like vitamins) to facilitate binding of the substrate in the active site. These elements are not consumed by the reaction and do not become incorporated into the product.
- Many enzymatic reactions are reversible. Under certain conditions, the enzyme can convert product(s) back into substrate(s).
Prerequisite Teacher Knowledge
In addition to the prerequisite student knowledge, teachers should understand how the components of an enzymatic reaction change through the course of the reaction. This will allow the instructor to facilitate intra- and inter-group discussions about the mechanistic changes to the enzyme during catalysis. Instructors should be cognizant of common student misconceptions (Supporting File S2: Enzyme Structure Function - Examples of Student Work) and be prepared to address these and other inaccuracies when roaming the room during the model planning and construction phase.
SCIENTIFIC TEACHING THEMES
Active Learning
This active learning Lesson requires constant student engagement. In small groups, students select topics to model and plan how to reflect each in a three-dimensional, dynamic model. The students in the group then take turns leading a presentation of their model to groups of peers. The students engage in discussion with presenters from other groups, asking questions and providing feedback. Finally, the students reflect on their learning through a series of open-ended questions.
Assessment
Learning is measured via both formative and summative assessments. During the Lesson, instructors provide feedback to correct or enhance student models. Students also receive formative peer feedback during the presentation phase of the Lesson. Following the Lesson, students reflect on their learning by responding to open-ended questions. Finally, information from the Lesson is evaluated via quiz and exam questions.
Inclusive Teaching
This Lesson leverages small student groups, where each student is involved in both model creation and presentation. Groups are generated randomly to promote diversity and to discourage grouping by existing relationships or student ability. The ideal group size of three students encourages participation of each group member while ensuring sufficient coverage of prior knowledge. During the presentation phase, group members who are more confident or outgoing are encouraged to present first; this allows more cautious or less confident members to observe before taking their turn presenting. The design of this Lesson exploits multiple learning styles, including audio, visual, tactile, and kinesthetic learning.
LESSON PLAN
Pre-class Preparation
Student preparation
Prior to the Lesson, the students should have a basic understanding of enzyme structure and function. We introduce relevant concepts and terminology in the previous class session, including active sites, substrates and products, competitive inhibition, allosteric regulation, and feedback inhibition. The students are also assigned to read the corresponding sections in their textbook and complete an online homework module hosted by the textbook publisher.
Instructor preparation
The instructor should acquire modeling compound in a variety of colors (enough for one can per student or 3 cans per group; 3- or 4-oz. cans work best). Student handouts should be printed and copied for distribution of one handout per student (Supporting File S1: Enzyme Structure Function - Student Handout). The instructor should determine which method of visibly random sorting they will use to sort students into random groups of three and acquire any materials necessary to do so. If portable white boards are being used as a backdrop for the models, they should be made available to the students (one or two boards per group). The instructor should prepare a sign-up method for topic selection in step 2. This could be writing the topics on the board or preparing paper sign-up sheets; make sure that the topics are evenly distributed based on the number of groups in your class.
In-class Activities
Group selection – 5 minutes
We have found that visibly random group selection enhances this activity because it eliminates students' perceptions of instructor bias when assigning groups. Although groups of three students per group is ideal, groups may be larger or smaller based on the course enrollment as long as the total number of groups is a multiple of four. Some suggestions for quickly visibly forming students into groups of three include the following:
- Before class, preload the roster into a random name generator (e.g., http://www.transum.org/software/RandomStudents/). Set the number of groups to a multiple of four (based on your enrollment divided by three). Project the website in the classroom so that the students can view the random group generation in action!
- Allow students to blindly select a card from a standard playing card deck and then form groups based on shared rank (e.g., all 2s in one group, all kings in another). Before class, preload the deck based on your class size, number of groups, and desired group size (e.g., remove one of each card for 13 groups of 3).
- Have the students "count off" by x, where x is the number of students divided by the desired group size, such that the total group number is a multiple of four.
Students should physically move to sit with their groups and obtain one handout (Supporting File S1: Enzyme Structure Function - Student Handout) per group member. To prevent students from becoming overwhelmed, instructors should discourage them from reading ahead to subsequent sections of the handout. The pagination of the handout is designed to facilitate this stepwise approach. The following steps mirror the directions in the student handout.
Step 1: CREATE (Creation of Fictional Enzymatic Reaction - 5 minutes)
This is the rough brainstorming/planning phase. Students should imagine an enzyme and the reaction it catalyzes. Encourage them to think about the big picture - enzyme and active site shape, type of reaction catalyzed, and the physical characteristics of the substrate(s) and product(s). They may be tempted to simply model a figure from their textbook, and although this is not a bad starting point, encourage them to consider the 3-D nature of their structural elements as well as any shape changes that will occur during the reaction. Feedback at this stage should mirror the questions in the handout. Resist the urge to make specific suggestions at this point although student ideas may be vague. For example, they may imagine an enzyme that breaks a shape into two smaller parts or one that combines two squares into a rectangle. The goal is for them to begin thinking about how the shape of each element will facilitate the reaction.
Step 2: REGULATE (Regulation of the Enzymatic Reaction - 5-10 minutes)
Each group will now select one factor that will regulate their enzyme. We have found that allowing the students to self-select topics increases their confidence, motivation, and engagement with the activity. To ensure even distribution of topics for the presentation component, provide a sign-up sheet on paper or on the board and evenly distribute the number of sign-up slots between the four topics. Encourage speedy sign-ups so that the remaining time can be used to brainstorm and plan how this regulatory element will impact the enzyme that they designed in step 1. The students may need to review the role of their chosen regulatory element before they can determine how to model its effect on their enzymatic reaction. During this time, try to visit each group so that you can address any questions they may have about their chosen mode of regulation.
Step 3: FABRICATE (Model Building and Presentation Rehearsal - 10-15 minutes)
Here, students will physically build their model. They have at least 3 required elements: enzyme (with an active site and potentially an allosteric site), substrate(s), and product(s). They may additionally need to create regulatory elements. They will then use each physical element to demonstrate two reactions. The first reaction is the "normal" (unregulated) reaction, while the second incorporates the effects of their regulator. The handout prompts students to focus on the structure of each element (e.g., an active site shape that is similar to that of the substrate) and any changes in structure that occur during the reaction (e.g., induced fit of the active site in substrate binding and changes in the substrate to create the product). It may help to remind students that they are building with modeling compound because it is malleable; it is easy to demonstrate shape changes in real-time as the reaction progresses. When modeling the regulated reaction, students should again focus on how the regulator affects the enzyme and its ability to catalyze the reaction. Inhibitors should be appropriately shaped based on their binding site, and allosteric regulators should affect the shape of the enzyme upon binding.
If portable white boards are available, students can build their models on the boards and label elements with a dry erase marker. If white boards are not available, the students may build the models directly on their desk or table or on a sheet of paper that can be labeled. Once the group feels that their model is complete, they should practice their presentation by manipulating the model and describing the reaction as it progresses. Encourage each student to practice as they will all take turns as the lead presenter in step 4. For examples of student work and misconceptions, see Figure 2 and Supporting File S2: Enzyme Structure Function - Examples of Student Work.

Figure 2. Example of student-created enzyme models. Here, the students are sharing their models with another group.
Step 4: DEMONSTRATE (Presentation and Revision - 20 minutes)
In the last 20 minutes of class, each group of students will rotate through three rounds of presentation and learning. During each round, the groups should use the pairing instructions summarized below (and included in Supporting File S1: Enzyme Structure Function - Student Handout) to ensure they learn about each of the other processes:
Round 1:
- Each Competitive Inhibition group will pair up with an Allosteric Inhibition group
- Each Allosteric Activation group will pair up with a Feedback Inhibition group
Round 2:
- Each Competitive Inhibition group will pair up with an Allosteric Activation group
- Each Allosteric Inhibition group will pair up with a Feedback Inhibition group
Round 3:
- Each Competitive Inhibition group will pair up with Feedback Inhibition group
- Each Allosteric Activation group will pair up with an Allosteric Inhibition group
During these three rounds, each student in a group will take a turn acting as the "lead presenter". In addition, each group will have the chance to learn about other topics that were modeled. If portable white boards were used, students can travel with their models to present their work. Otherwise, students should group up and travel to the different model locations.
During each round, the lead presenter of each group will describe and demonstrate the standard and regulated reaction. Students should be encouraged to ask questions of the presenters until they have a thorough understanding of the material. The instructor should also listen in on these presentations to correct any lingering errors or misconceptions (refer to Supporting File S2: Enzyme Structure Function - Examples of Student Work). Between each round of presentations, the groups will have the opportunity to revise their model and presentation based on the peer and instructor feedback that they received.
Reflection and assessment
Immediately following the activity (either during or after class, depending on how much time is available), the students were asked to reflect and respond to the following open-ended questions using online poll-response software:
- What is one new thing that you learned by listening to another group's presentation?
- What is one new thing that you learned by creating and presenting your own model?
These questions were designed to allow the students to reflect on their learning gains and to provide an entry point for review and studying.
Student comprehension of the concepts that were modeled was assessed on an end-of-unit exam as well as on the cumulative course final exam. Sample quiz and exam questions are provided below:
- Name the variable you altered in your enzyme model and briefly explain how you demonstrated it with modeling compound. Refer to the enzyme, substrate, active site, and product in your answer.
- Choose one of the following variables: competitive inhibition, allosteric effects, or feedback inhibition. Then, draw (or describe) how this variable changes/affects a typical enzymatic reaction.
- Indicate whether you agree or disagree with the following statement: Enzymes operate at a fixed rate that cannot be influenced by local conditions. Then, provide specific details that support your argument. Be sure to use examples that relate to biology, use appropriate terminology, and remember to be precise but concise.
- Indicate whether you agree or disagree with the following statement: An allosteric inhibitor that does not influence the three-dimensional shape of an enzyme will not inhibit the enzyme's typical activity. Then, provide specific details that support your argument. Be sure to use examples that relate to biology, use appropriate terminology, and remember to be precise but concise.
- A molecule binds to the active site of an enzyme, interfering with substrate binding. This is an example of _____________.
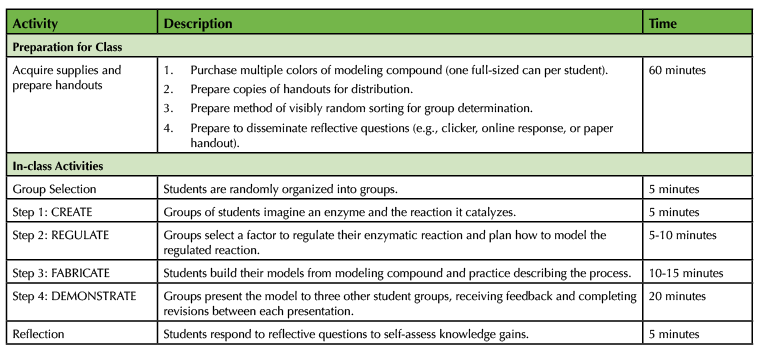
Table 1. Investigating enzyme structure and function - teaching timeline
TEACHING DISCUSSION
This activity has several benefits. First, the students actively engage with the material by physically creating and manipulating structures. Second, students teach their peers, which promotes mastery of content and allows the students to identify gaps in their knowledge that must be filled. The activity involves immediate formative assessment from peers and the instructor, which allows inaccuracies and misconceptions to be corrected before they are practiced. Because the activity is low-stakes, mistakes do not result in a grade penalty and become a learning opportunity rather than a stressful risk. Finally, the activity requires the students to apply their knowledge of enzymatic reactions to a novel scenario, which tests their understanding at one of the highest levels of Bloom's Taxonomy (17).
When surveyed, students overwhelmingly reported both enjoying and learning from the activity, and multiple students referenced their models when discussing enzymatic reactions on the final exam months later. In a mid-semester course survey, students were asked the following question: "Which in-class activity helped you learn the most? Why?" Many students referred to the enzyme modeling activity, and some of the responses are provided below:
- "I like the modeling activities because if you're confused about something you can see how someone else understands it."
- "It helped me put it all together."
- "It gave a visual to see how something worked even though we can't physically see it."
- "Actually having to shape parts and having to think about why it's shaped the way it is and how it functions helps me."
- "Explaining [enzymes] to other people really helped."
- "I liked the [modeling compound] because we got to see all types of enzyme reactions in a fun, easy to remember way."
- "It definitely helped me understand the topic."
- "Modeling helped me to take what [the instructor] says and put it into real life scenarios."
- "The modeling activities make sure you know and understand the information."
Adaptations
This activity has been successfully implemented by a single instructor in classes of up to 48 students. In larger classes, the number of students may prevent the instructor from providing useful feedback to all groups. In these situations, the instructor could either utilize the help of a teaching or learning assistant or complete the activity during smaller recitation sections.
For upper-level courses or class sessions lasting longer than 50 minutes, the Lesson can be expanded to include additional variables to be modeled. Examples of additional variables include the effect of temperature or pH, requirement of a cofactor or coenzyme, and reaction reversibility. Students can also be prompted to model additional details, such as induced fit and lowered activation energy.
If desired, this activity can be followed with an optional extension homework assignment. This option requires additional background information, which is described in the student prerequisite knowledge section. To complete the assignment, students should take home modeling compound and work individually to create new models from an expanded list of processes. Each student will then take photos and turn these images into multi-panel textbook figures, for which they will write corresponding figure legends to demonstrate mastery of the material. Examples of the homework instructions and grading rubric are provided (Supporting File S3: Enzyme Structure Function - Optional Extension Homework Activity and Rubric).
SUPPORTING MATERIALS
- S1. Enzyme Structure Function: Student Handout
- S2. Enzyme Structure Function: Examples of Student Work
- S3. Enzyme Structure Function: Optional Extension Homework Activity and Rubric
ACKNOWLEDGMENTS
The authors wish to thank Dr. Allison Jablonski, Dr. Kati Geszvain, and Dr. Jamie Brooks, who provided feedback after implementing this Lesson in their sections of introductory biology.
References
- U.S. Department of Health and Human Services, National Institutes of Health, National Institute of General Medical Science. 2007. The Structures of Life (NIH Publication No. 02-2778).
- Bretz SL, Linenberger K J. 2012. Development of the enzyme-substrate interactions concept inventory. Biochem Mol Biol Educ 40: 229-233.
- Linenberger KJ, Bretz SL. 2015. Biochemistry students' ideas about how an enzyme interacts with a substrate. Biochem Mol Biol Educ 43: 213-222.
- NGSS Lead States. 2013. Topical arrangement of the Next Generation Science Standards. Next Generation Science Standards: For States, By States. Washington, DC: The National Academic Press.
- American Association for the Advancement of Science. (2011). Vision and change in undergraduate biology education: a call to action. Washington, DC: AAAS.
- Passmore C, Gouvea J, Giere R. (2014). Models in science and in learning science: Focusing scientific practice on sense-making p. 1171-1202. In Matthews MR (ed), International Handbook of Research in History, Philosophy and Science Teaching. Springer.
- Aergerter-Wilmsen T, Coppens M, Jannsen F, Hartog R , Bisseling T. 2005. Digital learning material for student-directed model building in molecular biology. Biochem Mol Biol Educ 33: 325-329.
- Taylor I, Barker M, Jones A. 2010. Promoting mental model building in astronomy education. Int J Sci Educ 25: 1205-1225.
- Herman T, Morris J, Colton S, Batiza A, Patrick M, Franzen M, Goodsell DS. 2006. Tactile teaching: Exploring protein structure/function using physical models. Biochem Mol Biol Educ 34: 247-254.
- Hubbs NB, Parent KN, Stoltzfus JR. 2017. Models in the biology classroom: an in-class modeling activity on meiosis. Amer Biol Teach 79:482-491.
- Roberts JR, Hagedorn E, Dillenburg P, Patrick M, Herman T. 2005. Physical models enhance molecular three-dimensional literacy in an introductory biochemistry course. Biochem Mol Biol Edu 33: 105-110.
- Michael J. 2006. Where's the evidence that active learning works? Adv Physiol Educ 30: 159-167.
- Ambrose SA, Bridges MW, Dipetro M, Lovett MC, and Norman MK. 2010. How Learning Works. San Francisco: Jossey-Bass.
- Topping KJ. 2005. Trends in peer learning. Int J Sch Educ Psychol 25: 631-645.
- Clark MA, Douglas M, Choi J. 2018 Mar 28. Enzymes. In Biology 2e. OpenStax; [accessed 2019 Dec 19]. https://openstax.org/books/biology-2e/pages/6-5-enzymes
- Reece JB, Urry LA, Cain ML, Wasserman SA, Minorsky PV, Jackson R, Campbell N. 2017. P 153-161. In Campbell Biology, 11(th) ed. Pearson Higher Ed, Hoboken, NJ.
- Anderson LW and Krathwohl DR, eds. 2001. A Taxonomy for Learning, Teaching, and Assessing: A Revision of Bloom's Taxonomy of Educational Objectives. New York: Longman.
Article Files
Login to access supporting documents
Investigating Enzyme Structure and Function Through Model-Building and Peer Teaching in an Introductory Biology Course(PDF | 464 KB)
S1 Enzyme Structure Function - Student Handout.docx(DOCX | 26 KB)
S2 Enzyme Structure Function - Examples of Student Work.v2.docx(DOCX | 5 MB)
S3 Enzyme Structure Function - Optional Extension Homework Activity and Rubric.docx(DOCX | 19 KB)
- License terms

Comments
Comments
There are no comments on this resource.