Targeting Misconceptions in the Central Dogma by Examining Viral Infection
Editor: Lisa M. McDonnell
Published online:
Abstract
Understanding the central dogma and how changes in gene expression can impact cell function requires integration of several topics in molecular biology. Students often do not make the necessary connections between DNA structure, transcription, translation and how these processes work together to impact cell function. This lesson seeks to tie together these concepts through the use of data from primary literature, in the context of viral infection. This lesson asks students to think like scientists as they design experiments, make predictions and interpret and evaluate data from primary literature on how changes in the expression of a glucose transporter gene can alter the function of a cell through changes to glucose uptake and metabolism. This lesson incorporates the Vision and Change core concept of information flow and the core competency of quantitative reasoning. It also addresses The Genetics Society of America learning framework goal of Gene Expression and Regulation (How can gene activity be altered in the absence of DNA changes?). This lesson was taught in three sections of a small-enrollment undergraduate class and assessed summatively using a pre/post test and formatively using in class via personal response systems. This lesson describes the design, implementation and results of student assessment, and offers suggestions on how to adapt the materials to a variety of contexts including different class sizes, different units of introductory biology, and upper-level classes.
Citation
DeVito SR. 2021. Targeting misconceptions in the central dogma by examining viral infection. CourseSource. https://doi.org/10.24918/cs.2021.31Society Learning Goals
Genetics
- Gene Expression and Regulation
- How can gene activity be altered in the absence of DNA changes?
Lesson Learning Goals
- Students will determine how changes in gene expression via transcription and translation alter the function of a cell.
- Students will explore how scientists examine changes in gene expression to test a hypothesis about cell function, through making predictions and examining and interpreting original data.
Lesson Learning Objectives
Students will be able to:- Design an experiment to test glucose uptake in viral-infected cells compared to uninfected cells and graph the predicted results.
- Compare and contrast two glucose transport proteins.
- Predict whether viral infection will alter transcription and translation of infected cells.
- Interpret and draw conclusions based on primary data.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
Introduction
Students enter college-level introductory courses with the knowledge that traits are determined by genes, and have some understanding that genes contain information that can be passed down (1,2,3). Few students enter college-level courses with the knowledge that gene expression leads to protein production, and that this results in phenotypic variation (4,3). Students often understand that mutations lead to phenotypic changes, but understanding that cell function can change as levels of gene expression change without a mutation can be challenging (5). The central dogma, that information is transferred from DNA, to RNA, to proteins, indicates how genetic information is stored and transferred. Information flow is one of the five core concepts identified by the American Association for the Advancement of Science (AAAS) Vision and Change report (6). The Genetics Concept Assessment was developed to assess student understanding of genetics misconceptions (7). Student performance measured by this assessment revealed that one of the most common incorrect ideas students have is that different cells in individuals contain different genes (8). This misconception was also identified through the analysis of the Central Dogma Concept Inventory (9). Students often do not appreciate that it is the variation in gene expression that leads to different phenotypes within an individual, and not differences in genomic content. This lesson seeks to address this misconception through examining how gene expression and therefore cell function changes during the course of a viral infection, and to connect this information back to the central dogma.
In addition to misconceptions around the central dogma, another challenge in introductory biology courses is introducing primary literature and interpreting data. Quantitative reasoning skills, identified as a core competency in the by the American Association for the Advancement of Science (AAAS) Vision and Change report (6), are essential for the application of knowledge and therefore essential to many careers, including Biology (6,10). Research shows that including quantitative reasoning in undergraduate biology courses can help improve these necessary skills, along with reasoning in other disciplines such as math (11, 12). Complex terminology, complicated figures and unfamiliar techniques can leave students overwhelmed and frustrated (13), as many students struggle to interpret graphs and summarize trends in a biological context (11,14). While many lessons focus on reading and evaluating an entire paper from the primary literature, this lesson focuses on introducing students to analysis of data from primary literature, so that primary data can be interwoven with challenging biological concepts (15).
Misconceptions about the central dogma, while challenging, can be addressed in several places within an introductory biology curriculum. For example, the lessons from Pelletreau et. al and Connor address how mutations can impact different components of the central dogma (16,17). The lesson from Mann et. al helps students link genotype to phenotype in plants (18). The lesson described here addresses how gene activity can be altered in the absence of changes to the DNA in the context of viral infection. This lesson can be used as a bridge to understanding that endogenous changes in gene expression can alter cell function, such as how epigenetic changes alter transcription, and therefore translation and cell function.
This activity uses Human Cytomegalovirus (HCMV) to illustrate how gene expression can change in a cell, and how this influences cell function. While HCMV is a little-known virus, the virus makes many modifications to host gene expression, activating genes not normally expressed in infected cells and rewiring metabolic and other essential host pathways in order to obtain the macromolecules needed for viral replication (19). HCMV has a DNA genome and is in the herpes virus family. An estimated up to 90% of the worldwide population has been infected with HCMV (20). Most individuals are asymptomatic, however HCMV poses a serious risk to immunocompromised individuals and infants who are congenitally infected (20,21). Complications to immunocompromised individuals can include blindness, pneumonia, encephalitis, or a loss of transplanted organs (20). Twenty percent of children born with congenital infections develop complications which can include hearing loss, vision loss, cerebral palsy and cognitive impairment (21). By using this virus as a model, this lesson aims to help students explore how changes in gene expression can change cell function, creating a bridge between the central dogma and cell function.
Because this lesson also discusses protein function and transport of glucose, it can be used as an introduction to cell biology, genetics and/or metabolism. This lesson also ties in macromolecules by discussing sugars, RNA and protein. The use of primary data that shows changes in mRNA and protein levels helps students to visualize changes in transcription and translation while also practicing interpreting data from primary literature. Overall, this lesson aims to connect the central dogma to tangible changes in cell function made visible through data from primary literature.
Intended Audience
This lesson is intended for undergraduate introductory biology courses. It was given to students in a small enrollment (48 students) Introductory Biology course for majors and non-majors at the University of Delaware composed primarily of freshmen (n = 144, students divided into three sections). This activity has also been taught in two large-enrollment introductory biology courses; one for majors (with 650 students), and one for non-majors (with 850 students).
Required Learning Time
This lesson was designed for two 50-minute class periods but could be modified slightly to fit into one 75-minute period. See Table 1 for suggestions on formatting for different class periods.
Table 1. Timelines for progressing through the lesson. Classroom discussion open-response opportunities are identified by “TPSQ” (Think-Pair-Share Question). Clicker Questions are denoted by “CQ.”
| Activity | Description | Estimated Time |
|---|---|---|
| Preparation for Class | ||
| Introduction to the central dogma and membrane proteins. |
Students should have covered transcription, translation and membrane protein function prior to this activity. This activity works best as a method for students to synthesize these ideas together. Make one copy of the student worksheet for each student, or share via your learning management system or online platform of choice (Supporting File S1. Infection and Gene Expression - Instructor Resources on viruses, HCMV and GLUT proteins). Students should be familiar with the following terms through either reading or a previous class:
|
|
|
Pretest |
The pretest questions can be administered in two ways; either outside of class as a homework assignment or online quiz for completion points (recommended for completing the lesson in one 75 minute class), or in class using the slides provided if time permits (pre/post test questions and answers are provided in Supporting File S2. Infection and Gene Expression- Lesson Presentation Slides with Instructor Notes, and in the lesson slides, Supporting File S4. Infection and Gene Expression- Student Worksheet). | ~5 min |
| Class Session – Progressing Through the Activity | ||
|
1. Introduction to glucose transport proteins Slides 6-10 |
|
~12 min |
|
2. Introduction to Human Cytomegalovirus (HCMV) Slide 11 |
In small groups of 2-3, students should answer questions about HCMV on Part II of the worksheet (Supporting File S1. Infection and Gene Expression - Instructor Resources on viruses, HCMV and GLUT proteins). This can be done using outside resources, including the internet (~5min). This should be followed up with students reporting out, and a facilitated discussion on how viruses can have a DNA or RNA genome, and how immunocompromised individuals, pregnant women and their children, and newborn infants are at risk for this virus. Overall, HCMV is one of the most common causes of birth defects in the United States. |
~8 min |
|
3. The relationship between HCMV and cell metabolism; student experimental design and visualizing results Slides 12-15 |
|
~23 min |
|
4. Measuring GLUT gene expression at the mRNA and protein level Slides 16-24 |
|
~35 min |
|
5. Implications for treating HCMV and conclusions Slides 25-36 |
|
~5 min |
| Follow-Up | ||
| Post-test |
Post-test clicker questions (Supporting File S2. Infection and Gene Expression- Lesson Presentation Slides with Instructor Notes). If short on time or if completing the less in one 75-minute class, provide students with post-test questions outside of class |
~10 min |
Prerequisite Student Knowledge
Before this lesson, students completed a unit on DNA structure, transcription, translation, and protein structure including a summative exam. Immediately prior to this lesson, students were asked to complete an assigned textbook reading with questions on membrane proteins and active and passive transport, and participated in a short interactive lesson with clicker questions on membrane proteins and active versus passive membrane transport. Students had limited experience developing and testing hypotheses and graphing and interpreting data in the laboratory component of this course. Previous knowledge of viruses is not required, background information on viruses is introduced through the worksheet associated with this lesson.
Prerequisite Teacher Knowledge
Instructors should be familiar with techniques used to measure mRNA and protein levels, specifically RT-qPCR and Western blotting, and the basics of viral genomes, impacts on host cell function, and public health in the context of Human Cytomegalovirus (19). Resources on the basics of virology and more in-depth information on HCMV have been included in Supporting File S1: Infection and Gene Expression – Instructor Resources on viruses, HCMV and GLUT Proteins. Instructors would also benefit from understanding that infection of cells with HCMV leads to an increase in overall host cellular respiration similar to the Warburg effect observed in cancer cells. Instructors may find it helpful to review the primary paper from which the figures and hypothesis originated. More information about the figures used in this lesson is included in the instructor notes in Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, as well as in Supporting File S3: Infection and Gene Expression – Image References Table. Instructors should also have a basic understanding of tissue culture including that cells are incubated in a supplemental media that provides nutrients, including glucose, for survival.
Instructors should also be comfortable using technology to poll students on in-class questions and for administering pre/post-tests. For instructors without access to clickers, one can use a printed version of the pre/post-tests or display questions on a slide. To collect in-class student responses, instructors can use other polling techniques such as colored index cards where each color corresponds to a different answer option, raising hands (e.g., students raise hands if they selected answer choice A, if they selected choice B, etc.), or an electronic polling alternative such as Zoom polls, or Poll Everywhere.
Scientific Teaching Themes
Active Learning
Students actively engage in learning throughout the lesson. The provided worksheet (Supporting File S4: Infection and Gene Expression – Student Worksheet) contains several think-pair-share questions (abbreviated as TPSQ). In these sections, students should individually consider their own answers, and then pair up with another student for discussion. The output for these questions includes both short-answer response and graphing predictions of experimental outcomes.
This lesson includes many clicker questions that are used to probe student’s interpretation and understanding of how to draw conclusions from data from primary literature. In the case of a split student response, student discussion, re-polling and a group discussion can be used to address misunderstanding and misconceptions.
Lastly, this lesson includes a section where students research background information and answer guided questions on HCMV individually using the internet. This gives students the opportunity to explore the information on their own and find appropriate sources instead of listening to a lecture. Students can also discuss different websites they have found and any discrepancies they find in small groups as they are researching.
Assessment
Students were assessed using formative and summative questions in alignment with the learning objectives: designing an experiment, comparing and contrasting proteins, predicting the effect of infection on cell function, and drawing conclusions from primary data.
During the lesson: This lesson uses both clicker questions and think-pair-share questions for formative real-time assessment of student understanding throughout the lesson.
Pre/Post Assessment: Four questions were also used immediately before and after the lesson for administration of a pre/post-summative assessment. Pre/post-test questions are provided in Supporting File S5: Infection and Gene Expression - Pre/Post Test and Clicker Questions and Student Responses. These questions could also be administered on paper, or through a learning management system. Since the pre-test measures student understanding prior to the lesson and the same questions will be used for the post-test, the questions and answers should not be discussed before or during the lesson.
Inclusive Teaching
This lesson aims to foster an inclusive learning environment through the use of a variety of different methods of student engagement including the student worksheet, projected slides, and both instructor-led and student-led discussions. Using a variety of strategies engages a broader population of students (22). Students engage in multiple-choice clicker questions both individually and with class discussion, and in think-pair-share questions. Having students discuss with peers before sharing to the entire class helps to minimize student discomfort by allowing them to talk one-on-one with peers as opposed to speaking in front of the entire class, thus still encouraging students to collaborate and discourages competition (22). Whole class discussion in the “share” portion of the think-pair-share can be enhanced and made more equitable by calling on a variety of groups/individuals or quickly going around in a “whip around” (22). Students designed their own hypothesis and experiment, predicted the results through drawing, and observed and interpreted original data. This allowed students to be creative in their scientific approach and also explore previously published literature.
Lesson Plan
This lesson is designed for two 50-minute lectures, but could be taught in one 75-minute class by moving the pre and post-test outside of class. This lesson is intended to tie together the core concepts of the central dogma (transcription, translation and protein function) and apply the central dogma to a real-life experimental design to examine how changes in gene expression can impact cell function. This lesson can be used at the end of a unit on the central dogma, or follow a lesson on membrane transport and integral membrane proteins. Table 1 provides the progression of the lesson with estimated timing. This can also be used as a bridge between the conclusion of a unit on the central dogma and the beginning of a unit on metabolism.
Pre-Class Preparation
Students should be familiar with the processes of transcription, translation and membrane transport, specifically; active versus passive transport, diffusion, selective permeability of the cell membrane, and integral membrane proteins and their role in membrane transport.
Instructors will need to facilitate student discussions during this lesson. Additional instructional resources on the background of Human Cytomegalovirus and its impact on glucose transporters are provided in Supporting File S1: Infection and Gene Expression – Instructor Resources on viruses, HCMV and GLUT Proteins. Students do not need any background knowledge on Human Cytomegalovirus and/or viral structure and function.
Classroom Discussions Using Think-Pair-Share and Clickers
Think-Pair-Share (abbreviated in this paper as TPS) is a classroom-based active learning strategy in which the instructor poses a problem or question to students. Students then take a few minutes to individually work through the problem, and then work in pairs to compare their individual solutions and answer the question. This is followed by a whole-class discussion where students share their answers (22,23). This model allows for individual thinking followed by immediate feedback from peers and the instructor. Combining both peer-led and instructor-led discussion has been shown to yield greater students gains than either discussion on its own (25). This case study uses TPS both to facilitate discussion following closed-ended clicker questions, but also for open-ended TPS discussion questions written in the worksheet (Supporting File S4: Infection and Gene Expression – Student Worksheet). TPS can also be used with clicker questions for which students were split on their individual responses. Following the individual poll, which serves as the “think” portion, have students discuss in pairs, and then re-poll using clickers. Follow up with the “share” portion by having a whole-class discussion about why the incorrect answers are incorrect and any questions that arose during sharing. Whole-class discussion can be enhanced through inclusive techniques such as the whip around, calling on random students/groups, calling students by name, and others (22). Further ideas for implementing clickers in the classroom are described in Jane Caldwell’s paper (24) . Open-ended TPS questions are noted in the instructor slides and student worksheets as “TPSQ.” Clicker questions are noted in the instructor slides and student worksheets as “CQ.”
Progressing Through the Lesson
Assessing Prior Knowledge (~5 min)
The lesson begins by examining student prior knowledge about how glucose moves in and out of the cell, and how gene expression can be measured experimentally (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slides 1-5). These pre-assessment questions can be administered via clickers at the beginning of class, or as a pre-class homework if completing the lesson in one 75-minute session. It is helpful to tell students that these questions inform the instructor about what they know and don’t know so adjustments can be made to the lesson in real time. If students are divided in their answers, one can tell them they will discover the answer in the lesson as a way to maintain student engagement.
1. Introduction to Glucose Transport and Proteins (~12 min)
Following the pre-class assessment, or at the beginning of class if the pre-assessment was done outside of class, the instructor begins by informing students that they will be using the scientific method to design an experiment and make predictions about how viral infection might impact transcription and translation of transport proteins during infection (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 6). The instructor then introduces the concept of glucose transporters (abbreviated as GLUT) that are integral membrane proteins responsible for moving glucose across the membrane and into the cell, and that this glucose is required for production of ATP which provides energy for the cell (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 7). The instructor discusses how the GLUT proteins undergo a change in their shape, which is known as a conformational change, in order to move glucose into the cell. The instructor can use this opportunity to revisit protein function, and ask students what level of protein structure is changing during a conformational change (tertiary structure). This is also an opportunity to revisit selective permeability of membranes, and what properties limit glucose from freely diffusing through the cell membrane in sufficient quantities for cell survival. In order to activate student prior knowledge on the role of proteins in transporting molecules across the cell membrane, the instructor asks students to use their knowledge of protein transport to describe how GLUT1 works using the diagram on the worksheet (Supporting File S4: Infection and Gene Expression – Student Worksheet).
TPSQ1: Examine the following diagram of a Glucose Transporter (GLUT) in action and answer the following questions: A. What kind of transport do you think is occurring? (Hint, simple diffusion, facilitated diffusion, primary active transport, secondary active transport). B. What kind of membrane transporter do you think GLUT is? (Hint, channel, carrier, pump or antiporter)?
Students brainstorm individually for 2-3 minutes about the type of transport they think is occurring based on the provided diagram and what kind of membrane transporter they think GLUT is (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 8). After individual work, students pair up and discuss their answers for approximately 2-3 minutes. Following the paired discussion, the instructor can solicit answers from students. Typically, students recognize that the transport is not simple diffusion, but commonly think that because a membrane protein is involved in transport, it must be active transport. An important observation for students to make is that the concentration of glucose outside of the cell is higher than inside the cell, and that by moving into the cell via GLUT, glucose is moving down its concentration gradient towards an area of lower glucose concentration. Another clue that this is not active transport is that no ATP use is indicated, which is required for active transport.
Following this TPS activity, the instructor expands on the GLUT protein by explaining that there are several GLUT proteins that are in the same protein family because they share a similar structure and function due to a common evolutionary origin (26,27) (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 9). The focus of this lesson is on two of the GLUT proteins; GLUT1 and GLUT4 and the instructor reviews the differences between these two proteins (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 10). First, that they are expressed in different tissues; GLUT1 is expressed evenly in most tissues, and GLUT4 is expressed in fat tissue and skeletal muscle tissue but only moves to the cell membrane upon receiving a signal from insulin (26,27). The second difference is the rate of glucose uptake; GLUT4 can take up glucose at three times the rate of GLUT1 (26,27). The instructor transitions to the next part of the lesson by explaining that these GLUT proteins might play an important role in the infection of a cell by a virus known as Human Cytomegalovirus, or HCMV.
2. Introduction to Human Cytomegalovirus (HCMV) (~8 min)
The second component required for understanding the upcoming experimental designs and data is understanding the basics of HCMV. In order to explore HCMV and its impact on human health, students will investigate on their own, using the guided questions in the worksheet (Supporting File S4: Infection and Gene Expression – Student Worksheet). Discuss with students before the activity what types of websites are quality sources. Choosing a government website such as the Centers for Disease Control website, or a website that references peer-reviewed literature will provide them with trustworthy information. You may also ask students to individually research and answer the questions, and then compare their findings with their group members to see if their results are consistent.
Part II - Using any tools at your disposal (Internet, Wikipedia, CDC, etc.) answer the following questions: a. What family is this virus in? b. What kind of genome does HCMV have? (hint; DNA or RNA). c. What groups of people are at the greatest risk of illness from HCMV?
This information is available to the general public in places such as the CDC website or Wikipedia. Students individually research and answer the questions on the worksheet for approximately 5 minutes (Supporting File S4: Infection and Gene Expression – Student Worksheet). After a few minutes of individual research, students can begin to compare answers with each other, and this may help to reinforce the use of reliable sources. After about 5 minutes, the instructor brings the class back together to report out their answers. HCMV is in the Herpes family of viruses, and is related to (but not the same as) the more commonly known Herpes Simplex Virus-1, the virus known for causing cold sores, among other illnesses. This is an opportunity to discuss how viruses are grouped into families based on how closely they are related through evolutionary history. For example, there are many types of influenza viruses, but they are all in the same family. For question B, HCMV has a DNA genome. This will seem intuitive to students because they learned the central dogma that DNA is the heritable information that codes for mRNA which codes for proteins. Here, the instructor can introduce the concept of a viral RNA genome if desired. Some examples of viruses that have an RNA genome include influenza, HIV, Ebola, rabies, measles, coronaviruses, the common cold, and many others they may have heard of. Comparing and connecting HCMV to viruses of which students are already aware helps to keep them engaged. For question C, the people most at risk from HCMV include immunocompromised individuals, such as cancer patients or those with an HIV infection, and pregnant women and newborns who are at risk for congenital infection. The statistics of babies born with a congenital HCMV infection are surprising; one out of every 200 infants is born with a congenital HCMV infection and one in five of those will have long-term health problems including hearing loss (21,28). Despite the fact that many have not heard of it, HCMV is one of the most common causes of birth defects in the United States. This statistic really engages students, especially those who want to go into medicine. The instructor can then ask students if they came across any information on how to cure someone of HCMV. This leads to the discussion that because there is no vaccine and treatments are limited, the virus often becomes resistant in three months, and most treatments cannot be administered to infants (21,28). Therefore, it is important for scientists to gain a better understanding of how HCMV works, so we can develop better treatments for it.
Instructors may desire to put HCMV into context of other viruses which students have prior knowledge about, such as SARS-CoV2 or influenza. If the instructor opts to discuss this in the context of other viruses, students can research and answer the questions in Part II of the worksheet for not only HCMV, but for other viruses such as SARS-CoV2, and then compare and contrast the different genomes, families and public health risks of the viruses (Supporting File S4: Infection and Gene Expression – Student Worksheet). Recommended literature which provides an overview of SARS-CoV-2 for teachers is listed in Supporting File S1: Infection and Gene Expression – Instructor Resources on viruses, HCMV and GLUT Proteins (29,30).
3. The relationship between HCMV and cell metabolism; student experimental design and visualizing results (~23 min)
Once students understand the importance of investigating how HCMV infection works, the instructor transitions to aspects of HCMV we can study in order to better understand how HCMV infects cells. A group of scientists led by Alwine et. al observed that cells infected with HCMV generated more ATP than uninfected cells (31). If the course has not yet covered metabolism, the instructor should introduce the idea that ATP is the energy currency of the cell, is used as an energy source for a wide variety of cellular processes that keep the cell alive, and that it is generated in our cells through the breakdown of glucose. This ties HCMV and its use of ATP back to the topic of glucose uptake that started the lesson at the beginning of class. Based on this observation, Alwine et. al generated the hypothesis that “HCMV infected cells take up more glucose than uninfected cells” (31). Showing students how an observation leads to a hypothesis demonstrates the scientific method in practice (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 12). Next, the instructor transitions from hypothesis to experiment by introducing the tools that Alwine et. al used to test this hypothesis: human cells growing in petri dishes, supplemented with media. Media contains the nutrients cells need to survive in the petri dish, including glucose, and the amount of glucose in the media can be measured using glucose monitors (31). Students will synthesize the observations and tools provided to design an experiment to test Alwine et al.’s hypothesis.
Part III - TPSQ2 - Observation: HCMV infected cells generate more ATP for energy than uninfected cells. You want to test the hypothesis that “HCMV infected cells take up more glucose than uninfected cells.” Design an experiment to test this hypothesis using cells growing in a Petri dish. Be sure to include what you will measure, and what your control will be.
Given the tools discussed in the previous slide (human cells growing in petri dishes with media, media containing glucose, and glucose monitors) students work in groups designing an experiment to test the provided hypothesis that HCMV infected cells take up more glucose than uninfected cells. (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 12). Sometimes students struggle with visualizing the set-up of human cells growing in petri dishes and how they can be used in experiments. The instructor can walk around talking over ideas with individual groups of students. The goal is for the students to have two petri dishes of human cells; one control dish with human cells and no infection, the other with human cells and HCMV infection. Students should then propose putting media with an equal amount of glucose in each dish, and measuring how much glucose is left in the media after some period of time. Figure 1A shows the experiment students are asked to create. Glucose concentrations shown in Figure 1A are arbitrary, and included only for understanding. The quantitative change in glucose concentration are not important but rather the change in media glucose concentration over time. The goal is for students to understand that HCMV infected cells take up more glucose than uninfected cells, which will result in less glucose remaining in the media of infected cells compared to uninfected cells.
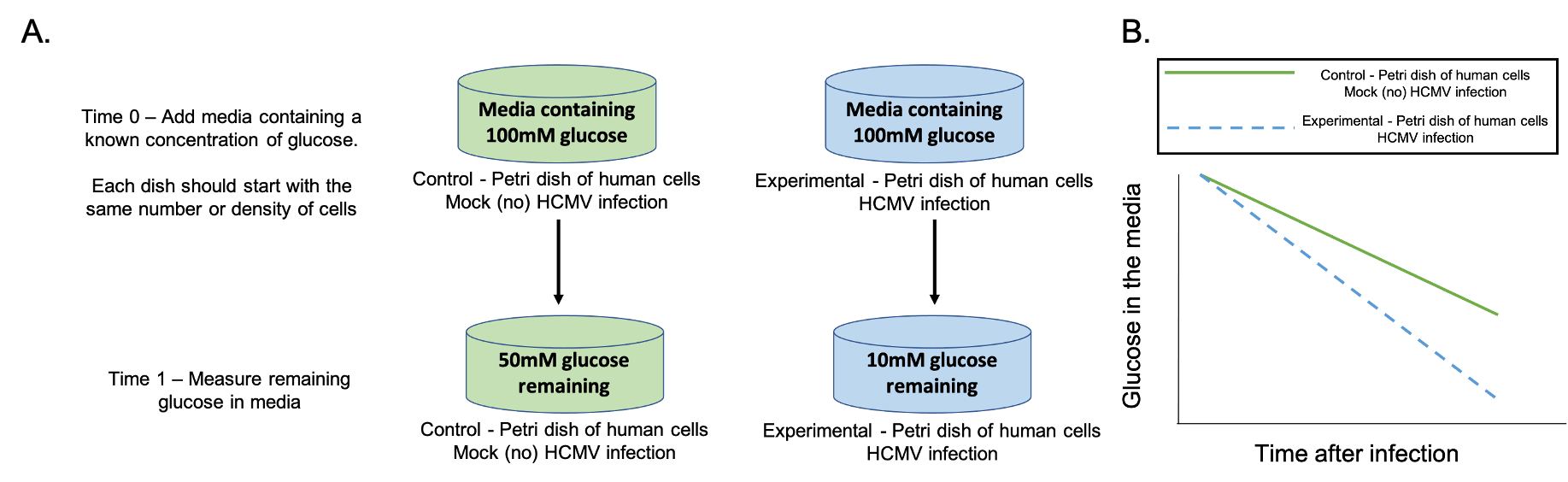
This is a good time to help students strengthen their experimental design skills. Students will often suggest measuring glucose or ATP inside the cell. Here the instructor may ask them how they would take such measurements without killing the cell, and if there are easier ways to measure glucose. Sometimes students want to have their experimental control be an empty petri dish without cells. In this instance, the instructor can ask them how they would know glucose uptake is different from that in a healthy cell. Ideally, students get to an experiment where they measure glucose remaining in the media in infected versus uninfected cells after a period of time.
TPSQ3 – Based on the experiment conducted by Alwine et al., draw a graph predicting how glucose levels in the media will change if their hypothesis is correct. Be sure to label your axis and label your results for your control and experimental groups.
Students sketch a graph of what they predict their experimental results will look like if the results support the hypothesis (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 13). Figure 1B shows the graph students are intended to draw. A common misconception is that glucose will go up in concentration. If this happens, the instructor can prompt students to sketch a cell in the petri dish, and show with arrows where glucose is going and how the glucose concentrations will change inside the cell versus outside the cell. This usually helps students realize that they are measuring glucose in the media outside of the cell, and as the cell takes up glucose, the glucose concentration in the media will decrease. Another misconception is that the uninfected control will be a straight line with a slope of zero. If this idea is voiced, the instructor can bring students back to the initial observation, and why the uninfected cells take up glucose. The instructor can ask students whether a healthy living cell needs to produce ATP, and if so, whether it needs to take up glucose. Alternatively, for a whole class discussion, the instructor can draw a graph showing the control line with a slope of zero on the board, and ask these questions to the class as a whole.
CQ1 – What can we conclude from this graph?
In order to check that students are connecting the experimental outcomes to the data on the graph and interpreting the data correctly, students answer a clicker question (CQ1) where they select the best conclusion based on the graph provided – A) infected cells took up more glucose than uninfected cells, B) infected cells took up less glucose than uninfected cells, or C) there was no difference in glucose uptake between the infected and uninfected cells. The provided graph is the original data from Alwine et. al showing their results from the same experiment that students designed in the previous question (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 14 and 15). If most students answer correctly (A, infected cells took up more glucose than uninfected cells), one can skip the peer discussion. The instructor can then ask students, “Based on what we’ve learned so far, how might a cell increase its glucose uptake?” Some students may respond in the context of the whole body, by mentioning food intake or insulin response. Others might mention increased diffusion of glucose across the membrane, or active transport of glucose across the membrane. Some also may suggest increasing the number of GLUT proteins present in the membrane. The instructor should work through these ideas to bring the class discussion back GLUT1 and GLUT4.
4. Measuring GLUT gene expression at the mRNA and protein level (~35 min)
TSPQ4 – If you wanted to measure the amount of GLUT and/or GLUT1 what would you want to measure?
Following the finding that infected cells take up more glucose than uninfected cells, Alwine et. al next asked how the cell was taking up the extra glucose (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 16). They hypothesized that HCMV infection stimulates the expression of GLUT4, leading to an increased uptake of glucose (31). Here the instructor revisits an earlier question regarding the difference between the GLUT1 and GLUT4 transporters. The instructor has students individually write down ideas on their worksheet for approximately 3 minutes, and then discuss with their peers (Supporting File S4: Infection and Gene Expression – Student Worksheet). This is followed with a whole class discussion. The first idea from students is typically to measure protein levels. If students do not suggest measuring mRNA, the instructor can ask students what molecule contains the instructions from the DNA to make the protein. The most common misconception is that DNA can be measured rather than mRNA, and that DNA content and amount varies from cell to cell. If students do not offer “measure DNA” as an answer to this question, you can ask the class “why would we not want to measure DNA levels?” This is a great opportunity to discuss that all cells in an organism contain the same DNA, but not all DNA is transcribed and translated the same in every cell. One can use the metaphor of the genome being like a library: libraries contain many books, but you do not read them all! Similarly, in a genome, one does not express every gene. Just as some people like to read specific books in the library such as mystery or nonfiction, certain cells only express certain genes. In our example, GLUT4 is only expressed in fat tissue and skeletal cardiac muscle cells in healthy cells. When HCMV stimulates expression of GLUT4, this is the expression of a gene in a tissue where it is not normally expressed
TPSQ5 – Measuring Gene Expression. 6. Why do you think Alwine et al. measured mRNA levels? What does that tell them? 7. Predict whether the amount of GLUT4 mRNA will increase, decrease or stay the same. 8. Predict whether the amount of GLUT1 mRNA will increase, decrease or stay the same. 9. Based on your predictions about mRNA, how do you think GLUT1 and GLUT4 protein levels will change?
Students work with peers to answer the questions in TPSQ5 and follow with a class discussion (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 17). mRNA levels will tell Alwine et. al if GLUT1 and/or GLUT4 are being transcribed and if those levels of transcription are changing during infection. For questions 7, 8 and 9 where students predict how mRNA levels of GLUT1 and GLUT4 mRNA and protein levels will change, the instructor can list the predictions from the class discussion as they are suggested. This helps students consider the possibilities while not giving away what was actually observed by the authors (and shown on the next slide).
CQ2 – How does HCMV infection impact GLUT mRNA levels?
In order to check that students are connecting their predictions about mRNA levels to graphical representations of data, students answer a clicker question (CQ2) where they select the best conclusion based on the graph provided – A) the amount of mRNA of GLUT4 and GLUT1 increases during infection, B) the amount of mRNA of GLUT4 and GLUT1 decreases during infection, C) GLUT4 mRNA increases during infection while GLUT1 mRNA decreases, or D) GLUT4 mRNA decreases during infection, while GLUT1 mRNA increases (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 18 and 19). If the majority of students answer correctly (C - GLUT4 mRNA increases during infection while GLUT1 mRNA decreases) peer discussion can be skipped and a follow up question asked about which mRNA was increasing and which was decreasing. The instructor can discuss how mRNA is measured via RT-PCR (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 20). This is also an opportunity to discuss the laboratory technique, especially if students will work on this technique as part of a lab or upper-level course.. However, it is not essential to the lesson if this topic is not relevant or there is not time.
To transition from measuring mRNA to protein, the instructor can ask students what else may vary if mRNA levels vary. Before discussing how GLUT1 and GLUT4 protein levels change, the instructor first discusses how protein is measured via Western blot (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 21). Because the Western blot images can be overwhelming, it is helpful to review this slide prior to showing students the image of the blot. The instructor then shows students the Western blot from Alwine et al. (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 22). Before asking students to draw conclusions from the Western blot, the instructor breaks down the blot by column and by row. It’s important to discuss with students why there are both positive and negative controls. The MIEP is a protein specific to HCMV not found in uninfected cells, and this shows that the uninfected cells are in fact not infected with HCMV. Actin is a protein present in all cells, and this row shows that there is a sample present in each lane, and the similar size Actin bands show that roughly the same amount of protein was put into each lane. The HPI indicates hours post infection, and the blot is showing samples from throughout infection. The M lanes indicate a “mock” infection. These are uninfected cells that went through the same treatments as the infected cells. The V lanes indicate the cells infected with HCMV.
CQ3 – How does infection impact protein levels?
Following the introduction to and break down of the Western blot data, students answer a clicker question (CQ3) where they select the best conclusion based on the Western blot data of GLUT1 and GLUT4 protein levels during healthy and infected cells – A) GLUT4 and GLUT1 protein levels increase during infection, B) GLUT4 and GLUT1 protein levels decrease during infection, C) GLUT4 protein levels decrease during infection while GLUT1 protein levels increase, or D) GLUT4 protein levels increase during infection while GLUT1 protein levels decrease - (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 23 and 24). In the class this lesson was taught, 73% of students answered correctly. The instructor asked students to briefly discuss and repoll, during which 98% of students answered correctly. GLUT1 protein levels decrease early during infection, and are barely detectable at 24 hours post infection compared to the uninfected cells. GLUT4 protein increases during the course of infection and is not detectable in the uninfected cells. The combination of the mRNA and protein data allowed Alwine et. al to conclude that HCMV is increasing the expression of GLUT4 during expression where it would be otherwise not expressed and decreasing the expression of GLUT1 protein.
5. Implications for treating HCMV and conclusions (~5 min)
CQ4 – Can we reduce HCMV replication by targeting GLUT4 – What can we conclude from this data?
The next figure returns to the big picture, of trying to stop or at least limit HCMV infection (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 25 and 26). The instructor explains that there are additional X and Y-axis labels that will be easier to understand. The Y-axis is time after infection as seen in the mRNA and protein data. The Y-axis is viral titer. This is how virologists measure the amount of viral replication that has occurred. Here, Alwine et. al treated infected cells with a drug that inhibits the function of the GLUT4 protein. The three lines indicate a normal control infection, infection with GLUT4 inhibition, and a third set where they inhibited GLUT4 infection starting at 24 hours post infection. Students can ignore the data set where the GLUT4 inhibitor was added at 24 hours post infection. The students are asked to answer a clicker question (CQ4) selecting the best conclusion from the data shown – A) viral replication is completely inhibited by GLUT4 inhibition, B) viral replication is reduced by GLUT4 inhibition, or C) viral replication was not affected by GLUT4 inhibition. In my class, 83% of students answered correctly. Students were then prompted to discuss with their peers, followed by a class discussion, including discussing how the graph might look different if GLUT4 inhibition completely inhibited viral replication. Because viral infection does not drop to zero, there is less new HCMV virus produced when GLUT4 is inhibited, but viral infection is not completely stopped. This result is often seen in HCMV studies. The virus does not rely on only one avenue of obtaining the resources it needs from its host cell in order to replicate itself. This is an important reason why scientists have not been successful in developing a treatment that stops HCMV infection, and why it is important to continue to study how HCMV manipulates its host cell during infection. The lesson is concluded with the summary slide (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slide 27).
Follow-Up (~10 min)
If completing in two 50-minutes sessions, administer the post-lesson clicker questions again (Supporting File S2: Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes, slides 28-36). If completing the lesson in one 75-minute session, administer these as homework outside of class.
Teaching Discussion
Formative and summative assessments were used to reflect on the effectiveness of this lesson plan. Here, I discuss student responses to pre/post multiple-choice questions, and exam questions and how they help to inform the misconceptions that were observed during the lesson and whether progress in debunking these misconceptions was made.
Student Performance and Challenging Student Misconceptions
Pre/Post and Exam Multiple Choice Questions
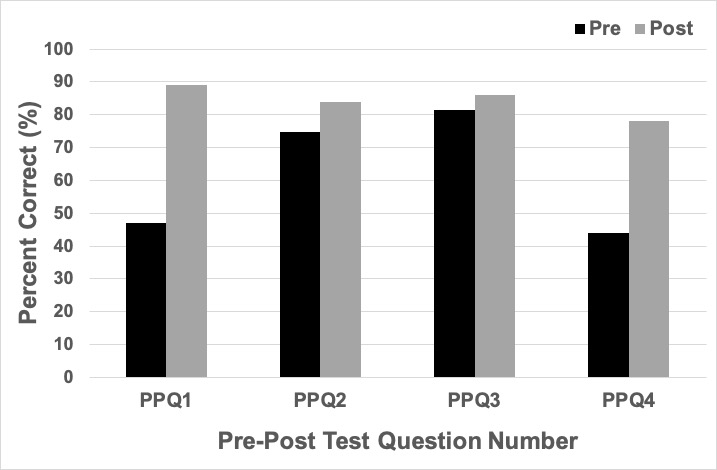
Introductory biology students answered four pre/post multiple-choice summative assessment questions (abbreviated as PPQ). The first three questions focus on understanding the role of proteins in membrane transport, and a lesson on proteins and membrane transport immediately preceded this activity and the pre-test questions. The fourth question targeted the common misconception that DNA amounts and DNA composition vary from cell to cell in one organism. The percent correct for each question for those students who completed all components of this activity is shown in Figure 2 (n = 123).
Across all three sections of this class, the overall average score on the pre-test questions was 62% and the average post-test score 84%. The normalized gain for the overall average score on the pre/post questions was calculated using the following formula (32): [% of students who scored correct on the post-questions - % of students who scored correct on the pre-questions] / [100% - % of students who scored correct on pre-questions]. The normalized gain for the pre/post-questions is
Table 2. Student performance and learning gains on pre/post assessment questions.
| Pre/Post-Test Scores | |
|---|---|
| Overall Average Pre-Test Score | 62% |
| Overall Average Post-Test Score | 84% |
| Normalized Gain for Overall Scores on Pre/Post Test |
0.58 |
| Averaged Normalized Change for Overall Scores for Individual Students |
0.57 |
| Pre/Post Question 1 Normalized Gain Score |
0.8 |
| Pre/Post Question 2 Normalized Gain Score |
0.35 |
| Pre/Post Question 3 Normalized Gain Score |
0.26 |
| Pre/Post Question 4 Normalized Gain Score |
0.61 |
Misconceptions in Active versus Passive Transport
The first three pre/post questions focused on transport across a membrane, and the role of proteins in transport. In my class, this activity immediately followed the lesson on membrane proteins and transport, and the activity gave students practice in analyzing the role of proteins in membrane transport using the specific example of glucose transport through GLUT1 and GLUT4. The first question asks students “how might glucose enter the cell; through active transport, passive transport, or simple diffusion?” This requires students to look at the size and polarity of glucose to determine how likely it is to diffuse through the cell membrane without assistance. 47% of students answered this question correctly in the pre-test questions, compared to 89% on the post-test questions. Most students who missed this question on both the pre-and post-questions selected active transport, but the large increase in correct answers suggests that most students who missed the pre-question gained an understanding of how the GLUT proteins facilitate movement of glucose into the cell, and that proteins can facilitate diffusion. There may be a misconception that any large molecules that cannot diffuse directly through the plasma membrane require active transport to cross the membrane. The second question asks a yes or no question; “do you think enough glucose can freely diffuse across the plasma membrane to meet the cell’s needs?” 75% of students answered correctly in the pre-test questions, and 84% in the post-test questions. This indicates that the majority of students came into this activity understanding that glucose is limited in its ability to diffuse freely through the membrane. The third question was not something previously covered in this class but introduced the concept of membrane proteins that vary in the rate that they allow molecules to cross the membrane. This question asked “T/F: membrane proteins involved with facilitated diffusion can vary in the rate with which they allow their specific molecules to enter/leave the cell.” Surprisingly, 81% of students answered this question correctly on the pre-test, and 86% answered correctly on the post-test questions. This question was intended to make students think about a new concept without additional instruction, and to engage them in an upcoming concept. Questions 2 and 3 had fairly high scores on the pre-test, limiting understanding of whether the lesson improved their knowledge of these concepts. However, it also shows that students had the prior knowledge needed to complete the lesson. These pre/post questions may be useful if it has been some time since students have discussed diffusion across a membrane and facilitated diffusion. This set of questions about membrane transport and what they remembered from this activity was diagnosed on the following exam which was two weeks after this activity. On the exam, students were asked the question “Glucose entering the cell through the GLUT4 transporter is an example of” with the answer choices being simple diffusion, facilitated diffusion, primary active transport, or secondary active transport. 78% of students correctly answered facilitated diffusion. 22% of students incorrectly answered either primary or secondary active transport. This may indicate remaining confusion about active versus passive transport, or may indicate that some students were absent during this activity, or failed to study this concept.
Misconceptions on the Central Dogma
The fourth question is intended to target a misconception that has come up in every class in the past three years of teaching this lesson: the idea that DNA varies in make-up and amounts from cell to cell in an organism. The fourth question (PPQ4) asked, “How can you experimentally measure gene expression?” The answer options were; measuring DNA levels, measuring mRNA levels, measuring protein levels, measuring mRNA and protein levels, or measuring DNA, mRNA and protein levels. Note that a better wording for the first choice is “measuring genomic DNA levels” so students do not confuse genomic DNA with cDNA made during RT-PCR. On the pre-test, 44% of students answered correctly. 40% of students incorrectly answered that DNA, mRNA and proteins can be used to measure gene expression. This highlights the misconception that DNA varies with gene expression changes. 78% answered correctly on the post-test. 11% of students answered incorrectly that DNA, mRNA and protein can be used to measure changes in gene expression. This is a substantial increase in correct answers, suggesting that this activity may be helpful for some students in debunking this misconception. However, 11% of students were still confused at the end of the activity about the concept that DNA is the same in every single cell of an organism. Once can address this misconception during TPSQ4 “how can we measure GLUT expression” if it has not yet come up in student discussions.
This misconception was addressed on the next exam which was two weeks after this activity. Students were asked on the exam “how can you experimentally measure the expression of a gene that codes for a membrane protein?” The answer options were measuring DNA levels, measuring mRNA levels, measuring protein levels, measuring mRNA and protein levels, or measuring DNA, mRNA and protein levels. On the exam question, 85% of students answered correctly, with those answering incorrectly choosing either protein only (8.7% of students), or DNA, mRNA and protein (4.3%). This indicates that the misconception about DNA levels changing with gene expression continued to decrease after this activity. An option for instructors that changes the question but addresses this same misconception would be to ask “Why would measuring genomic DNA not tell you whether a gene is expressed” with the answer being “Because a gene may be present in the genome, but not expressed.”
Additional Suggestions to Enhance Learning
Based on the class discussions in three iterations of this activity, the following are the two misconceptions and challenges that should be targeted and directly discussed with students. 1) DNA in cells varies with gene expression changes. For this challenge, it is useful to have a targeted full-class discussion explicitly addressing that DNA in every cell in an organism has the same composition, but every cell transcribed and translates different genes from that DNA by TPSQ4. Suggestions for debunking this misconception are described in “Progressing through the lesson.”
2) Moving large molecules requires active transport. The first figure in the worksheet is a good time to revisit the concept that active transport can be identified by the use of ATP, and moving a molecule to an area of higher concentration (Supporting File S4: Infection and Gene Expression – Student Worksheet). Students seem to have an easier time identifying active versus passive transport once they have had some practice in looking for these two components.
Possible Adaptations
This case study can introduce either a unit on cell biology or metabolism. For introducing metabolism, the use of glucose to produce ATP can be discussed in more detail to introduce the concept of cellular respiration. For introducing cell biology, one can discuss how DNA methylation and histone acetylation might be used by HCMV to decrease expression of GLUT1, and increase expression of GLUT4. This lesson could also be adapted to an upper-level virology course by increasing the focus on virology and the techniques used in the paper, and used to revisit the central dogma as review. It can also be used to introduce the concept of viruses manipulating host cell metabolism for replication, and other viruses could be discusses as well since many viruses have the characteristic of altering metabolism during infection. In a small class, this lesson would benefit from whole class discussions for the TPSQ’s. Students can also draw their experimental set-up and predicted results for TPSQ2 and TPSQ3 on the board. This lesson also works well in larger classes. In this setting, the group work component for the think-pair-share and clicker questions are essential. A larger class would benefit from the presence of peer mentors if available, who can facilitate student discussions and help students work through misconceptions. If one does not have such resources, it is important to explicitly address any misconceptions or misunderstandings that may exist when reviewing the think-pair-share and clicker questions. The clicker questions interspersed in the case study are intended to help pull discussions together and verify that students are understanding the concepts, which is especially necessary in a larger classroom.
Conclusions
This interactive case study uses clicker questions, written questions, and graphing to synthesize the scientific method, the central dogma and viral infection into one lesson. Students experience how the scientific method frames the experimental study, and challenges them to use quantitative reasoning skills to design experiments and interpret original, published data. This lesson can help students gain confidence in their ability to be scientists by breaking down primary literature into manageable pieces, while also helping them to repair misconceptions about the central dogma.
SUPPORTING MATERIALS
- S1. Infection and Gene Expression – Instructor Resources on viruses, HCMV and GLUT proteins.
- S2. Infection and Gene Expression – Lesson Presentation Slides with Instructor Notes.
- S3. Infection and Gene Expression – Image References Table.
- S4. Infection and Gene Expression – Student Worksheet.
- S5. Infection and Gene Expression – Pre/Post Test and Clicker Questions and Student Responses.
Acknowledgments
This study was conducted under the University of Delaware IRB 1413165-3; Evaluation of ISLL programs and courses.
I’d like to thank James Alwine, the author of “Human Cytomegalovirus Activates Glucose Transporter 4 Expression to Increase Glucose Uptake During Infection” for his permission to reuse the figures from his paper.
I’d like to acknowledge the participation and contributions of my students who participated in this lesson and have provided valuable feedback over the years.
References
- Lewis J, Leach J, Wood-Robinson C. 2000. All in the genes? – young people’s understanding of the nature of genes. J Biol Educ. 34:2, 74-79.
- Marbach-Ad, G. 2001. Attempting to break the code in student comprehension of genetic concepts. J Biol Educ. 35:4, 183-189.
- Reinagel A, Bray Speth E. 2016. Beyond the Central Dogma: Model-Based Learning of How Genes Determine Phenotypes. CBE Life Sci Educ. 15:1-13.
- Lewis J. 2004. Traits, genes, particles and information: re-visiting students’ understanding of genetics. Int J Sci Educ. 26:2, 195-206.
- Bray EB, Shaw N, Momsen J, Reinagel A, Le P, Taqieddin R, Long T. Introductory Biology Students’ Conceptual Models and Explanations of the Origin of Variation. CBE Life Sci Educ. 13:529-539.
- American Association for the Advancement of Science. 2009. Vision and change in undergraduate biology education: a call to action. Washington, DC: American Association for the Advancement of Science.
- Smith, MK, Wood WB, Knight JK. 2008. The Genetics Concept Assessment: A New Concept Inventory for Gauging Student Understanding of Genetics. CBE Life Sci. Edu. 7:422-430.
- Smith MK, Knight JK. 2012. Using the Genetics Concept Assessment to Document Persistent Conceptual Difficulties in Undergraduate Genetics Courses. Genetics. 191:21-32.
- Newman DL, Snyder CW, Fisk JN, Wright KL. 2016. Development of the Central Dogma Concept Inventory (CDCI) Assessment Tool. CBE Life Sci. Edu. 15:1-14.
- National Research Council (NRC). 2003. BIO2010: Transforming undergraduate education for future research biologists. Washington, DC: National Academic Press.
- Bray EB, Momsen J, Moyerbrailean GA, Ebert-May D, Long T, Wyse S, Linton D. 2010. 1, 2, 3, 4: Infusing Quantitative Literacy into Introductory Biology. CBE Life Sci Educ. 9:323-332.
- Hester S, Buxner S, Elfring L, Nagy L. 2014. Integrating quantitative thinking into an introductory biology course improves students’ mathematical reasoning in biological contexts. CBE Life Sci. Edu. 13:54-64.
- Round J, Campbell MA. 2012. Figure Facts: Encouraging Undergraduates to Take a Data-Centered Approach to Reading Primary Literature. CBE Life Sci. Edu. 12:39-46.
- Picone C, Rhode J, Hyatt L, Parshall T. 2007. Assessing gains in undergraduate students’ abilities to analyze graphical data. TIEE. 5:1-54.
- Jebanesan D, Ashok A. 2016. “Reading groups” in an undergraduate biology course: A peer-based model to help students develop skills to evaluate primary literature. CourseSource.
- Pelletreau KN, Andrews T, Armstrong N, Bedell MA, Dastoor F, Dean N, Erster S, Fata-Hartly C, Guild N, Greig H, Hall D, Knight JK, Koslowsky D, Lemons PP, Martin J, McCourt J, Merrill J, Moscarella R, Nehm R, Northington R, Olsen B, Prevost L, Stoltzfus J, Urban-Lurain M, and Smith MK 2016. A clicker-based study that untangles student thinking about the processes in the central dogma. CourseSource.
- Connor, M. 2015. Follow the Sulfur: Using Yeast Mutants to Study a Metabolic Pathway. CourseSource.
- Mann JW, Larson J, Pomeranz M, Knee EM, Shin D, Miller JA, Price, CG, Crist DK, Grotewold E, and Brkljacic J. 2017. Linking Genotype to Phenotype: The effect of a mutation in gibberellic acid production on plant germination. CourseSource
- Rodríguez-Sánchez I, Munger J. 2019. Meal for Two: Human Cytomegalovirus-Induced Activation of Cellular Metabolism. Viruses. 11:273.
- Burny W, Liesnard C, Donner C, and Marchant A. 2004. Epidemiology, pathogenesis and prevention of congenital cytomegalovirus infection. Expert Rev Anti Infect Ther. 2(6): p. 881-94.
- Cannon MJ. 2009. Congenital cytomegalovirus (CMV) epidemiology and awareness. J Clin Virol. 46 Suppl 4: p. S6-10.
- Tanner KD. 2013. Structure Matters: Twenty-One Teaching Strategies to Promote Student Engagement and Cultivate Classroom Equity. CBE Life Sci. Edu. 12:322-332.
- Kothiyal A, Majumar R, Murthy S, Iyer S. 2013. Effect of think-pair-share in a large CS1 class: 83% sustained engagement. Proceedings of the 9(th) Annual ACM International Computing Education Research Conference, San Diego, CA.
- Caldwell J.E. 2007. Clickers in the Large Classroom: Current Research and Best-Practice Tips. CBE Life Sci. Edu. 6(1).
- Smith MK, Wood WB, Adams WK, Wieman C, Knight JK, Guild N, Su TT. 2009. Why peer discussion improves student performance on in-class concept questions. Science. 323:122-124.
- Landini MP. 1984. Early Enhanced Glucose Uptake in Human Cytomegalovirus-infected Cells. J Gen Virol. 65:1229-1232.
- Mueckler M, Thorens B. 2013. The SLC2 (GLUT) Family of Membrane Transporters. Mol Aspects Med. 34:121-138.
- Pass RF. 2001. Cytomegalovirus. Fields’ virology 4th edition. NY: Knipe DM, Howley PM, Eds.; Lippincott-Williams and Wilkins.
- Ludwig, S, & Zarbock, A. 2020. Coronaviruses and SARS-CoV-2: A Brief Overview. Anesthesia and analgesia, 131(1): 93–96.
- Astuti I, Ysrafil. 2020. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 14(4): 407-412.
- Yu Y, Maguire TG, Alwine JC. 2011. Human Cytomegalovirus Activates Glucose Transporter 4 Expression to Increase Glucose Uptake During Infection. J. Virology. 85:1573-1580.
- Hake RR. 1998. Interactive-engagement versus traditional methods: a six- thousand-student survey of mechanics test data for introductory physics courses. Am. J. Phys. 66:64-74.
- Marx JD, Cummings K. 2007. Normalized change. Am. J. Phys. 75:87-91.
Article Files
Login to access supporting documents
DeVito-Targeting Misconceptions in the Central Dogma.pdf(PDF | 388 KB)
S1. Infection and Gene Expression - Instructor Resources on viruses HCMV and GLUT proteins.docx(DOCX | 19 KB)
S2. Infection and Gene Expression- Lesson Presentation Slides with Instructor Notes.pptx(PPTX | 21 MB)
S3. Infection and Gene Expression- Image References Table.xlsx(XLSX | 10 KB)
S4. Infection and Gene Expression- Student Worksheet.docx(DOCX | 8 MB)
S5. Infection and Gene Expression- PrePost Test and Clicker Questions and Student Responses.docx(DOCX | 180 KB)
- License terms

Comments
Comments
There are no comments on this resource.