Meiosis Remodeled: Inclusion of New Parts to Poppit Bead Models Enhances Understanding of Meiosis
Editor: Leocadia Paliulis
Published online:
Abstract
A long-standing tradition uses strings of poppit beads of different colors to model meiosis, especially to show how segments of paired homologous chromosomes are recombined. Our use of orthodontic latex bands to model cohesion of sister chromatids, and plastic coffee stirrers as microtubules, extends what can normally be achieved with ‘standard’ commercial kits of beads, so emphasizing the importance of four key elements of meiosis: (a) the role of chromosome replication before meiosis itself begins; (b) pairing and exchange (chiasma formation) of homologous chromosomes during meiosis I; (c) centromere (kinetochore) attachment and orientation within/on the spindle during meiosis I and meiosis II; and (d) the differential loss of arm and centromere cohesion at onset of anaphase I and anaphase II. These are essential elements of meiosis that students best need to visualize, not just read and think about. Bead modeling leads them in that direction, as our gallery of figures and accompanying text show.
Primary image: Unassembled components of ‘PoppitMeiosis’ – a poppit bead exercise aimed at student learning of meiosis. Beads are snapped together to model bivalent chromosomes (on the right side), with double-stick tape (top) representing the synaptonemal complex, orthodontic latex bands representing cohesion rings, and coffee stirrers representing microtubule bundles that connect centromeres to the spindle poles.
Citation
LaFountain JR, Rickards GK. 2022. Meiosis remodeled: Inclusion of new parts to Poppit Bead models enhances understanding of meiosis. CourseSource. https://doi.org/10.24918/cs.2022.2
Society Learning Goals
Genetics
- Nature of Genetic Material
- What are the molecular components and mechanisms necessary to preserve and duplicate an organism’s genome?
Lesson Learning Goals
The scientific teaching context of this lesson is in the fields of cell biology and genetics, focusing primarily on concepts of chromosome behavior during meiosis and thus transmission genetics and patterns of inheritance. Students will learn
- the components and mechanisms necessary for successful meiosis;
- of replication of chromosomes through to the formation of haploid gamete cells;
- of the importance of chiasma formation to recombination of homologues;
- that chiasma formation and centromere (kinetochore) behavior are key elements to proper segregation of homologous chromosomes and genetic recombination.
Lesson Learning Objectives
Students will:
- be able to compare and contrast mitosis and meiosis;
- be able to explain effectively, in a professional manner, how meiosis works to an uninformed audience, such as peers who are not biology majors;
- gain expertise in explaining on a professional level the nuances of such features of meiosis as synapsis, chiasma formation, syntelic and amphitelic orientation; controlled loss of sister chromatid cohesion as the trigger for anaphase I and anaphase II;
- become familiar with sources of errors in meiotic mechanisms and thus defective chromosome and gene transmission.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
Introduction
Many students have difficulty understanding how meiosis works, attributable we submit to misconceptions ingrained from earlier times in their education and to uncertainty about how features of meiosis are different from those of mitosis. Not surprisingly, then, most instructors charged with the task of teaching meiosis use some sort of hands-on modeling to attempt to dispel misunderstanding and enhance learning. Modeling offers a way of putting principles into practice to achieve a proper ‘meiosis understanding’. A long-standing tradition in this respect uses strings of poppit beads of different colors to model meiosis, especially to show how segments of paired homologous chromosomes are recombined. We note that poppit beads have long been endorsed in the Advanced Placement curriculum; and bead modeling is still recommended in the U.S. 2019 revised curriculum (Investigation 7).
Recent publications concerning challenges teachers face when attempting to build a good understanding of meiosis will be found in references (1, 2). The efficacy of the modeling approach to meiosis teaching has also been investigated (3, 4), using modeling materials ranging from strips of paper (5), pipe cleaners (4), pool noodles (6) or wires (7) to recently innovated virtual animations (8). Most relevant, a meiosis concept inventory has been developed (9), aimed at investigating student understanding of general features of meiosis, including (a) ploidy, (b) relationships amongst DNA, chromosome structure and chromosome number, and (c) when and where meiosis takes place.
Specifically, we have found - and demonstrate quantitatively in this article - that many students at undergraduate junior/senior levels express uncertainty about the importance of, and roles played by, chromosome replication, sister chromatid cohesion, chiasma formation and kinetochore (centromere) orientation in the meiotic process. The purpose of the second division of meiosis also seems to be a major point of misunderstanding. Because of all this uncertainly, the overall level of understanding held by students tends to be superficial and vague, lacking the clarity and depth expected. Thus their ‘meiosis education’ is not as complete as it can and should be; and students therefore often miss out on appreciating the sheer beauty and vitality of the overall process of meiosis and its separate components.
We attempt to overcome some of these problems by providing here an improved, we believe, poppit-bead modeling exercise that we have found to be strongly impactful on student understanding of meiosis. It builds significantly on the use of poppit beads to track exchange (recombination) of homologous chromosomes, by using orthodontic elastic bands and plastic coffee stirrers to model sister chromatid cohesion and kinetochore/microtubule attachments to the spindle, respectively. We emphasize how bivalents are constructed for and proceed through meiosis, with emphasis on (a) the vital role of chromosome replication before meiosis itself begins, and thus the ‘math of meiosis’; (b) pairing and exchange (chiasma formation) of homologues; (c) kinetochore attachment to the spindle during meiosis I and meiosis II; and (d) the differential loss of arm and centromere cohesion at onset of anaphase I and anaphase II. These essential elements of meiosis need best be visualized, not just talked about or read about as occurring during meiosis. Bead modeling offers students hands-on exercises that lead them in that direction.
When our improved modeling approach was first implemented, we quickly became well satisfied with its success, based on verbal feedback from students, and student reviews have consistently been positive in this respect. Nevertheless, we have now used a ‘before/after’ assessment tool to gauge quantitatively how impactful bead modeling is.
The 45-question meiosis survey presented here (S1. Meiosis remodeled – Meiosis survey PowerPoint slides) is aimed at evaluating how well our students understand the main features of meiosis. We do not see this survey as an inventory akin to that of Kalas et al. (9), though we can envision how others might use it, either in part or in full, to survey their students on these matters. Rather, the survey’s questions were formulated for the purpose of gauging knowledge at two times: first, prior to modeling, then second, after students have worked with the models. Thereby we offer strong evidence that modeling does indeed enhance understanding of meiosis.
Intended Audience
As noted in the Introduction, the Advanced Placement curriculum used in high schools has a long-standing strategy of using poppit beads for teaching meiosis. Students at that level in their biological science education should, therefore, find our remodeled version of PoppitMeiosis familiar, as well as both instructive and enjoyable. We are confident this will also be so for students at the introductory college level. Our own experience has been with BIO majors, who benefited from Poppit even though they had previously completed a major’s course in basic genetics. Lastly, in our sharing PoppitMeiosis with our university colleagues, we have witnessed how our modeling approach has also edified them. Thus, we present PoppitMeiosis with the potential to be valuable broadly, as above, as well as in the contexts of courses in introductory genetics, advanced genetics, or introductory or advanced cell biology.
Required Learning Time
PoppitMeiosis has been developed in a senior-level cytogenetics laboratory course, which has three 2hr meetings per week. For PoppitMeiosis the better part of three of these meetings is used for (1) the before survey, (2) instruction devoted to the lesson as described below, and (3) the after survey. These are sessions when the entire class is engaged in the same activity. Additionally, students are active at other times working on their own, building models and acquiring microscope images of meiosis for assessment of their work in the form of a PowerPoint presentation. Typically, the entire meiosis course project – making slides of meiosis, microscopy and modeling – takes about three weeks, of which several days are devoted to modeling and assessment. For more detail, refer to the recommended timeline presented at the end of the lesson.
Prerequisite Student Knowledge
‘Meiosis remodeled’ aims at improving student understanding of the mechanisms underlying the recombination and segregation of chromosomes during meiosis. The emphasis is on ‘improved understanding’ (implying some prior basic knowledge) of key elements concerning chromosome replication, synapsis, chiasma formation and chromosome segregation, and thus genetic recombination. Students involved with any curriculum that has already exposed them to the importance and general outcome(s) of meiosis would be adequately equipped to take on PoppitMeiosis. The lower the level of preparation the greater would be the need for guidance by someone more knowledgeable and mastered. But PoppitMeiosis itself is flexible; and educators themselves will be able to assess the extent to which their students are prepared for, and thus likely to benefit from, an engagement with it.
Prerequisite Teacher Knowledge
Our Poppit lesson is intended primarily for an audience that already has some understanding of the basics of meiosis, and thus that criterion would be the minimal pre-requisite for an instructor. We recognize, however, that the understanding of meiosis amongst instructors themselves varies considerably, stemming from their own prior exposure to the topic and/or their interest in such things as chromosome structure, chromosome segregation, and recombination and transmission of genes. For those with expertise in the subject, Poppit will, we believe, satisfy a longing, which we ourselves have had, for a teaching tool of the likes that Poppit provides. For those who understand the importance of meiosis to Mendelian genetics but are not well versed in its underlying mechanisms, Poppit, we believe, will unquestionably be helpful. Poppit is intended to be an instructive tool, firstly for those wanting understanding and desirous of ‘passing it on’, and secondly for those onto whom it has been passed. Information derived only from textbooks in many cases does not do justice to the process of meiosis, and figures purporting to properly show the process often convey more confusion than clarification (which is why, in the first place, we put PoppitMeiosis together). Poppit is a ‘lesson on meiosis’, structured in a way that will inform and update participants, whilst also righting some of the wrongs gotten from earlier study. We are hopeful that teachers themselves will gain new understanding from PoppitMeiosis, and that this will spark a desire and enthusiasm to introduce it to their students.
Scientific Teaching Themes
Active Learning
The effectiveness of PoppitMeiosis lies in its hands-on nature. The exercise emphasizes the way bivalents are constructed for and proceed through meiosis, with emphasis on (a) the vital role of chromosome replication before meiosis itself begins, and thus the ‘math of meiosis’; (b) pairing and exchange (chiasma formation) of homologues; (c) kinetochore attachment to the spindle during meiosis I and meiosis II; and (d) the differential loss of arm and centromere cohesion at onset of anaphase I and anaphase II. These essential elements of meiosis need best be visualized and even touched, not just talked or read about. PoppitMeiosis offers students hands-on experience that leads them in that direction.
Assessment
In the Teaching Discussion section of this article, a method of assessment is presented in detail. A before/after survey uses a set of 45 meiosis/mitosis (control) related questions to evaluate student knowledge. The resulting data demonstrate unequivocally the value of PoppitMeiosis.
Inclusive Teaching
PoppitMeiosis is designed to be adaptable for a wide range of curricula, and under the widest of circumstances – in a lecture course or seminar, in a classroom, in a laboratory setting, and for ‘self-teaching’ in a home situation. The lesson employs instructional materials that are easily and widely available, and with which students can work at their own pace and depth, depending on their individual backgrounds, needs, learning abilities and styles. PoppitMeiosis encourages learning and understanding of chromosomes in meiosis, the teaching of which has been a challenge for generations of educators. Poppit’s hands-on approach is inviting to students of virtually all learning styles and abilities. It offers ways they can quickly engage with the material at hand and therefore be encouraged to talk, listen, and interact on the task. PoppitMeiosis thus naturally creates an atmosphere that improves understanding of meiosis and thereby enhances appreciation of the significance of meiosis, as it relates to all sexually reproducing organisms: plants, animals and humans.
Lesson Plan
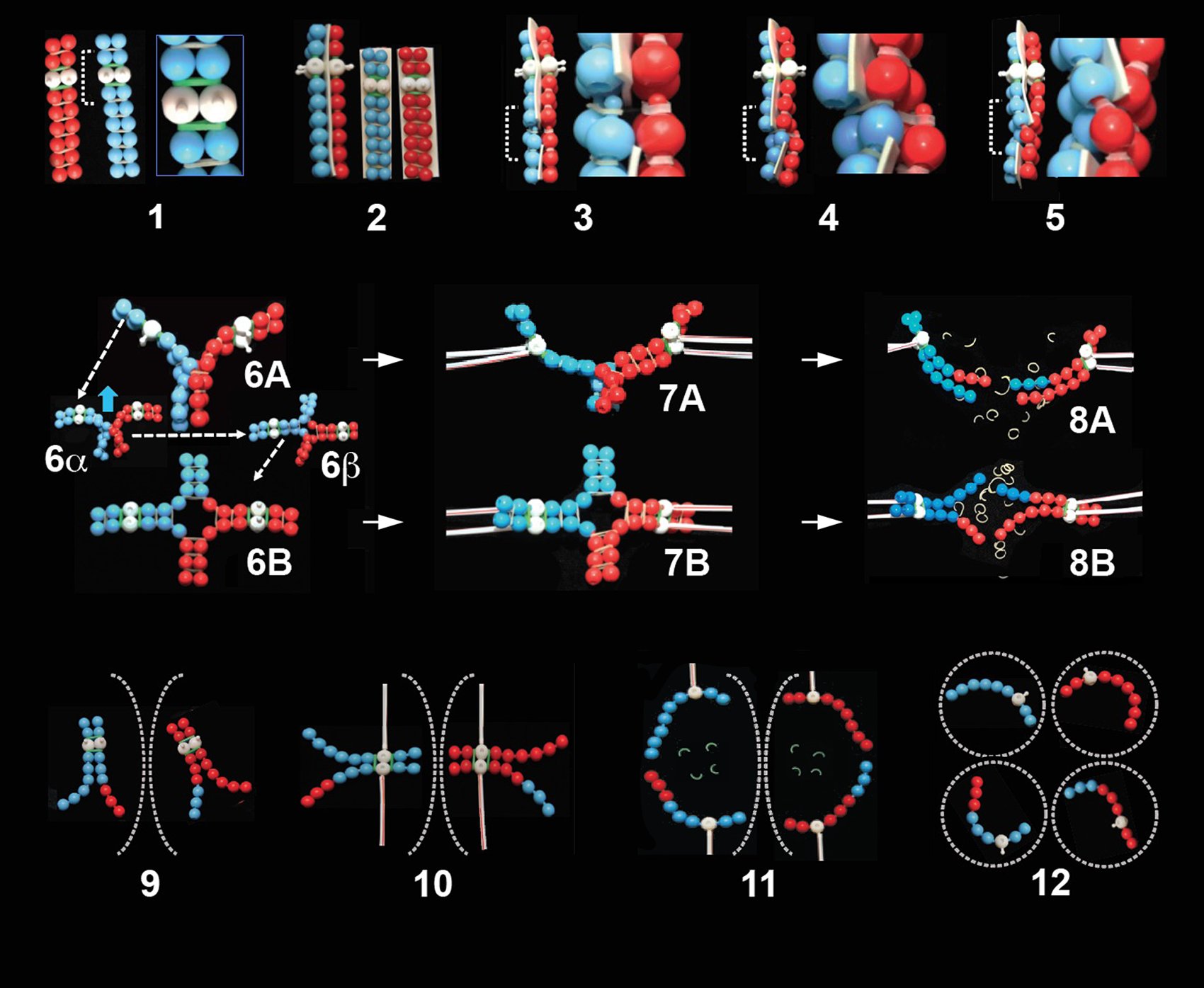
Before undertaking PoppitMeiosis, instructors should assemble the components shown in Figure 1. That figure also shows an assembled di-chiasmic bivalent, to illustrate what a finished product of the modeling exercise looks like.

Materials used in Poppit modeling
Poppit beads: These are available from a wide variety of vendors. In addition to standard colored beads having one hole and one knob, white beads with a knob and five holes are needed to model kinetochores (white knobs) and their attachments to microtubules (coffee stirrers). Poppit (pop) beads from Ward’s Science (Catalog number # 470041) work well, though note that 5-hole white beads need to be purchased separately (Catalog number #470023-858).
Orthodontic latex elastic bands: These are used to represent cohesion protein complexes that link sister chromatids formed during chromosome replication. The size of the bands is critical, because once attached they must provide sufficient tension to hold two strands of beads (sister chromatids) firmly together. Bands ~¼ inch diameter, medium thickness from Amazon are recommended. In addition to standard, neutral-colored bands, we use others of different colors (‘neon elastics,’ also from Amazon) to model centromere cohesion and the guardian protein Shugoshin.
Double stick mounting tape: This is used to model the synaptonemal complex. We use Scotch Permanent Mounting Tape available in rolls 1 x 125 inches.
Coffee stirrers: These are used to represent kinetochore microtubules that connect chromosomes to the spindle poles. The inner diameter of a stirrer must fit snugly on the knob, so that if pulled on an attached stirrer will not slide off. Dixie brand stirrers (5-inch unwrapped stirs/straws) from Office Depot meet this requirement.
Instructor Preparation for PoppitMeiosis
The following text, along with Figures 2 and 3, provide a ‘PoppitGuide’ for instructors to use when presenting PoppitMeiosis to students. Main features of meiosis are linked to Poppit in a way that shows how it rectifies misconceptions and lack of understanding. We also reference relevant articles, to assist instructors in acquiring more information if desired.
During PoppitMeiosis we use PowerPoint slides equivalent to those of Figure 3, each feature presented on a separate slide. Students are not referred to this article or its figures until they have completed Sessions 1 through 4 or 5. We recommend instructors make their own slides to get prior hands-on experience with PoppitMeiosis itself, as well as with intricacies of putting together the poster presentation, if they opt for that type of assessment.
The Importance of an Attentive Instructor
With all the necessary materials at hand (see above), and a pre-lesson behind them, a PoppitInstructor leads students through the exercise at an appropriate level of detail. In our case, students have already participated in PoppitMitosis, using the same materials supplied for PoppitMeiosis. Consequently, much of the necessary terminology (e.g., chromatid, cohesion, kinetochore, etc.) has either already been introduced or reviewed quickly. A ‘Pre-Poppit survey’ (see Timeline for the lesson) will provide evidence of their level and correctness of their understanding.
Most participants, therefore, have little difficulty in following the oral instructions given below, and therefore need little further guidance. Nevertheless, it is important to check on students’ mastery of a completed step before they go forward to the next step. The exercise is very ‘in-person’, encouraging student-instructor and, indeed, student-student, interaction. Key points, as detailed in the descriptive sections given below, are communicated by word and action. For our laboratory course, meetings given after Poppit review what students have learned. They then complete a poster project, which includes both their own cell phone images of PoppitMeiosis plus microscope images of chromosomes ‘doing the real thing’.
Warm-ups for PoppitMeiosis
PoppitChromosomes in meiosis and mitosis
Before embarking on PoppitMeiosis itself, we advise taking time to reacquaint students in pre-lesson form with (i) what chromosomes are in real life and thus what PoppitChromosomes represent, and (ii) the basics of meiosis in real life and thus what PoppitMeiosis can achieve. We also recommend points be made on the advantages of using Poppit beads for demonstrating chromosome behavior, and their limitations. The following comments aim to assist in these respects.
Throughout, we refer to a complementary activity – PoppitMitosis – which entails similar hands-on bead modeling aimed at understanding mitosis. We view PoppitMeiosis as a stand-alone lesson. Nevertheless, there is merit in preceding it with a lesson that includes presentation of mitosis using strings of poppit beads to cover: (a) chromosome replication/sister chromatid formation; (b) the establishment of cohesion rings for maintaining connection between sister chromatids; (c) attachment of sister chromatid centromeres at their kinetochores by microtubules to opposite poles of a spindle; (d) sister chromatid separation, triggered at the onset of anaphase by loss of cohesion; and that (e) the resulting division produces daughter cells having the same DNA, same chromosomes and same set of genes as the parent cell. We propose the following pre-lesson material to provide enhancement to what students can achieve through the running of PoppitMeiosis alone.
Real-life chromosomes
A real-life eukaryotic chromosome, in its pure genetic essence, is a single long strand of duplex DNA – a double helix in the classic Watson and Crick form. But the end-to-end (telomere-to-telomere) length of a chromosome as usually observed under the microscope at metaphase of mitosis is, roughly speaking, about 10,000 times shorter than the length of the DNA it contains. Alternatively put, a metaphase chromosome measuring 5 micrometers contains about 5 centimeters of DNA! This is because the DNA of a chromosome is extensively ‘packaged’ to make it fit the space within the interphase nucleus it occupies, and then packaged still further when it prepares for division.
For most of the time of its existence, a chromosome is in a somewhat amorphous state, lacking a clearly definable form, seemingly unraveled within its nucleus (10). It is in its interphase stage of its cell cycle – so-called because it is in-between two successive rounds of cell division – between the telophase of its previous division and the prophase of its next one. Only when a cell is triggered to enter division do its chromosomes transform into the discrete, rod or ‘sausage’-shaped structures that we can then see distinctly and separately under the microscope.
The centromere of a chromosome is an intrinsically fixed portion of a chromosome’s DNA (11). It possesses the highly specialized function of organizing onto itself a kinetochore, which is the chromosome’s ‘spindle attachment body’, and thus one of its functions is to mediate the chromosome’s movement. It is usually possible to identify the location of a chromosome’s centromere because it produces a constriction (waist!) thereby giving the chromosome the appearance of having two ‘arms.’ The relative lengths of these arms vary considerably between different chromosomes, but it is essentially fixed for a given chromosome. The centromere produces its kinetochore after it exits interphase and prepares for cell division (mitosis or meiosis). The kinetochore attaches to the spindle during establishment of metaphase, and then disintegrates after anaphase. Following chromosome replication in the lead-up to division, each of the two sister chromatids has its own centromere and organizes its own kinetochore.
Comment.
Packaging of DNA in a chromosome, based on its wrapping around histone proteins, is a topic that is widely and usually well covered in textbooks; also see (12).
Transformability – its ability to change its structural (and functional) state depending on the stage of its cell’s cycle, and its own chromosome cycle – is a defining characteristic of a chromosome. Chromosome transformations are highly correlated with their genetic activity, their genes being active (‘turned on’ or potentially ‘turned on’) in interphase. In contrast, genes are largely inactivated during mitosis and meiosis. When in either mitosis or meiosis, the dense packing of DNA puts a chromosome into a state that allows it to be moved around easily without entanglement and breakage.
The following definitions may be helpful.
Chromosome: a thread-like (interphase) or rod-shaped (mitosis, meiosis) structure consisting in eukaryotes of DNA and proteins and comprising a linear sequence of genes.
Centromere: that specialized segment of a chromosome’s DNA that provides a site for the assembly of the chromosome’s kinetochore.
Kinetochore: a microscopic disc-shaped structure composed of diverse proteins that function in attaching the chromosome at its centromere to microtubules of the spindle. Some of these proteins play a role in moving chromosomes.
PoppitChromosomes
In PoppitMeiosis (and PoppitMitosis) a string of poppit beads is used to model the highly packaged DNA and associated histone proteins, as shown in Figure 2A. Sister chromatids (chromosome replicas as presented in Figure 2B) are represented by the same bead color, homologous chromosomes by a differently colored string of beads. A white bead represents the chromosome’s centromere; the knob projecting from it represents the kinetochore.
Comments.
Some of the desirable attributes of using poppit beads to model meiosis are as follows.
- A string of poppit beads has an integrated linear structure not greatly unlike that of a DNA double helix with its linear series of genes. It has a sausage-like shape and three-dimensionality that resembles a real-life chromosome.
- Poppit beads offer flexibility in deciding which type of chromosome to model. This will depend on its length (number of beads) in relation to its DNA quantity/number of genes, and its ‘shape’, in relation to the position of its centromere - whether sub-metacentric (as in Figure 2A), metacentric (as in Figure 1) or otherwise (acrocentric, telocentric).
- The plug/unplug feature of poppit beads confers the capability of modeling chiasma formation/genetic recombination between homologous chromosomes during meiosis.
- The joining stems of a string of beads are ideal for placement of orthodontic latex bands representing cohesion proteins binding together sister chromatids.
- The knobs of white beads provide ideal sites (kinetochores) for coffee stirrers (microtubules) representing chromosome attachment to the spindle.
Despite its many positive attributes, Poppit lacks the ability to model the transformable nature of chromosomes – their ability to change from an unraveled (dispersed) state to a contracted (condensed) state – as they transition from interphase to division, and likewise from condensed to dispersed state as they transition from division into the subsequent interphase. That point is presented more explicitly in the following section.
Chromosome replication: sister chromatids
Transformability is a crucial property of chromosomes in their replication because DNA must be in an unraveled, seemingly dispersed state for DNA polymerase to have access to it for its replication. As noted, it is not possible to represent this important aspect of ‘real’ chromosome behavior in Poppit. Thus, in Figure 2 the portion labeled 2A represents the stage of meiosis pre-chromosome replication, whilst 2B represents post-replication. In both figures, however, chromosomes are presented in their highly contracted ‘sausage’-shaped form, as if there had been no change in this form during their transitioning from 2A to 2B. In real life, the chromosomes in 2A would have dispersed, then replicated, and then condensed again by the time they get to 2B, the latter occurring after a long pre-meiotic interphase during which replication took place.
Note also that in real life, pairing (synapsis) of homologues and subsequent chiasma formation are key events of meiosis that take place well prior to their adopting their highly contracted ‘sausage-shaped’ state.
In math, multiplication must precede division, if numbers are to be maintained. In somewhat similar fashion, chromosomes must be replicated (copied, duplicated) prior to entering mitosis or meiosis, otherwise proper division cannot proceed. Thus, the ‘before replication’ portrait of a chromosome prior to entering division shows each chromosome existing as a single entity - a single DNA duplex (Figure 2A). The ‘after replication’ portrait shows that each chromosome has doubled to produce a pair of entities - a pair of DNA duplexes (Figure 2B). For the relationship of the two chromosome replicas to each other we use the term sister chromatids.
If students have had experience with PoppitMitosis they will recognize that the chromosome models after DNA replication, as represented for PoppitMeiosis in Figure 2B, are the same as they were for PoppitMitosis. The chromosome replicas (sister chromatids) of each homologue are what separate from each other to opposite poles of the spindle during anaphase of mitosis. The pathway of replicated homologues is, however, quite different, as students will see in PoppitMeiosis.
It is, therefore, at two times in the progression of meiosis that we see a chromosome in its most easily understandable form, when it is in its un-replicated state and has just a single DNA duplex. These are: (i) at anaphase of the mitosis that precedes meiosis (Figure 2A), and (ii) during and after anaphase of the second division of meiosis (Figure 2D). Thus, by definition, un-replicated means a single chromosome, a single duplex of DNA, a single sequence of genes! A metaphase (and prophase) chromosome is in its replicated form, defined as a pair of sister chromatids, two DNA duplexes (one for each chromatid), two sequences of genes (one for each chromatid). The word – chromosome (singular) – is freely used to cover both situations, for when the chromosome is in its un-replicated or its replicated state.
Comments.
DNA replication, and thus production of sister chromatids, is a prerequisite for meiosis that is undertaken during its ‘pre-meiotic interphase’, i.e., the interphase immediately prior to the cell’s entry into meiosis. It is, unfortunately, an aspect of meiosis often neglected in classroom presentations. Its omission commonly complicates – if not making it impossible to understand properly – what is achieved, and how it is achieved, when the number of chromosomes is reduced during meiosis. Because of prior chromosome replication, two divisions of meiosis are necessary for a reduction to be achieved. The second division of meiosis is as important in this respect as the first, as our presentation of the math of meiosis bears out.
The following definitions may be helpful.
DNA/chromosome replication: the process of making copies of DNA, during which a single strand of DNA is used as a template for the semi-conservative synthesis of a complementary strand, thus overall forming two new (half old, half new) DNA duplexes. Thus, from an original DNA duplex two replica duplexes, and thus two replica chromosomes, are made. The resulting chromosome replicas are referred to as ‘sister chromatids.’
Chromatid: one member of an associated pair of chromosome replicas (sister chromatids).
Interphase: that portion of the cell’s cycle during which its genes are active, or potentially active, and its DNA/chromosomes in a somewhat diffuse, seemingly amorphous form and not visibly engaging in division (mitosis or meiosis). The stage of a cell’s life cycle when DNA replication occurs.
Sister chromatid cohesion
At the molecular level, sister chromatids produced by DNA replication are held together (‘glued’ is an appropriate term) both at their centromeres and all along their arms by a special class of proteins known as cohesins (13). Cohesins are assemblies of protein molecules that form nanoscale rings that link the two DNA duplexes of sister chromatids, forming hundreds of such linkages per sister chromatid pair along the length of their DNA. Cohesin rings form in association with sister chromatids at the time of DNA replication in interphase.
Cohesin rings form the basis of what is referred to as ‘sister chromatid cohesion’, a phrase that implies an adhesive connection at the boundaries between sister chromatids, all along their lengths. What is important about cohesion is that sister chromatids remain linked together as they proceed through prophase, prometaphase, and metaphase of cell division. Cohesion is removed only at the start of anaphase, thereby allowing chromatids to separate and move to the spindle poles to which they are attached. In meiosis, cohesin links between sister chromatids are especially critical for the maintenance of a chiasma /chiasmata, and then for controlled entry of chromosomes into anaphase I and anaphase II, as covered in detail in PoppitMeiosis.
Comments.
Cohesion of sister chromatids is often omitted entirely, or only casually referred to, in presentations of meiosis (and mitosis). Figure 2B correctly illustrates sister chromatids not only positioned side-by-side but also physically linked together, all along their lengths, by cohesin rings in the form of orthodontic bands. That particular facet of PoppitMeiosis represents in our view a significant improvement over the ‘magnetic centromeres’ that are used to represent cohesion in commercially available bead kits. With latex bands, students not only witness the existence of cohesion between sisters, but they also get a good impression of its strength, as it is impossible to separate homologues for the first division of meiosis without first cutting the bands that bind them.
Bivalents
In biology, the term ‘bivalent’ is used for a chromosome construct in meiosis in which homologous chromosomes, in their replicated (sister chromatid) state, are associated (synapsed) longitudinally in pairs. After pairing, the two homologues of a bivalent then usually form at least one chiasma (and often several chiasmata), which continues to hold the homologues together after pairing forces have lapsed.
Comments.
Unfortunately, presentations of meiosis, in textbooks and thus in lessons, very often ‘get chiasmata all wrong’, in terms of their formation, structure and purpose. An important aim of PoppitMeiosis is to present the correct situation. For this we strongly recommend a pre-lesson with students in which an instructor has in hand three pre-made Poppit items to show the audience. These are illustrated in Poppit Figure 2B referring specifically to a pair of replicated homologous chromosomes and in Figure 3, step 7 highlighting two views of one bivalent. Students should first be reminded of how the two replicated homologues would have behaved in mitosis (ideally after having experienced PoppitMitosis).
The pre-lesson would then continue with the instructor referring visually to the bivalent. The commentary at that stage should include two key points: (i) the two homologues in the bivalent are identical to the pair of replicated homologues in mitosis, and (ii) the PoppitBivalent represents the conjoined homologues as they would appear at metaphase of the first division of meiosis. Also, the point should be made that the actual ‘making’ of the bivalent in real life occurs during early stages of meiosis, when the chromosomes have not yet achieved such a fully condensed state. Later (if warranted), while running PoppitMeiosis, the instructor might introduce the significance and stages of meiotic prophase (Leptonema, Zygonema, Pachynema, Diplonema, Diakinesis) when steps in the making of the bivalent are taken. For images of cells in meiosis with interpretative drawings, see Chapter 2 in (14).
The following definitions may be helpful.
Homologous chromosomes/homologues: chromosomes of a diploid (or polyploid) organism that are essentially identical to each other, in terms of their length, centromere position and DNA and thus gene content and distribution. They may carry different alleles of a particular gene.
Bivalent: a construct formed in meiosis when two replicated homologous chromosomes pair (undergo synapsis) with each other, and then usually become further linked via formation of a chiasma (plural: chiasmata).
Chiasma: An ‘X’ or cross-shaped arrangement of sister chromatids in a bivalent, generated at the point at which exchange of segments has taken place during crossing-over/recombination.
Running PoppitMeiosis
Replication and the math of meiosis
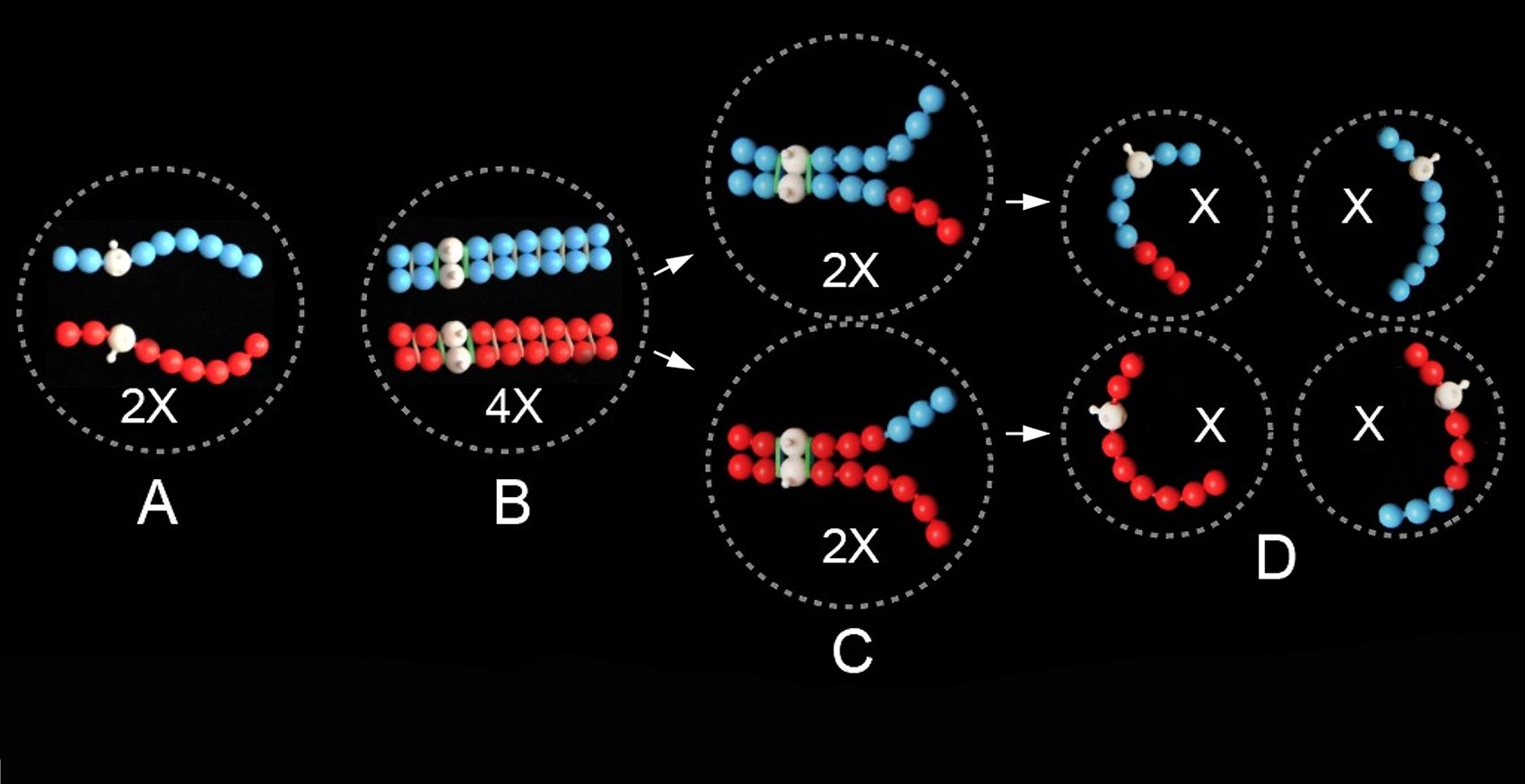
The beginning of PoppitMeiosis is shown in Figure 2A, where red and blue strings of poppit beads model a homologous pair of chromosomes. Their centromeres, represented by white beads, have been placed off-center (i.e., sub-metacentric), thus creating chromosome arms of different lengths. PoppitMeiosis with this pair of chromosomes will, eventually, result in either a red or a blue bead-string (or a recombinant thereof) in the cells formed, not both together in the one cell (Figure 2D).
Chromosome replication in the lead-up to meiosis is modeled in Poppit by each single string of beads becoming a double string, representing a pair of sister chromatids (as chromosome replicas) glued to each other all along their lengths (Figure 2B). Note that whatever color of bead is used for the single string, and whatever its chosen length and position of its white bead (for metacentric, acrocentric or telocentric cases) before replication, that arrangement is repeated in the replica string, to model genetic identity of sister chromatids.
Comments
(i) The essence of meiosis is embodied in the phrase the math of meiosis. This is given by the three-step ‘equation’ 2X → 4X → 2X → X, which accounts for the halving of a set of chromosomes from diploid, with two of each type, to haploid, with only one of each type. The first step of the equation, 2X → 4X, represents chromosome replication (i.e., formation of sister chromatids). It results in, effectively, a doubling of the number of chromosomes [see Comment (ii) below]; Figure 2, A → B. Step 2 of the equation, 4X → 2X, represents the first division of meiosis, which halves the chromosome number (and DNA content and gene copy number) from replicated diploid (4X) to replicated haploid (2X); Figure 2, B → C. Then, finally, step 3 of the equation, 2X → X, represents the second division of meiosis, which halves everything again, from replicated haploid to un-replicated haploid; Figure 2, C → D. Note that in the math of meiosis, X represents all of the following: (a) chromosomes, (b) DNA quantity (C-value), and (c) gene copy number. For each of these components, a diploid cell has a content of 2X prior to replication, and 4X afterwards; and then 2X then X over the two divisions of meiosis.
(ii) Throughout Poppit, and thus specifically for the math of meiosis, we recommend the shorthand system for chromosome numbers that, most informatively, uses X to indicate haploid (one of each type of chromosome), 2X for diploid (two of each type), 4X for tetraploid (four of each type) etc., reserving n for the gametic and 2n for the corresponding zygotic (somatic) chromosome number. Humans are diploid organisms, with 2n = 2X = 46 (and n = X = 23); cultivated potatoes are tetraploid, with 2n = 4X = 48 (and n = 2X = 24). Meiosis can take place only in zygotic (2n) cells. It starts with chromosomes in their replicated state, and thus, in a diploid organism, with a chromosome number at the tetraploid (4X) level – i.e., with, effectively, four of each type of chromosome, as two pairs of sister chromatids. The implication is that we view each chromatid of an associated pair of sisters as being a chromosome in its own right. This approach makes full sense, both in general and with the need for chromosome replication in the lead-up to meiosis (and mitosis!), and then the need for two divisions, not just one, for meiosis to produce haploid gametes in a diploid organism (and diploid gametes in a tetraploid organism!). One can rightly ponder why biology, in the leadup to meiosis, goes to the trouble of doubling chromosome number by replication, only then to be counter-faced with the need for two divisions to reduce the number by half. Why not, one might retort, pair un-replicated homologues, followed by a single division in meiosis? As far as is known, no organism in biology has ‘cottoned-on’ to such a simplified form of meiosis. We suggest the reason is that replication (formation of sister chromatids) is the mechanism nature has opted for, coupled with chiasma formation, to cohesively glue homologues together, thereby ensuring their cooperation and thus orderly separation into different cells during meiosis.
Cohesion of sister chromatids
The sticking together of sister chromatids – PoppitCohesion – is modeled by orthodontic latex bands connecting paired strings of plugged-together beads, with a band on each pair of knobs (Figure 2B; Figure 3, feature 1). Uncolored bands represent cohesion around chromatid arms; colored bands represent cohesion specifically around their centromeres (green bands in Figure 3, feature 1). The distinction is crucial to subsequent divisions of meiosis, when uncolored bands are lost in the first division, with colored ones remaining intact; then colored ones lost in the second division (refer below - Loss of cohesion: the anaphase trigger).
Comments.
(i) Maintenance of chromosome and thus genetic stability through sexual reproduction demands that homologous chromosomes and their sister chromatids be distributed in a highly ordered manner during meiosis. Each chromosome doing its own thing, going it alone, when and how, simply would not work! Cooperation is the name of the game, so that, concerning the product of meiosis in a diploid organism, each comes to contain but a single copy of each chromosome type. Cohesion between sister chromatids (and subsequent synapsis of and chiasma formation between homologous chromosomes) are the channels through which cooperation takes.
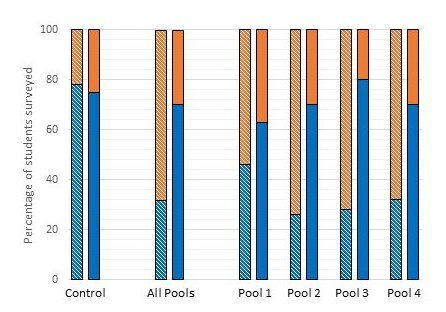
(ii) The principles of cohesion – their importance, formation and controlled loss - are well researched aspects of meiosis that have yet to be incorporated routinely into standard biology texts. Not unexpectedly, many students initially have difficulty grasping their significance, but then respond well after being exposed to Poppit (see survey data, Figure 4, Pool 4).
(iii) Cohesion between sister chromatids is established during chromosome replication through a not-yet-fully understood mechanism whereby pre-formed cohesin proteins encircle newly produced DNA helices (15, 16, 17). However achieved, sister chromatids are, at the start of meiosis, held tightly close to each other, all along their lengths, by cohesion bonds. These bonds remain intact through prophase and metaphase, until separase-mediated proteolysis of cohesin allows sister chromatids to separate from each other, thereby triggering the onset of anaphase. Sister chromatid cohesion is paramount to both the cooperative attachment of chromosomes to the metaphase I and metaphase II spindles and then to their separation during anaphase I and anaphase II (refer below - Loss of cohesion: the anaphase trigger).
Pairing of homologues
The basic principle of pairing (synapsis) of homologous chromosomes is a well-known feature of meiosis, usually well covered in standard biology texts (for examples, see 18, 19). It is modeled in Poppit as the intimate coming together of red and blue pairs of bead-strings, representing replicated maternal and paternal chromosomes (Figure 3, feature 2). PoppitPairing (best done by pairs of students, each having command of one homologue) is achieved by aligning homologues bead-for-bead, their alignment held in place with a strip of double-stick mounting tape representing the synaptonemal complex (SC).
Comment.
During Poppit we give explanation of the existence (when formed, when lost) and the important role (recombination, chiasma formation) of the SC in meiosis and biology in general (20, 21, 22).
Chiasma formation
Illustrations of the formation of a PoppitChiasma start in Figure 3, feature 3, where two differently colored bead-strings undergo breakage. Particularly for this and subsequent steps of PoppitMeiosis, it is recommended that students work in pairs, so as to ‘tango together’ to achieve proper formation of a chiasma. In this first step, the DNA of homologues about to undergo chiasma formation must be broken at a common locus, which is achieved in Poppit by disconnecting (un-popping) beads at the same point in individual non-sister chromatids of paired homologues. Note that PoppitBreakage involves double-stranded breaks in DNA of actual chromosomes, since each single strand of beads represents a chromosome entity comprising double-stranded DNA.
After breakage, chromosome continuity is then re-established by re-joining (re-popping) the bead strings, but now such that complete strings are formed of both colors, representing recombinant chromosomes (Figure 3, features 4, 5; also in feature 12).
The place where breakage-reconnection is made is the ‘cross-over’ (recombination) site; it becomes apparent in a bivalent as a chiasma. We emphasize in Poppit that opening the bivalent as in Figure 3, features 6A, 6B does not produce, it only reveals, the chiasma, which is a really important point.
Poppit well demonstrates that sister chromatid cohesion (represented by latex bands) in the presence of breakage and re-joining between homologues (modeled as un-popping and re-popping) are the twin features that continue to hold the bivalent together as an intact unit, after the synaptonemal complex has disappeared. The chiasma has formed a covalent (shared) cohesion bond between homologues. During Poppit modeling of chiasma formation, students come to the realization, often for the very first time, of a demanding interplay taking place amongst cohesion, breakage of chromosomes and their rejoining. It is necessary to un-plug/break and re-plug/rejoin an appropriate pair of chromatids, all the time keeping sister chromatids cohered together, rather than parting them. Thus, a chiasma is formed, resulting in both recombination and construction of a bivalent. Then, after opening out their bivalent into a crossroad configuration, the importance of sister chromatid arm cohesion distal to the chiasma in maintaining bivalent integrity becomes crystal clear.
Comments.
(i) We remind students during Poppit that chiasma formation takes place inside the early prophase I nucleus, and results in each bivalent having at least one chiasma (‘chiasmata’ if more than one has formed). A bivalent with two chiasmata, as presented in Figure 1, helps to confirm that the overall shape of a metaphase I bivalent depends on the number and position of its chiasmata.
(ii) Both prior experience and survey data presented below indicate considerable misunderstanding concerning the importance of chiasma/ta in meiosis (23, 24). There are, we suggest, two distinct aspects to this misunderstanding, namely concerning chiasma number (how many are formed) and concerning chiasma structure (their method of formation). In most organisms, chiasma position along the length of a pair of synapsed chromosomes is essentially random. But this randomness is not to be confused with randomness of occurrence, in that every synapsed pair of homologues must form at least one chiasma (25, 26). The purpose of this ‘always at least one chiasma’ concerns the chiasma’s mechanical role in meiosis, which is to ensure that paired chromosomes remain bound together, and thus can communicate with each other, after loss of the synaptonemal complex, through to metaphase I and up to the start of anaphase I. Our students are made to confront this issue head-on: chiasma formation is essential; it is needed for maintenance of bivalent integrity; proper completion of meiosis depends on it. Many students depart PoppitMeiosis with a clearer understanding of the issue, though some struggle to grasp the situation fully (survey data Figure 4, Pool 2). This contrasts with an alternative outcome in which a pair of homologues synapse but then, following loss of the synaptonemal complex, abnormally fail to form a chiasma. In such cases the two homologues are univalent (27), and their subsequent segregation is error prone. Note that just a few organisms, including males of Drosophila fruit flies, are ‘achiasmate’, in that bivalents result from only synapsis; there is no subsequent chiasma formation. In PoppitMeiosis we consider only bivalents in which at least one chiasma has formed, not the few exceptions.
(iii) In Figure 3, feature 3 (and in features 4 and 5) a portion of the double-stick tape (representing the SC) has been cut away at the site of the Poppit chiasma, to provide ‘access’ for rejoining of unplugged beads. An alternative to cutting away a piece of tape is to use narrower (less than the recommended 1-inch thick) double-stick tape, though this can create unwanted loosening and disconnection of beads downstream and upstream from the chiasma. Regardless of whether one opts for tape cutting, or use of narrower tape, regular re-pinching of beads to the tape on either side of the forming chiasma helps to maintain the desired situation. Moreover, we tell our students that nature itself has similar issues to resolve, during formation of a real-life chiasma - i.e., how to maintain structural integrity of chromatids during their breakage, and how to gain access through the SC for their rejoining. Science has yet to provide us with answers to such ‘problems.’
Attachment of chromosomes to the spindle
Poppit also homes in on the importance of connections that chromosomes make to the spindle during the first and second divisions of meiosis. Use of plastic coffee stirrers as microtubules of the spindle helps in removing misunderstanding. PoppitAttachment to the spindle is made by inserting one end of a coffee stirrer, representing in life a bundle of 20 or so spindle microtubules (28), onto the knob (kinetochore) of a white bead (centromere). This is illustrated in Figure 3 features 7A, 7B for a mono-chiasmic bivalent (and Figure 1 for a di-chiasmic bivalent).
Chromosomes that separate from each other during the first division of meiosis (see following section) remain connected to each other by centromere cohesion. Now in separate cells (Figure 3, feature 9) they are connected to the second division spindle by coffee stirrers attaching sister kinetochores to opposite poles in amphitelic orientation [rather than syntelic orientation, defined in Comments (ii) below]; Figure 3, feature 10. This ensures that sister kinetochores, and thus their associated chromatids, move to opposite poles of the spindle during anaphase II.
Comments.
(i) Bivalent attachment to the metaphase I spindle has to be made in a very precise manner, because a bivalent has four centromeres/kinetochores to contend with, one for each of its four chromatids (29). The essentials are then twofold, namely -
(a) sister chromatid kinetochores must cooperate, so as to do the same thing; they must attach to one and the same pole of the spindle (referred to in recent texts and research articles as ‘syntely’ or ‘syntelic attachment’); and
(b) the two pairs of syntelic kinetochores must attach to opposite poles [referred to overall as ‘di-syntely’ or ‘di-syntelic attachment’ (30) or, in earlier literature, as ‘co-orientation’].
Di-syntely ensures that sister kinetochores, and thus their associated chromatids, move to the same pole of the spindle during anaphase of the first division of meiosis. Homologues, therefore, separate to opposite spindle poles.
(ii) A bivalent at metaphase I is in a ‘ready to go’ state, i.e., correct attachment to the spindle has been achieved, but sister chromatid arm cohesion continues to hold everything together. This is attested in Poppit when students tug on the centromeres of a bivalent such as that of Figure 3, feature 7A, B: the induced stretching of latex bands distal to the chiasma makes an informative and lasting impression on students.
(iii) Syntely is no longer appropriate for attachment to the spindle for metaphase II. Here, instead, sister chromatid kinetochores attach to opposite poles of the spindle (referred to as ‘amphitely’ or amphi-attachment or, in earlier literature, as ‘auto-orientation’) and then part ways as they segregate from each other.
Loss of cohesion: the anaphase trigger
PoppitSeparation of a bivalent into its two half-bivalents during anaphase I is illustrated in Figure 3, feature 8A, B. It is set in motion by using scissors to cut neutral (but not colored) latex bands, representing loss of arm cohesion. This allows half-bivalents to separate to opposite spindle poles, with their chromatids moving to the same pole (because of their syntelic spindle attachment).
Subsequently, PoppitAnaphase II is triggered, by cutting of, this time, the colored latex bands (Figure 3, feature 11). This allows each chromosome to move to the spindle pole to which its kinetochore is connected (31). Second division chromosomes end up in four separate cells, each of which is haploid, now with just one of the two homologues that were together in the same cell at the start of meiosis (Figure 3, feature 12). Halving of the number of chromosomes has been achieved.
Comments.
(i) Controlled loss of sister chromatid cohesion is a central feature in the overall mechanism of meiosis. As the ‘glue’ that maintains the integrity of a bivalent up to its attachment to the spindle, cohesion must be removed for anaphase to take place. Its loss is the trigger that sets anaphase separations into motion (16), thus converting 4X to 2X during anaphase I, then 2X to X during anaphase II.
(ii) Triggering of both anaphase I and anaphase II depends on the action of Separase enzymes, which cleave rings of cohesin proteins. Vitally, loss of cohesion of chromatid centromeres is prevented during anaphase I by their cohesin molecules being protected against proteolysis by the ‘guardian protein’ Shugoshin (32, 33).
Supplementing Poppit with Videos
Students are very responsive to the viewing of live-cell videos of meiosis. These have potential to enhance understanding beyond what modelling alone can accomplish. Making such videos, in either plants or animals, is not easy. It has been achieved in a few species, for males rather than females largely because of accessability and numbers of female cells available for culture. Videos of male meiosis may be found in The Cell Image Library. A strongly recommended one is CIL 10729 (enter into search line), which shows two primary spermatocytes of the crane-fly Nephrotoma suturalis undergoing meiosis. One cell starts at metaphase I, the other at anaphase I (the chromosomes of earlier stages being much less visible and thus not recorded). Both cells proceed through the two divisions of meiosis to produce haploid spermatids. Viewing such a video might be followed by information in diagram form and discussion on the equivalent process in females.
Recommended Timeline for PoppitMeiosis
The timeline for PoppitMeiosis itself will vary depending on the setting in which it is presented. The table below includes reference to the 2-hour sessions in our laboratory setting wherein Session 3 is shown to provide ample time to complete all steps of the lesson (i.e., ‘Running Poppit’ in its entirety).
For a standard lecture, seminar or classroom setting (lasting say 50 minutes) running PoppitMeiosis may need to be spread over multiple sessions. In such cases, a Poppit Pre-lesson and Introduction might occupy one class session, and then Poppit DNA/chromosome replication and bivalent formation in a following session, as would the remaining steps involving loss of cohesion and segregation. Instructors will quickly learn what works best for their particular circumstances.
Table 1 shows the timeline for each session. Instructors should assume that a Pre-lesson and PoppitMitosis have already been undertaken. The Meiosis Survey is supplied in Supporting Material (S1. Meiosis remodeled – Meiosis survey PowerPoint slides).
Table 1. Teaching timeline for the lesson by each session.
| Session 1 | Administer Meiosis Survey. Instructor obtains BeforePoppit data. |
| Session 2 | Review survey results (30 – 45 minutes). Each student identifies aspects of meiosis that are misunderstood and/or confusing. Most students already have a feeling for their individual misunderstandings, after taking the survey. An overall review adds to realization, whilst preserving anonymity. |
| Session 3 | Poppit instruction (2 hours). Instructor uses guidance provided in this article to present PoppitMeiosis. Student progress is checked at each stage of the project, to ensure modeling is being done correctly. |
| Session 4 | Students work on their own (1 hour). The aim is to build Poppit models and take cell phone images to document the process, for use either (a) in a laboratory report/assignment and/or (b) in a poster focusing on the use of Poppit and how meiosis works. This may be done outside set classroom hours. |
| Session 5 | Students assemble Poppit images (1 hour) into their report or poster, and write the text to explain their images. This may be done outside set classroom hours. |
| Session 6 | Administer Meiosis Survey (45 minutes). Instructor obtains AfterPoppit data. |
Teaching Discussion
Notwithstanding our personal conviction of the impact of Poppit on student learning, we are acutely aware of a need to ‘prove the point.’ Thus we attempted to assess the issue using two different strategies: (1) how well students perform in a ‘Meiosis’ PowerPoint poster presentation, and (2) how well they perform in a set of survey questions presented to them before and then again after involvement with Poppit. For the poster presentation, after receiving instruction with Poppit, students were required to ‘start from scratch’ and make their own cell-phone images of the steps in meiosis, in a way resembling Figure 3. This proves to be a very convincing way for instructors to assess competence/understanding that students have realized from Poppit. Nevertheless, such assessment is necessarily somewhat subjective. Our survey, results from which are presented below, was designed to obtain a more definitive, quantitative view of Poppit’s impact.
Poppit Survey
Design
A set of 45 multiple-choice questions was presented to students both prior to participating in PoppitMeiosis (hereafter termed ‘BeforePoppit’) and then again afterwards (‘AfterPoppit’). Questions were formatted as a PowerPoint presentation and shown to students during a regular class session. Students were allotted one minute to answer each question. The survey in its entirety, plus an answer key, is included as Supporting Material (S1. Meiosis remodeled – Meiosis survey PowerPoint slides). Of the total, 35 questions were designed to assess understanding of meiosis, whilst 10, covering features of mitosis, were designed to act as an internal control. We deemed results from the control questions unlikely to be influenced significantly by PoppitMeiosis. That expectation was based on the fact that three weeks prior to the survey students had completed a project on mitosis in Vicia faba root meristem cells. For that project, the importance of mitosis-specific concepts were studied, such as amphitelic kinetochore attachment to the spindle in metaphase, and cohesion of sister chromatids and its loss at anaphase onset.
Two types of survey questions, Type 1 and Type 2, were used. Type 1 questions were in the standard multiple-choice form, offering (i) the correct answer and (ii) four incorrect answers. In contrast, Type 2 questions offered (i) the correct answer, (ii) three incorrect answers and (iii) an ‘I am uncertain …’ option. The latter was included to discourage guessing, which students were informed would be unhelpful for the purpose of the survey. For analysis, we grouped the meiosis-specific questions into four pools, as shown below in the section titled: Partitioning meiosis-specific questions.
Table 2. Composition of question groupings. Meiosis Survey questions, categorized according to control and four meiosis-specific themes identified at left of the table, for Type 1 and Type 2 questions.
| Type of question | Questions | Number |
|---|---|---|
| Control group (mitosis-specific questions) | ||
| Type 1 | 26, 39, 40, 41 | 4 |
| Type 2 | 2, 36, 37, 38, 42, 44 | 6 |
| Pool 1 (DNA replication and math of meiosis) | ||
| Type 1 | 27, 31, 33 | 3 |
| Type 2 | 34, 35, 45 | 3 |
| Pool 2 (synapsis, bivalents and chiasma formation | ||
| Type 1 | 7, 9, 28 | 3 |
| Type 2 | 6, 10, 12, 14, 15, 17, 19, 20, 43 | 9 |
| Pool 3 (kinetochore attachment and orientation) | ||
| Type 1 | not included | 0 |
| Type 2 | 4, 5, 11, 16, 21, 23 | 6 |
| Pool 4 (cohesion and loss thereof) | ||
| Type 1 | 8, 25, 29, 30, 32 | 5 |
| Type 2 | 1, 3, 13, 18, 22, 24 | 6 |
| Totals | ||
| Type 1 | 15 | |
| Type 2 | 30 | |
| Survey total | 45 | |
Subjects
Students enrolled in a senior-level cytogenetics lab course BIO 420 at the University at Buffalo (UB) were recruited using a protocol approved for use on human subjects by the Institutional Review Board of UB (Meiosis study 00002563). Enrollments in this course typically total around 15 (average for the past 5 years). To increase the sample size of the study, therefore, data were obtained from three sequential offerings of the survey, held during (a) the Fall semester of 2018 (F’18), (b) the Spring semester of 2019 (S’19) and (c) the Fall semester of 2019 (F’19). To ensure anonymity, students were given free choice as to whether they took part in the survey, and participation was, accordingly, less than 100% of enrollments. Moreover, a few students elected to take part in assessment BeforePoppit but not AfterPoppit, or vice versa. When combined, the three survey offerings provided for analysis a total of 77 ‘student cases’ and 3,465 answers (to 2,310 Type 1 and 1,155 Type 2 questions). Throughout, student answer sheets (bubble sheets) were assessed anonymously.
We imagined that our three surveys together would provide good variation in student ability, in terms of ‘raw’ talent, attitude to learning, response to visual cues, responsiveness to assessment etc., thus giving ‘substance’ and relevance to our cause. Whilst not pursuing the issue in depth, we did test for variation between surveys by contingency chi-squared analysis. The results, shown in Table 3, indicate the presence of statistically significant differences (<0.01 probability) between surveys for the ‘more challenging’ Type 2 questions, but not for more ‘straight forward’ Type 1 questions.
Table 3. Individual survey results. Before/after survey results for individual semesters (F’18, S’19 and F’19) for Type 1 and Type 2 questions. Results of heterogeneity chi-squared analysis given at base of each sub-table.
| Survey | BeforePoppit | AfterPoppit | Total | ||||
|---|---|---|---|---|---|---|---|
| Correct | Uncertain | Incorrect | Correct | Uncertain | Incorrect | ||
| Type 1 questions | |||||||
| F’18 | 115 | 0 | 80 | 141 | 0 | 69 | 405 |
| S’19 | 111 | 0 | 84 | 129 | 0 | 66 | 390 |
| F’19 | 99 | 0 | 81 | 123 | 0 | 57 | 360 |
| Total | 325 | 0 | 245 | 393 | 0 | 192 | 1,155 |
| Contingency Χ2 = 2.3, degrees of freedom (d.f.) 6, probability (p.) >>0.05, not significant (ns) | |||||||
| Type 2 questions | |||||||
| F’18 | 145 | 198 | 47 | 322 | 44 | 54 | 810 |
| S’19 | 144 | 166 | 80 | 267 | 42 | 79 | 780 |
| F’19 | 105 | 217 | 38 | 273 | 31 | 56 | 720 |
| Total | 394 | 581 | 165 | 864 | 117 | 189 | 2,310 |
| Contingency Χ2 = 42.2, degrees of freedom (d.f.) 10, probability (p.) <0.01 | |||||||
For presentation and analysis, we show only combined data from all three surveys together. Their individual data are available on request.
Overall results and ‘control’ questions
When all questions are considered together, survey results show an overwhelmingly positive response to student involvement with Poppit, registering an ‘improvement’ of from 43% to 72% correct answers given (731/1,710 BeforePoppit, 1,257/1,755 AfterPoppit; X2 = 295, d.f. 1, p. <<0.001). When separated out, the 10 internal control questions showed essentially no significant difference between BeforePoppit and AfterPoppit, neither for Type 1 nor Type 2 questions; Table 4. Particularly noteworthy is the fact that even the presence of an ‘I am uncertain’ option has little effect on the Before/After outcome for control questions. We are justified, therefore, in using these control data as a base for comparisons with the remaining 35, meiosis-specific questions.
Table 4. Before/After results for control questions, Type 1, and Type 2 questions for three semester combined. Results of heterogeneity chi-squared analysis given at base of table; abbreviation as for Table 3.
| Correct (%) | Uncertain (%) | Incorrect | Uncertain + Incorrect | Total | |
|---|---|---|---|---|---|
| A. Type 1 questions (4) | |||||
| Before | 133 (88) | 19 | 152 | ||
| After | 120 (77) | 36 | 156 | ||
| B. Type 2 questions (6) | |||||
| Before | 164 (72) | 38 (17) | 26 | 228 | |
| After | 174 (74) | 25 (11) | 35 | 234 | |
| C. Type 1 +Type 2 (10) | |||||
| Before | 297 (78) | 83 | 380 | ||
| After | 294 (76) | 96 | 390 | ||
| Before/after tests. A. X2 = 5.8, d.f. 1, p. ~0.02; B. X2 = 4.2, d.f. 2, ns; C. X2 = 0.8, d.f. 1, ns | |||||
Meiosis-specific questions
Two main general points arise in regard to the meiosis specific questions of Table 5.
Table 5. Before/After results from meiosis-specific question, Type 1, and Type 2 questions for three semesters combined. Results of heterogeneity chi-squared analysis given at base of table; abbreviation as for Table 3.
| Correct (%) | Uncertain (%) | Incorrect | Total |
Correct as % of correct + incorrect * |
|
|---|---|---|---|---|---|
| A. Type 1 questions (15) | |||||
| Before | 192 (46) | 226 | 418 | 46 (192/418) | |
| After | 273 (64) | 156 | 429 | 64 (273/429) | |
| B. Type 2 questions (20) | |||||
| Before | 230 [25] | 543 [60] | 139 | 912 | 62 (230/369) |
| After | 690 [74] | 92 [10] | 154 | 936 | 82 (690/844) |
|
Type 1 vs. Type 2 tests. BeforePoppit, X2 = 21, d.f. 1, p. <<0.01; AfterPoppit, X2 = 52, d.f. 1, p. <<0.01 * i.e. excluding the ‘uncertain’ responses to Type 2 questions |
|||||
(i) The ‘Uncertain’ effect.
Presence of an ‘I am uncertain’ option to a question in itself substantially changes the ratio of correct to incorrect answers at the BeforePoppit stage of assessment, from about half (46%) correct to two thirds (62%) correct; refer far right of Table 5. This may be because for a significant number of questions a significant number of students are ‘guessing’ – and, given that 3 or 4 of the 5 answer choices for each question are incorrect, these students are guessing mostly incorrectly. A similar effect (64% vs. 82% correct) is shown for the AfterPoppit stage of assessment.
(ii) The ‘Poppit’ effect.
The overall beneficial effect of Poppit is abundantly clear from the data of Table 5. Firstly, the percentage of correct answers has increased, most impressively we note, from 46% to 64% for Type 1 questions, and from 25% to 74% for Type 2 questions (refer far left of Table 5). Secondly, and correspondingly, the percentage of ‘uncertain’ answers to Type 2 questions decreased from 60% to but 10%. We submit that that is an impressive result, indicating that Poppit does indeed go a long way to removing uncertainty and promoting understanding of the principles of meiosis.
We note that the beneficial effects of Poppit are less marked (though still clearly present) for Type 1 questions, compared to those of Type 2. Presumably this reflects the fact that a level of uncertainty still exists after Poppit. We presume this is because a significant number of students continue to guess and guess incorrectly.
Partitioning meiosis-specific questions
We were interested in the extent to which students find different aspects of meiosis more or less challenging. Thus, we divided the overall data set of meiosis-specific questions into four Pools, containing questions relating to the following.
Pool 1: chromosome replication and the math of meiosis;
Pool 2: synapsis, bivalents and chiasma formation;
Pool 3: kinetochore attachment to and orientation on the metaphase spindle; and
Pool 4: cohesion and its loss during anaphase I and anaphase II.
Table 2 indicates specific questions included in each Pool.
Figure 4 presents data for individual Pools 1 through 4, with Type 1 and Type 2 questions combined, for comparison with equivalents for ‘Control’ (far left) and ‘All Pools’ (all four pools combined). The BeforePoppit data are on the left of each pair of bars, the AfterPoppit data on the right. Each bar indicates the percentage of survey participants that answered the included questions correctly, compared to those that chose either the incorrect answer or the uncertain option (which for the purpose of this figure were scored as not correct).
Equivalent data for Type 1 and Type 2 questions examined separately are shown in Figure 5. For Type 1 questions (left set of bars) each bar shows correct and incorrect answers (‘I am uncertain’ not being an option). For Type 2 questions (right set) each bar indicates the percentage of participants that gave the correct answer, an incorrect answer or, now in this case, selected the ‘I am uncertain’ response.
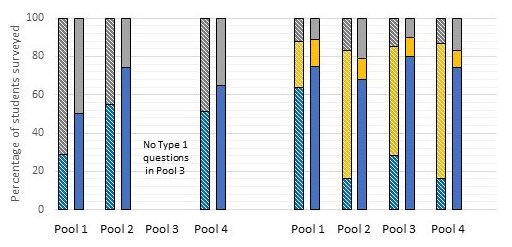
The data of Figures 4 and 5 show unequivocally that Poppit is impactful for all four aspects of meiosis represented by Pools 1 – 4, for both Type 1 and Type 2 questions. However, the Poppit effect is ‘felt’ most strongly for Type 2, Pool 3 and Pool 4 questions, in that these show the greatest normalized gains (34) in correct answers (0.72 and 0.70, respectively); and felt least strongly for Type 1, Pools 1 and 4 and Type 2, Pool 1 questions (normalized gains ~0.30). Correspondingly, Pools 2-4 Type 2 questions show particularly marked decreases in uncertainty.
Student Evaluation
In addition to the survey results presented above, we obtained supporting quantitative evidence that Poppit modeling is indeed effective in teaching meiosis from responses to a Likert-style (35) item in end-of-course evaluations conducted by UB. The Poppit item added to the standard survey read: ‘Poppit bead modeling of meiosis was instrumental in my achieving a better understanding of the mechanisms of meiosis than I had prior to this course’. On a 5-point scale, 29 respondents out of 38 strongly agreed (scale value 5) and another 9 agreed (scale value 4), whilst none disagreed, indicating a very favorable response to Poppit.
Summary
With jointly over eighty years of experience in teaching and research in cell biology and genetics, we appreciate well that hands-on ‘teaching tools’ can have a marked impact on student understanding and learning. Addition of new elements to the use of strings of colored beads to assist understanding of meiosis motivated us here to share some of this experience – with what we refer to overall as ‘PoppitMeiosis’ - with others. To this end, we have presented a modern version of how to use poppit beads to model meiosis, and the results of a BeforePoppit/AfterPoppit survey of its impact. Thus –
(a) We have reported statistically significant, and what we see as impressive, evidence that Poppit modeling is, indeed, an impactful tool to use in the seemingly ever ongoing difficulties associated with student understanding of meiosis. The results of our survey indicate an overall highly significant improvement in understanding stemming from student participation in Poppit, for both traditional ‘select the correct answer’ Type 1 questions and those less conventional Type 2 questions that contain an ‘I am uncertain …’ option.
(b) Our survey data as presented in Figure 5 indicate that our students have considerable difficulty in answering Type 1 questions. Many such questions include diagrams requiring students to both interpret correctly and then make a judgment based on that interpretation. Such double-barrelled questions seem to ask a lot of students, even with Poppit. Also, several Type 1 questions required students to recognize a feature as being important to meiosis and then to correctly place it in a sequence of events. Difficulty in such chronology questions suggests a need for greater understanding of both the events themselves and where they fit in the overall sequence constituting meiosis.
(c) For Type 2 questions, the high percentage of students selecting the ‘uncertain’ option BeforePoppit (Figure 5) comes as no surprise to us. Perhaps specifically for our survey, some students ‘hedged their bets’, or simply were not willing to commit to a definite answer, given the option of not doing so. We suggest, however, that mostly the reason is that for a significant number of questions a significant number of students are genuinely confused and thus simply ‘guessing’ at the answer; and these students are guessing mostly incorrectly, when forced to commit to a definite answer. But we are pleased by the fact that uncertainty levels decrease very sharply to as low as less than 10% AfterPoppit. Moreover, this decrease is matched mostly by a corresponding increase in correct rather than incorrect answers AfterPoppit. We submit that that is an impressive result, indicating that Poppit does indeed go a long way to removing uncertainty and promoting correct understanding of the principles of meiosis. We do not claim that Poppit is the only modeling exercise that will achieve such a result, only that it is at least one effective, nay very effective, way of promoting proper understanding. We therefore highly recommend it, or an equivalent, be adopted generally, rather than relying solely on verbal presentation, reading and studying textbook diagrams on the topic. Proper hands-on modeling is demonstrably effective, as evident from our survey, and via student evaluation responses.
(d) Nevertheless, it is clear from our survey results that even AfterPoppit as much as perhaps 50% of students either are still ‘uncertain’ or still have not ‘got it right’, for Type 2 and especially for Type 1 questions, in spite of our best efforts with Poppit. But we accept that our students have varying interests and commitments to the subject, and thus it may not be possible ever to get to or close to 100% correct responses, even though that is what we should strive for.
SUPPORTING MATERIALS
S1. Meiosis remodeled – Meiosis survey PowerPoint slides
Acknowledgments
Jim Stamos, whose expertise and dedication to the task we gratefully acknowledge and applaud, made the illustrations for this article. Many a discussion over many a year on ‘things meiosis’ with RN (Neil) Jones (Aberystwyth University, Wales) and, in particular, with JL (Oof) Oud (University of Amsterdam, Netherlands) indirectly, but most meaningfully, helped in putting Poppit together. Shirley Pledger (School of Mathematics, Statistics and Computer Science, Victoria University of Wellington, Wellington, New Zealand) provided helpful advice on statistical matters. Thank you.
References
- Newman DL, Catavero CM, Wright LK. 2012. Students fail to transfer knowledge of chromosome structure to topics pertaining to cell division. CBE – Life Sciences Education 11, 425-436. doi: 10.1187/cbe.12-01-0003
- Wright LK, Catavero CM, Newman DL. 2017. The DNA triangle and its application to learning meiosis. CBE – Life Sciences Education. 16: ar50, 1-14. doi: 10.1187/cbe.17-03-0046
- Metzger KJ, Yowler JY. 2019. Which way is better? Comparison of two interactive modeling approaches for teaching meiosis in an introductory undergraduate biology course. The American Biology Teacher 81, 98-109. https://doi.org/10.1525/abt.2019.81.2.98
- Hubbs NB, Parent KN, Stoltzfus JR. 2017. Models in the biology classroom: An in-class modeling activity on meiosis. The American Biology Teacher 79, 482-491. https://doi.org/10.1525/abt.2017.79.6.482
- Newman DL, Wright LK. 2017. Meiosis: A play in three acts, starring DNA sequence. CourseSource. https://doi.org/10.24918/cs.2017.9
- Locke J, McDermid HE. 2005. Using pool noodles to teach mitosis and meiosis. Genetics 170, 5-6. https://doi.org/10.1534/genetics.104.032060
- Oud JL, Rickards GK. 1999. Understanding mitosis and meiosis: an interactive education tool (CD-ROM). Springer-Verlag Berlin & Heidelberg GmbH & Co. KG.
- Goff EE, Reindl KM, Johnson C, McClean P, Offerdahl EG, Schroeder NL, White AR. 2017. Efficacy of a meiosis learning module developed for the virtual cell animation collection. CBE – Life Sciences Education 16, 1-12. https://doi.org/10.1187/cbe.16-03-0141
- Kalas P, O’Neill A, Pollock C, Birol G. 2013. Development of a meiosis concept inventory. CBE – Life Sciences Education 12, 655-664. doi: 10.1187/cbe.12-10-0174
- Misteli T. 2008. The arrangement of chromosomes in the nucleus. Nature Education 1:167. https://www.nature.com/scitable/topicpage/chromosome-territories-the-arrangement-of-chromosomes-in-3025/
- O’Connor C. 2008. Chromosome segregation in mitosis: the role of centromeres. Nature Education 1:28. https://www.nature.com/scitable/topicpage/chromosome-segregation-in-mitosis-the-role-of-242/
- Annunziato A. 2008. DNA packaging: Nucleosomes and chromatin. Nature Education 1:26. https://www.nature.com/scitable/topicpage/dna-packaging-nucleosomes-and-chromatin-310/
- O’Connor C. 2008. Mitosis and cell division: Stages of mitosis. Nature Education 1:188. https://www.nature.com/scitable/topicpage/mitosis-and-cell-division-205/
- Jones RN, Rickards GK. 1992. Practical Genetics. John Wiley & Sons.
- Nasmyth K. 2011. Cohesin: a catenase with separate entry and exit gates? Nature Cell Biology 13, 1170-1177. https://www.nature.com/articles/ncb2349
- Clift D, Marston AL. 2011. The role of Shugoshin in meiotic chromosome segregation. Cytogenet. Genome Res. 133, 234-242. https://doi.org/10.1159/000323793
- Makrantoni V, Marston AL. 2018. Cohesin and chromosome segregation. Current Biology 28, R688-R693. doi: 10.1016/j.cub/2018.05.019
- Brooker R. 2021. Genetics: analysis and principles. McGraw Hill Learning, 7th edition.
- Pierce BA. 2020. Genetics: A conceptual approach. Macmillan Learning, 7th edition.
- Moens PB. 2001. Synaptonemal complex. In Brenner, S. & Miller, J.L. (Eds.), Encyclopedia of Genetics. Academic Press. doi: 10.1006/rwgn.2001.1265
- Kleckner N. 2006. Chiasma formation: chromatin/axis interplay and the role(s) of the synaptonemal complex. Chromosoma (Berl.) 115, 175-194. https://doi.org/10.1007/s00412-006-0055-7
- Spindler MC, Filbeck S, Stigloher C, Benavente R. 2019. Quantitative basis of meiotic chromosome synapsis analyzed by electron tomography. Sci. Rep. 9, 16102. https://doi.org/10.1038/s41598-019-52455-4
- Moens PB. 2001. Chiasma/ta. In Encyclopedia of Genetics. Eds. S. Brenner and J.H. Miller. Academic Press. doi: 10.1006/rwgn.2001.0189
- Klutstein M, Cooper JP. 2014. The chromosomal courtship dance – homolog pairing in early meiosis. Curr. Opin. Cell Biol. 26, 123-131. https://doi.org/10.1016/j.ceb.2013.12.004
- Zickler D, Kleckner N. 2015. Recombination, pairing, and synapsis of homologs during meiosis. Cold Spring Harb. Perspect. Biol. 7, a016626. doi: 10.1101/cshperspect.a016626
- Grelon M. 2016. Meiotic recombination mechanisms. Comptes Rendus Biologies 339, 247-251. doi: 10.1016/j/crvi/2016.04.003
- Sakakibara Y, Hashimoto S, Nakaoka Y, Kouznetsova A, Höög C, Kitajima TS. 2015. Bivalent separation into univalents precedes age-related meiosis I errors in oocytes. Nat. Commun. 6, 7550. https://doi.org/10.1038/ncomms8550
- Rieder CL. 1981. The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma (Berl.) 84, 145-158. https://doi.org/10.1007/BF00293368
- Miller MP, Amon A, Ünal E. 2013. Meiosis I: When chromosomes undergo extreme makeover. Curr. Opin. Cell Biol. 25(6), 687-696. doi: 10.1016/j.ceb.2013.07.009
- LaFountain JR Jr, Oldenbourg R. 2004. Maloriented bivalents have metaphase positions at the spindle equator with more kinetochore microtubules to one pole than to the other. Molecular Biology of the Cell 15, 5346-5355. https://doi.org/10.1091/mbc.e04-06-0524
- Gregan J, Spirek M, Rumpf C. 2008. Solving the Shugoshin puzzle. Trends in Genetics 24, 205-207. doi:https://doi.org/10.1016/j.tig.2008.02.001
- Watanabe Y. 2005. Shugoshin: guardian spirit at the centromere. Curr. Opin. Cell Biol. 17(6), 590-5. doi: 10.1016/j.ceb.2005.10.003
- Watanabe Y. 2012. Geometry and force behind kinetochore orientation: lessons from meiosis. Nature Reviews Molecular Cell Biology 13, 370-382. https://doi.org/10.1038/nrm3349
- Marx JD, Cummings K. 2007. Normalized change. Am. J. Physics 75, 87-91. https://doi.org/10.1119/1.2372468
- Likert R. 1932. A technique for the measurement of attitudes. Archives of Psychology 140, 5-55.
Article Files
Login to access supporting documents
LaFountain-Meiosis Remodeled Poppit Beads.pdf(PDF | 1 MB)
S1. Meiosis remodeled Meiosis survey PowerPoint slides.pptx(PPTX | 2 MB)
- License terms

Comments
Comments
0 Like
Carolyn Wetzel @ on
I have a question about the prep for this activity. How many of each item (popbeads, stirrers, etc) are needed per student? Thanks, Carolyn
Copy link Report abuse
0 Like
Sharleen Flowers @ on
Hi, Carolyn. Thank you for your question! Here's the response from the authors:
For di-chiasmic bivalents, metacentric chromosomes having 8 beads per arm or 16 total are recommended.
Put the chiasma in the space marked by the arrow (see attached image file).
Copy link Report abuse