Sequences and Properties of Gluten Proteins From Different Grains: A Virtual-Friendly Protein Laboratory
Editor: Kristin Fox
Published online:
Abstract
The COVID-19 pandemic has forced much university instruction into virtual modes. As a result, many laboratory courses have either dropped content altogether or replaced hands-on activities with simulations and “dry” labs. Our goal was to create a structured, hands-on laboratory in protein sequence, interactions, and function that students can perform either in their own homes for a virtual laboratory, or in a standard teaching lab during in-person instruction. This laboratory explores the sequences of proteins from grain glutens (the protein matrix of flour, consisting of glutenin and prolamin proteins) and correlates sequences with the physical properties of bread dough. In this laboratory, students examine the sequences of glutenin proteins from various grains (wheat, spelt, rye, and rice), prepare dough from one of these four flours, extract gluten, and compare the physical properties of the gluten between grains, and between untreated and cysteine-treated samples to examine the importance of disulfide bonds in gluten structure. The students are expected to correlate these properties with the sequences of the gluten proteins and the relevant sequence-dependent noncovalent intermolecular interactions. Assessment consists of a simple worksheet and (for virtual laboratories) a student-submitted video of the resulting gluten sample. We find this laboratory successfully demonstrates protein sequence-structure-function relationships with a minimum of resources, is highly customizable, is accessible and interesting to students in a range of study disciplines, and is generally appreciated by students desiring hands-on laboratory activities during virtual instruction.
Primary image: Gluten extracted from wheat flour, demonstrating the extensible and plastic nature of this complex protein network.
Citation
Jones EM, Fogle EJ. 2022. Sequences and properties of gluten proteins from different grains: A Virtual-friendly protein laboratory. CourseSource. https://doi.org/10.24918/cs.2022.13Society Learning Goals
Biochemistry and Molecular Biology
- Macromolecular Structure Determines Function and Regulation
- What factors contribute to the size and complexity of biological macromolecules?
- What factors determine structure?
- How are structure and function related?
- What is the role of noncovalent intermolecular interactions?
- How is structure (and hence function) of macromolecules governed by foundational principles of chemistry and physics?
Lesson Learning Goals
Students will- understand the structure of gluten and its importance in the structure and properties of breads and other grain-based foods.
- know the general sequence features of gluten proteins.
- understand the importance of covalent and noncovalent intermolecular interactions to the properties of gluten.
- understand the relationship between gluten protein sequences and diseases of gluten sensitivity, such as celiac disease.
Lesson Learning Objectives
Students will be able to- relate the properties of bread dough to the gluten protein content and the sequence of the gluten proteins.
- compare the sequences of different gluten proteins and correlate sequence features with intermolecular forces and interactions.
- qualitatively predict the relative elasticity and extensibility of various flour doughs based on the lengths and sequences of the gluten proteins, and semiquantitatively measure these properties.
- explain the importance of intermolecular disulfide bonds in the properties of gluten networks.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
Introduction
The global coronavirus pandemic has forced educators and students of all types to adjust to remote and online learning. This transition has been particularly challenging for students and educators in the sciences, as laboratories are difficult or impossible to replicate completely in a virtual or distance format. Simulated and virtual laboratories, in which students perform approximations of actual laboratory actions through an electronic interface, have grown in complexity and popularity in recent decades, and show similar gains in student conceptual learning to in-person laboratories (1). However, maintaining student motivation and satisfaction is often a challenge with college- and graduate-level virtual laboratories (2, 3), as many students prefer a hands-on learning experience.
For our department, a particular challenge was creating accessible and engaging remote-learning labs for non-science majors, as such students often struggle with motivation even in a face-to-face laboratory. During the first academic year of the COVID-19 pandemic, we invested considerable effort in creating hands-on home or “kitchen” biochemistry activities for an upper-division non-majors biochemistry course (CHEM 313: Survey of Biochemistry and Biotechnology). The goals for the laboratories were 1) to illustrate and reinforce principles of biochemistry that are emphasized in the existing curriculum, 2) to be equally achievable and safe at home as in a teaching laboratory, and 3) to be inexpensive and engaging, to maximize accessibility and student engagement. One of the most important learning goals for the course is for students to develop a strong understanding of protein structure and function, and to relate macromolecular function to basic principles of chemistry and physics, such as bonding and intermolecular forces. We therefore sought to develop a kitchen laboratory that illustrates these concepts using inexpensive materials and that can be ported back to the in-person laboratory as the need for remote instruction eases. This laboratory, in which students extract gluten from baking flour and relate the properties of the gluten to the sequence of the component gluten proteins, is a result of these efforts.
Gluten (Figure 1) is the major protein component of cereal grains, and is responsible for the extensibility and elasticity of baked goods (4). Gluten is a complex mixture of glutamine- and proline-rich proteins, consisting of a low-molecular-weight fraction soluble in 50-70% aqueous ethanol (prolamins) and an insoluble polymeric fraction (glutenins, also known as glutelins). The prolamins (known as gliadins in wheat and secalins in rye) are the primary immunogenic agents in diseases of gluten sensitivity, such as celiac disease (5). The glutenin fraction consists of disulfide-crosslinked high- and low-molecular-mass glutenin proteins (HMM and LMM glutenins, respectively), which consist of globular N- and C-terminal domains separated by a Gln/Pro-rich repetitive domain of variable length, as reviewed by Shewry et al. (6). The network structure of HMM glutenins is shown schematically in Figure 1B. The repetitive domains form sequential intermolecular hydrogen bonds (“trains”, shown as groupings of dotted lines in Figure 1B), interspersed with hydrated segments (“loops”). The gluten network provides a viscoelastic matrix that retains moisture, with the ratio of loops to trains influencing the stiffness of the dough (6, 7). Grains lacking the glutenin proteins, such as maize and rice, do not produce such a network and thus lack a spongy texture when baked.
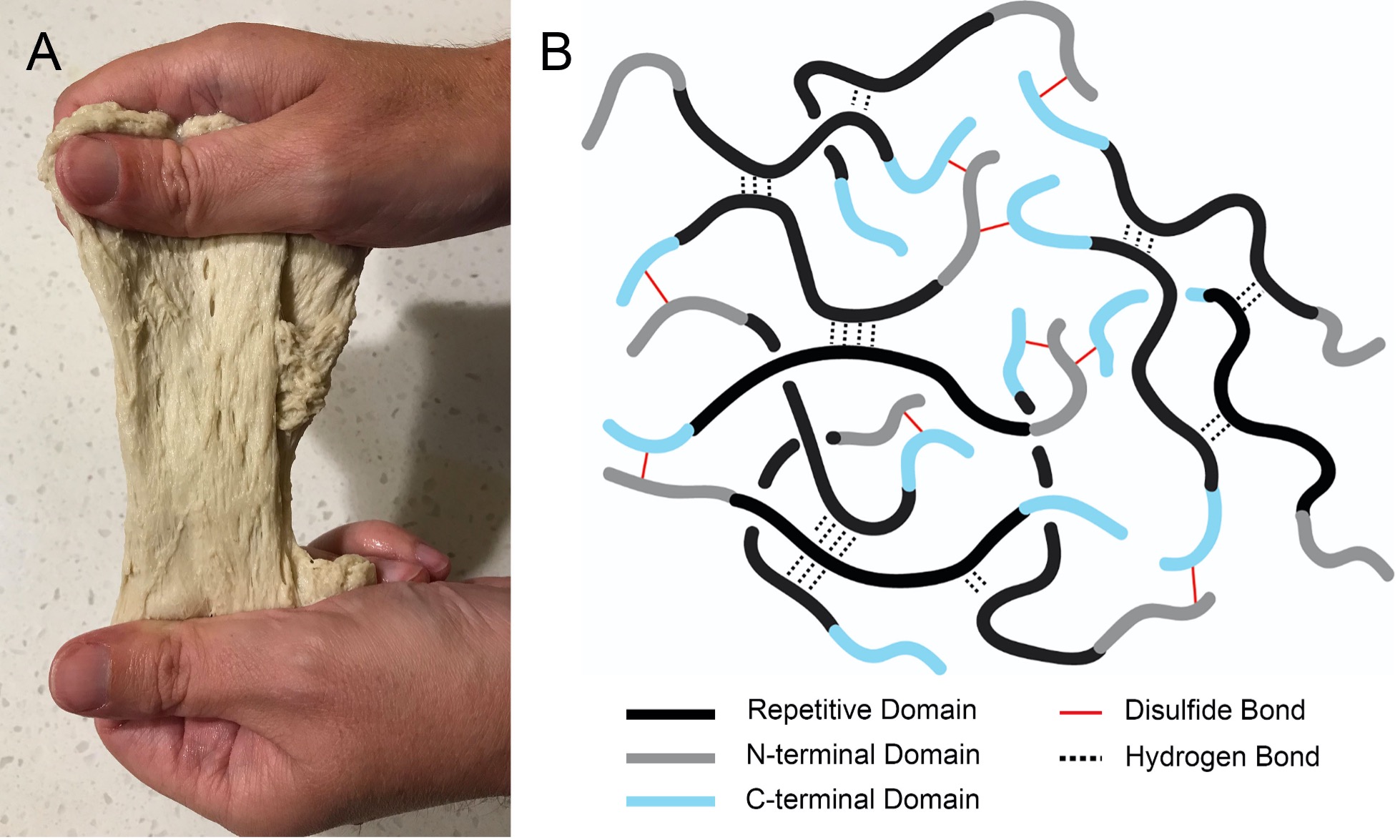
The processes involved in the formation of the gluten network remain poorly understood. Nonetheless, it is generally believed that hydration and kneading of dough increases the extent of intermolecular contacts in the “trains” of the repetitive domains, including intermolecular β-sheet formation, entanglements, and sidechain hydrogen bonding between the many Gln residues in both glutenins and prolamins (6–9). The rigidity of the gluten network is provided partly by these hydrogen bond interactions and partly by the intermolecular disulfide crosslinks between the N- and C-terminal domains of the HMM glutenins (6) (Figure 1B). LMM glutenins (omitted for clarity from Figure 1B) also link to the HMM chains by disulfide bridges and contribute to the hydrogen bonding network. Thus, a higher content of glutenins, and a higher degree of disulfide crosslinking, is associated with stiffer and less viscous dough. Disulfide reducing agents, such as cysteine and glutathione, are therefore often used in large-scale baking to improve dough workability by breaking the crosslinks (10). In summary, three major properties of flour influence the mechanical properties of the gluten network: The content of glutenins (particularly the HMM chains), the length and sequence of the repetitive domains of these glutenins, and the degree of intermolecular disulfide bonding (6).
This laboratory allows students to examine the relationship between these properties of gluten proteins and the physical characteristics of bread dough, using both a hands-on gluten extraction activity and a database search of gluten proteins and their sequences. Since full sequence data is available for gluten proteins from a broad range of important grains, including a full, annotated proteome database for wheat gluten proteins (11), it is possible to incorporate these data into a simple online bioinformatics activity in parallel with the hands-on activity. Students prepare dough from flour of various pre-selected grains, extract the gluten, and compare the mechanical properties between different flours, which can then be explained on the basis of the different protein sequences. Optionally, the importance of disulfide bonds in gluten properties can be examined by assigning some students to prepare dough with added cysteine as a disulfide reducing agent. For a virtual (home) lab, each student prepares one dough formulation and shares data with the rest of the class asynchronously, while results can be compared in real-time in an in-person laboratory. Lesson plans for in-person and virtual variants of the laboratory are provided below (see Table 1), and a summary of results for a virtual lab section is provided in the Discussion. The hands-on portion of this laboratory is broadly similar to gluten structure-property activities described previously for courses in food science and general chemistry(12, 13); however, the present laboratory incorporates a greater emphasis on gluten sequence analysis while eliminating the need for specialized equipment (e.g., kitchen mixers). We therefore feel this laboratory better aligns with the specific learning goals of nonmajors biochemistry or general-organic-biochemistry courses and is more easily portable to distance and virtual learning than similar laboratories.
Table 1. Teaching timeline for gluten laboratory.
| Activity | Description | Estimated Time | Notes |
|---|---|---|---|
| Before-lab preparation | |||
| Assign conditions to students | Assign a flour and cysteine treatment condition to each student and ask for verification | Varies | Unnecessary for in-person lab. For virtual lab, this should be done 2 weeks prior to due date in case supplies must be ordered. If possible, consider pre-assembled “kits” students can pick up. |
| Introductory videos | Students watch videos about gluten and general lab procedure | 10 minutes | Was followed by 10-minute online quiz (optional) to verify preparedness. |
| Lab activity | |||
| Introductory Lecture | Brief overview of lab procedure | 5 minutes | In-person lab only |
| Preparing dough | Mix yeast, water, cysteine, flour; knead for 10 minutes, measure diameter | 15-20 minutes | Rotate kneading between students (in-person lab) |
| Proofing | Allow dough to rise, covered, about 25 °C | Up to 90 minutes | Optional, but recommended |
| Bioinformatics activity | Obtain glutenin sequences from UniProt, sequence alignment, compute protein parameters | 30-45 minutes | Can be done while dough rises |
| Gluten extraction | Measure diameter after rise; rinse dough under running water | 20-30 minutes | Rotate task between students (in-person lab) |
| Gluten properties | Stretch gluten, perform “pull test”, share data with class | 10-20 minutes | Sharing data with class for in-person lab only; virtual lab students upload video and data to LMS |
| Clean up | Discard gluten, rinse utensils, scrape countertops | Varies | |
Intended Audience
This activity was designed for an upper-division, non-majors undergraduate course in general biochemistry and biotechnology, with laboratory. The course is taken primarily by students majoring in sciences other than biology, chemistry, or biochemistry; and by majors in the nutritional and agricultural sciences, including nutrition, food science, animal science, and crop science. We believe the activity would also be appropriate for an introductory biology laboratory for majors.
Required Learning Time
Although this laboratory was intended as a home “kitchen” activity, it was designed to fit into a three-hour laboratory block, plus time outside of class for pre-lab reading and the assessment worksheet after lab. Pre-lab preparation (videos and a short online quiz) is expected to take about 20 minutes for each student. The lab itself can be completed in three hours (significantly less if the dough rising step is omitted). The post-lab assessment activity is expected to take 30-45 minutes per student. An approximate timeline is provided in the sample lesson plan shown in Table 1.
Prerequisite Student Knowledge
Students performing this laboratory have already been exposed to fundamentals of intermolecular forces important to biological molecules, including hydrogen bonding, dipole-dipole interactions, ion-dipole interactions, and the hydrophobic effect. The structures and properties of the amino acids have also been introduced by this point in the course, as well as the hierarchical nature of protein structure (primary-secondary-tertiary-quaternary), chemical factors influencing protein stability, and the relationship of protein structure to the intermolecular forces mentioned above. Though not specifically addressed in the course, students were also asked to watch a short video describing the relationship of gluten protein sequence and structure to diseases of gluten sensitivity, e.g., celiac disease (see Supporting File S1: Gluten proteins – List of relevant YouTube video links).
Prerequisite Teacher Knowledge
Instructors should be well-versed in protein structure and sequence analysis. A familiarity with basic bioinformatics tools, such as multiple sequence alignments and locating protein and gene sequences and domains in databases such as UniProt and PROSITE, is desirable. Though not essential, hands-on experience with baking is helpful, as this will allow the instructor to anticipate difficulties the students may encounter during dough mixing, kneading, and proofing.
Scientific Teaching Themes
Active Learning
Before the activity, students will watch videos briefly explaining the structure and properties of gluten. Working in teams (in a classroom) or individually (in virtual learning), students will then prepare dough from an assigned grain to extract the gluten. During the activity, students will examine and align sequences of glutenin proteins from the different grains being examined in the lab and from these sequences and the background reading will qualitatively predict the properties of the various glutens being examined. After successful extraction of gluten, students will examine the physical properties of the gluten samples and share their data either in real-time (in-person lab) or prepare videos and share their data asynchronously (virtual lab). A complete set of student instructions is provided in the Supporting Materials (Supporting File S2. Gluten proteins – Student instructions and Supporting File S3. Gluten proteins – Brief instructional video including pull test demonstration). Because all students rely on all other students for a complete set of data, the laboratory is inherently hands-on and collaborative even if conducted in a virtual format.
Assessment
Assessment consisted of a worksheet containing free-response questions relating to the data acquired in the lab (see Supporting File S4. Gluten proteins – Student worksheet). Students were also asked to provide a brief video in which the mechanical properties of their isolated gluten samples were demonstrated by pulling or otherwise deforming the gluten. This video was used to assess the success of the students’ gluten extractions and to suggest improvements.
Inclusive Teaching
A major consideration in the design of this laboratory was accessibility for nearly all students, regardless of income level, living arrangement (on-campus versus with family), or background. Therefore, inexpensive and easily available kitchen ingredients and tools are used, and there is no need for any electronic instrument other than a smartphone or digital camera. Instructors are encouraged to consider using flours from the culinary traditions of diverse cultures; sequences of glutenin proteins (or analogues) from a variety of domesticated grains can be found in UniProt. Additionally, the connection of protein structure to cooking and food crops makes the laboratory engaging and relatable to a wide range of students, particularly those in applied disciplines related to biology, including nutrition, food science, and agricultural sciences. The emphasis on tactile, as opposed to visual/auditory sensory input, makes this laboratory particularly helpful for students with visual or hearing impairments; these students may further benefit from subtitling or narration of the instructional videos as deemed appropriate by the instructor.
Lesson Plan
Materials
See Supporting File S5. Gluten proteins – Complete list of materials for a list of all reagents and equipment. Briefly, students will need a ruler, kitchen implements (mixing spoon, bowls, cup, measuring spoons) and dough ingredients (flour, yeast, water, salt, and optionally cysteine), plus access to running water. For virtual laboratories, instructors may assemble all materials ahead of time in “kits” for each student to pick up; if this is not possible, students will need to obtain their own supplies. While the materials are mostly inexpensive (no more than $20 for a student using the most expensive flour, and considerably less for common flour), some items may have to be ordered, such as cysteine capsules. Thus, we strongly recommend that students be assigned a condition well ahead of the due date (2 weeks minimum) and be given an option to request an “inexpensive condition” (e.g., common wheat flour and no cysteine) in case online ordering is not feasible (e.g., due to lack of a credit/debit card).
The Laboratory
Pre-class preparation
An approximate timetable and list of tasks for the laboratory (both virtual and in-person formats) is provided in Table 1. The amount of pre-class setup will depend greatly on whether the activity is being performed in-person or remotely. For virtual instruction, which students perform individually, students are assigned a flour and condition (see below) at least two weeks before lab. A week before lab, links are posted to three online videos (see Supporting File S1. Gluten Proteins – List of relevant YouTube video links) providing background on gluten, a brief reading is assigned on the structure and properties of gluten, and students are asked to complete a 10-minute, 3-question online quiz in the learning management system to demonstrate preparedness. For in-person laboratories, students should be divided into partners or groups ahead of time, and a brief introductory lecture summarizing the day’s tasks should be given at the beginning of lab. While the lab contains no significant safety hazards, gluten sensitivities and grain allergies may be a concern; a list of all materials used in lab should be provided to students ahead of time and students with such allergies should be given the option to avoid contact with allergenic grains or to perform a home version of the lab, using a nonallergenic flour of the student’s choosing.
Extraction of gluten
We used four flour types: Wheat (all-purpose flour), spelt, dark rye, and rice as a gluten-free control. Typical protein and glutenin contents of these grains are summarized in Table 2. Dough was made from each flour in the presence and absence of L-cysteine at 0.1% w/w dry mass (this value was chosen for the convenience of using half a 500 mg L-cysteine capsule with 250 g of flour; this concentration of cysteine is about an order of magnitude higher than that used in commercial baking). For the virtual lab, students were assigned one of these flours, with or without cysteine. For an in-person lab, students should work with a partner or group.
Table 2. Approximate protein and glutenin contents of the grains used in this study.
| Grain | Total protein, mg/g* | Glutenin, % of total protein† | Reference |
|---|---|---|---|
| Common wheat (Triticum aestivum) | 100 | 16.6 | (19) |
| Spelt (Triticum spelta) | 133 | 19.0 | (19) |
| Rye (Secale cereale) | 133 | 9 | (20) |
| Rice (Oryza sativa) | 7.5 | N/A |
*Taken from nutrition facts label. †From reference in “Reference” column; note that values given are averages of several cultivars. N/A = not applicable (does not contain glutenins). Note that “wheat” flour is often a mixture of cultivars; common wheat is listed here for simplicity.
To make dough, 160 mL (2/3 US cup) of water at ~35-40 °C is placed in a bowl or other mixing container and 3.6 g (1 teaspoon) dry yeast and 3 g (1/2 teaspoon) of sodium chloride (salt) are added. For dough samples with cysteine, 250 mg or ½ capsule of L-cysteine should be added at this point. The mixture is stirred until mostly homogeneous, then flour is added little by little and stirred to make a paste. A total of 250 g of flour is used for each sample; this is just under 2 US cups for wheat, spelt, and rye flours and about 1 ¾ US cups of rice flour (measurements need not be precise). The dough will soon become too viscous to stir, and the remaining flour should be folded in by hand. Once all flour has been combined, the dough ball is kneaded (see link in Supporting File S1 for technique video) for a minimum of 10 minutes to form the viscoelastic gluten network (rice dough, lacking gluten, will crumble rather than stretch when kneaded). In an in-person lab, students making cysteine-treated and non-treated dough of the same grain should be asked to trade off with one another at the kneading stage, to demonstrate how cysteine affects the kneading characteristics of certain flours. After 10 minutes, the dough is shaped into a ball, its diameter measured, and covered with a porous cloth to rise (proof) for ~90 minutes, at 25-30 °C if possible (if time is limited, the proofing step can be omitted; however, it provides a dramatic demonstration of the differences between gluten and non-gluten samples and the presence and absence of cysteine). While the dough rises, students may perform the bioinformatics portion of the lab (see below).
After the dough has risen, students measure the diameter of the dough ball to quantitate the amount of rise (if the ball is no longer spherical, it should be rolled back up before measuring). The dough ball is then squeezed and stretched under a trickle of running water to remove starch and bran (for dark rye or whole-grain flours, a wire strainer is recommended to catch bran). This process will take a minimum of 15-20 minutes; to minimize fatigue, this task should be rotated between students in an in-person lab, and remote students may take a rest break by leaving the dough in a bowl of water. Gluten extraction is complete when the rinse water is clear and the gluten ball has a uniform texture with no lumps. Rice dough will probably dissolve completely leaving no gluten ball; students assigned rice flour should be gently reminded this is a possibility.
At this point, students make a brief video demonstrating the mechanical properties of the gluten. A semiquantitative measure of gluten extensibility (the “pull test”) may be made at this point (see Supporting File S3. Gluten proteins - Brief instructional video including pull test demonstration). Once all data is recorded, students upload their data and video to the learning management system (virtual lab) or share it with the rest of the class (in-person lab) and clean up. Typical results are shown and described in Supporting File S6: Gluten proteins – Description and photos of typical results.
Sequence alignment and properties of glutenin proteins
To relate the physical properties of gluten to the amino acid sequence of gluten proteins, students obtain glutenin sequences from the UniProt database, perform a multiple sequence alignment, and calculate various chemical properties using online tools (see Supporting File S2. Student instructions for complete instructions). For illustrative purposes, a single high-molecular-mass glutenin sequence is compared from each flour type (glutenins 1D from wheat and spelt, and the homologous glutenin 1R from rye; students should be reminded that gluten contains many different glutenins but that these are representative). Rice does not express glutenins, but the glutelin 1D serves as the closest homologue from rice. The four protein sequences are then aligned (students save and/or print out the alignments) and students answer questions about the similarities between sequences. The same sequences are then fed into the ProtParam tool (14) to calculate total number of residues, percentage of Gln and Cys residues, and (optionally) hydrophilicity, based on the grand average of hydropathy (GRAVY) score (15). From these properties and the sequence similarities, based on the background reading, students should be able to predict which grains will produce the most elastic/extensible glutens and which the least, and which flours will be most and least affected by cysteine.
Assessment
Assessment is based on a worksheet (Supporting File S4. Gluten proteins – Student worksheet) and the videos submitted by students. For virtual labs, the videos are a critical component of assessment as this is the only reliable way to determine if the student extracted gluten properly (students who did not should be given another chance, with feedback). Videos are optional for in-person labs since the instructor can provide real-time feedback. An important component of assessment is the sharing of data between groups/students; therefore, timeliness is essential for all students to be able to complete the worksheet, and instructors should consider penalties for late work for this reason.
Teaching Discussion
General Observations
Results of the gluten extraction from the eight conditions studied are summarized in the Supporting Materials. Briefly, gluten could be extracted from all flours except rice, and cysteine treatment made the extracted gluten more liquid-like and less extensible and elastic. In the case of rye flour, the extracted gluten was a noncohesive, non-extensible mush with little difference between cysteine and non-cysteine samples. The most common difficulty encountered by students was a tendency to stop the rinse too early, resulting in lumpy, starch-contaminated gluten balls. Thus, we recommend supervising this process in an in-person lab, and having students rotate the monotonous rinsing task. For the virtual lab, students should be reminded that they can leave the gluten ball in a bowl of water if a break is desired. Nonetheless, from the student-submitted videos it was evident that a majority of virtual-lab students did successfully isolate gluten.
In a preliminary version of this lab, students were shown their peers’ videos with a description of flour type and were asked to rank the flour types in order of increasing gluten content based on the characteristics of the gluten balls shown in the videos. The results were disappointing, which is why we developed the somewhat more quantitative “pull test” and added the measurement of dough balls before and after rising. The dramatic effect of cysteine on the dough properties was successfully related to the presence of intermolecular disulfide bonds as diagrammed in Figure 1B.
Based on the multiple sequence alignments, it was recognizable to most that wheat and spelt glutens would have the most similar physical properties, followed by rye, with rice having quite different properties. This point could probably be further emphasized by aligning a wider variety of glutenin proteins from the grains being examined, although this was not attempted. It was also evident, based on the amino acid composition calculated in ProtParam, that gluten elasticity correlates with Gln content, though not absolutely (rye, whose Gln content is intermediate between wheat and spelt, yielded a less elastic gluten ball, due to its lower total glutenin content). The most likely problem with this portion of the assignment is students not recognizing which parameter(s) from the calculation are the most important to gluten properties, as ProtParam returns some irrelevant information (e.g., absorptivity coefficients). Thus, it is important to provide adequate framing of the protein property calculations so that students know what to look for. In particular, many students will miss the correlation between a very negative hydropathicity (GRAVY) score and an extended conformation capable of intermolecular associations as happens in gluten; giving examples of GRAVY scores for some globular proteins is one way to provide hints on this topic (or alternately, these questions may simply be omitted from the worksheet).
Suggested Variations
This activity was designed for a single laboratory session. A more enriching and in-depth experience can be obtained by extending the activity across multiple lab sessions. For example, the bioinformatics portion of the lab can be made into an activity unto itself, in which students (working alone or in teams) find, align, and analyze sequences of multiple glutenin and prolamin proteins from the grains being studied, and from the results formulate their own hypotheses about which grains will yield the most gluten and which will be most sensitive to cysteine. These hypotheses can then be tested in the hands-on part of the lab in which gluten is actually isolated. To link the activity more closely to nutritional and medical sciences, students may also be given the sequences of various epitopes responsible for celiac disease (11), asked to find these sequences in gluten proteins, and suggest which grains would be most and least immunogenic. Since this goal was tangential to the primary learning objective of the lab (relating amino acid sequence to protein physical properties), this was not attempted. Additionally, the extracted gluten from the various flours can be subject to SDS-PAGE analysis to demonstrate the relative amounts of high- and low-molecular-mass glutenin proteins in each sample, as demonstrated elsewhere (16). However, this activity would generally be limited to an in-person lab.
Even for a single lab session, there are other variations and variables that can be explored. Other grains (or even non-grain baking flours, such as tapioca) can also be examined, based on student interest. Since other flour types may not have gluten-like proteins, the bioinformatics portion of the lab may not be applicable in these cases, but allowing a wider variety of flour types to be examined may increase student interest in the subject matter, a particularly important consideration for virtual or distance learning. Factors such as salt content, yeast type and amount, and water temperature can also be examined as variables in determining gluten properties. Salt content, in particular, is known to have dramatic effects on the viscoelastic properties of gluten (17, 18), and in preliminary experiments it was found that omission of salt noticeably affects the texture of dough (data not shown). Since salt affects the ionic (ion-ion and ion-dipole) interactions in biomolecules, students may be able to correlate the effects of salt to other features of the glutenin sequences. This was not attempted in our laboratory.
SUPPORTING MATERIALS
- S1. Gluten proteins – List of relevant YouTube video links
- S2. Gluten proteins – Student instructions (both virtual and in-person variants included)
- S3. Gluten proteins – Brief instructional video including pull test demonstration
- S4. Gluten proteins – Student worksheet
- S5. Gluten proteins – Complete list of materials
- S6. Gluten proteins – Descriptions and photos of typical results
Acknowledgments
We appreciate the ongoing support of our technician, Andrea Laubscher, and the enthusiasm and patience of our students as we improvised during our unplanned excursion into virtual learning.
References
- de Jong T, Linn MC, Zacharia ZC. 2013. Physical and virtual laboratories in science and engineering education. Science 340:305–308. doi: 10.1126/science.1230579.
- Dyrberg NR, Treusch AH, Wiegand C. 2017. Virtual laboratories in science education: students’ motivation and experiences in two tertiary biology courses. J Biol Educ 51:358–374. doi: 10.1080/00219266.2016.1257498.
- Serrano-Perez JJ, González-García L, Flacco N, Taberner-Cortés A, García-Arnandis I, Pérez-López G, Pellín-Carcelén A, Romá-Mateo C. 2021. Traditional vs. virtual laboratories in health sciences education. J Biol Educ (epub ahead of print). doi: 10.1080/00219266.2021.1877776.
- Hu X, Cheng L, Hong Y, Li Z, Li C, Gu Z. 2021. An extensive review: How starch and gluten impact dough machinability and resultant bread qualities. Crit Rev Food Sci Nutr (epub ahead of print). doi: 10.1080/10408398.2021.1969535.
- Balakireva A V, Zamyatnin AA. 2016. Properties of gluten intolerance: Gluten structure, evolution, pathogenicity and detoxification capabilities. Nutrients 8:644.
- Shewry PR, Halford NG, Belton PS, Tatham AS. 2002. The structure and properties of gluten: An elastic protein from wheat grain. Philos Trans R Soc B Biol Sci 357:133–142. doi: 10.1098/rstb.2001.1024.
- Mioduszewski Ł, Cieplak M. 2021. Viscoelastic properties of wheat gluten in a molecular dynamics study. PLoS Comput Biol 17:1–19. doi: 10.1371/journal.pcbi.1008840.
- Singh H, MacRitchie F. 2001. Application of polymer science to properties of gluten. J Cereal Sci 33:231–243. doi: 10.1006/jcrs.2000.0360.
- Lancelot E, Fontaine J, Grua-Priol J, Assaf A, Thouand G, Le-Bail A. 2021. Study of structural changes of gluten proteins during bread dough mixing by Raman spectroscopy. Food Chem 358: 129916. doi: 10.1016/j.foodchem.2021.129916.
- Sahi SS. 2012. Applications of natural ingredients in baked goods, 318-332. In Baines D, Seal R (ed), Natural Food Additives, Ingredients and Flavourings, 233. Woodhead Pub., Cambridge, UK. doi: 10.1016/B978-1-84569-811-9.50014-1.
- Bromilow S, Gethings LA, Buckley M, Bromley M, Shewry PR, Langridge JI, Clare Mills EN. 2017. A curated gluten protein sequence database to support development of proteomics methods for determination of gluten in gluten-free foods. J Proteomics 163:67–75. doi: 10.1016/j.jprot.2017.03.026.
- Miles DT, Bachman JK. 2009. Science of food and cooking: A non-science majors course. J Chem Educ 86:311-315. doi: 10.1021/ed086p311.
- Cheng SC, Ziffle VE, King RC. 2020. Innovative food laboratory for a chemistry of food and cooking course. J Chem Educ 97:659–667. doi: 10.1021/acs.jchemed.9b00465.
- Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. 2005. Protein identification and analysis tools on the ExPASy server, 571-607. In Walker JM (ed), The Proteomics Protocols Handbook. Humana Press, Totowa, NJ.
- Kyte J, Doolittle RF. 1982. A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132. doi: 10.1016/0022-2836(82)90515-0.
- Pirinelli AL, Trinidad JC, Pohl NLB. 2016. Introducing students to protein analysis techniques: Separation and comparative analysis of gluten proteins in various wheat strains. J Chem Educ 93:330–334. doi: 10.1021/acs.jchemed.5b00311.
- Chen G, Ehmke L, Sharma C, Miller R, Faa P, Smith G, Li Y. 2019. Physicochemical properties and gluten structures of hard wheat flour doughs as affected by salt. Food Chem 275:569–576. doi: 10.1016/j.foodchem.2018.07.157.
- Chen G, Ehmke L, Miller R, Faa P, Smith G, Li Y. 2018. Effect of sodium chloride and sodium bicarbonate on the physicochemical properties of soft wheat flour doughs and gluten polymerization. J Agric Food Chem 66:6840–6850. doi: 10.1021/acs.jafc.8b01197.
- Geisslitz S, Longin CFH, Scherf KA, Koehler P. 2019. Comparative study on gluten protein composition of ancient (einkorn, emmer and spelt) and modern wheat species (durum and common wheat). Foods 8:409. doi: 10.3390/foods8090409.
- Gellrich C, Schieberle P, Wieser H. 2003. Biochemical characterization and quantification of the storage protein (secalin) types in rye flour. Cereal Chem. 80:102-109. doi: 10.1094/CCHEM.2003.80.1.102.
Article Files
Login to access supporting documents
Jones-Sequences and properties of gluten proteins from different grains.pdf(PDF | 333 KB)
S1. Gluten proteins -List of relevant YouTube video links.docx(DOCX | 15 KB)
S2. Gluten proteins -Student instructions.docx(DOCX | 344 KB)
S3. Gluten proteins -Brief instructional video.mp4(MP4 | 68 MB)
S4. Gluten proteins -Student worksheet.docx(DOCX | 55 KB)
S5. Gluten proteins -Complete list of materials.docx(DOCX | 19 KB)
S6. Gluten proteins -Descriptions and photos of typical results.docx(DOCX | 15 MB)
- License terms

Comments
Comments
There are no comments on this resource.