Using Yeast to Make Scientists: A Six-Week Student-Driven Research Project for the Cell Biology Laboratory
Published online:
Abstract
Traditionally-trained undergraduate students often lack an understanding of science as an active process that yields the information presented in their textbooks. One result has been a call for more research experiences built into traditional introductory undergraduate courses, now commonly referred to as course-based undergraduate research experiences (CUREs). The laboratory module presented in this paper used an established four-step pedagogical framework to simplify and streamline the development and implementation process of a CURE in an introductory biology laboratory setting. A unique six-week CURE was designed for undergraduates enrolled in a cell biology lab that employs Saccharomyces cerevisiae as a eukaryotic model organism. Students address a research problem that is of interest to the scientific community: Do select chemicals in the environment have adverse effects on the mitotic cell division? Students are first introduced to S. cerevisiae, its life cycle, morphology, growth curve generation and analysis, and the laboratory techniques required to cultivate this organism. Working in groups, students then act as scientists to research primary literature, ask an original question, develop a testable hypothesis, collaborate with peers, design and conduct an experiment, analyze and interpret data, and present their work to their peers. In addition, students are involved in multiple levels of iterative work, including addressing problems or inconsistencies, ruling out alternative explanations, and/or gathering additional data to support assertions.
Citation
Goudsouzian, L.K., McLaughlin, J.S., and Slee, J.B. 2017. Using Yeast to Make Scientists: A six-week student-driven research project for the Cell Biology laboratory. CourseSource. https://doi.org/10.24918/cs.2017.4Society Learning Goals
Cell Biology
- Methods & Tools of Cell Biology
- How do the methods and tools of cell biology enable and limit our understanding of the cell?
Science Process Skills
- Process of Science
- Locate, interpret, and evaluate scientific information and primary literature
- Pose testable questions and hypotheses to address gaps in knowledge
- Plan, evaluate, and implement scientific investigations
- Interpret, evaluate, and draw conclusions from data
- Construct explanations and make evidence-based arguments about the natural world
- Address novel questions through authentic research experiences
- Modeling/ Developing and Using Models
- Build and evaluate models of biological systems
- Quantitative Reasoning/ Using Mathematics and Computational Thinking
- Apply the tools of graphing, statistics, and data science to analyze biological data
- Communication and Collaboration
- Share ideas, data, and findings with others clearly and accurately
Lesson Learning Goals
- Appreciate the utility of a model organism
- Acquire skills in experimental techniques
- Develop a scientific proposal
- Execute an experiment
- Interpret data
- Communicate experimental results
Lesson Learning Objectives
- Learn about basic S. cerevisiae biology
- Use sterile technique
- Perform a yeast viability assay
- Use a spectrophotometer to measure growth of S. cerevisiae
- Perform a literature search
- Calculate concentrations of chemicals appropriate for S. cerevisiae
- Generate S. cerevisiae growth curves
- Troubleshoot experimental difficulties
- Perform statistical analysis
- Present findings to an audience
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
Mitotic cell division is a central topic in many undergraduate biology courses. It is critical for students to understand that cell proliferation is responsible for the growth of all eukaryotic organisms, and that the regulation of the cell cycle is necessary for every cell and organism to function normally. Perturbations of cell proliferation are best known for causing cancer, but are also implicated in other pathologies, including atherosclerosis, viral and bacterial infections, and neurodegenerative disorders (1). We have designed an inquiry-based, six-week laboratory module based on mitotic reproduction of the model organism, Saccharomyces cerevisiae. S. cerevisiae is a eukaryotic, unicellular model organism that lends itself well to an undergraduate teaching laboratory because it reproduces rapidly, is resistant to mechanical insults, and is inexpensive to maintain. In this laboratory module, students select non-toxic chemicals that may have potential adverse effects on the cell cycle and examine the impact of these chemicals on yeast mitotic division.
We devised this laboratory module in keeping with the guidelines set forth in the AAAS Vision and Change Report (2), which called upon educators to introduce more student-centered and inquiry-based undergraduate research experiences in life science courses. An additional goal was to develop our laboratory experience as a unique Course-based Undergraduate Research Experience (CURE) for students studying cell biology. Research has shown that CUREs increase and broaden participation of students in scientific research and train them in the essential elements of the scientific process (3-7) A CURE also effectively involves students from the entire course in addressing a research question or problem that is of interest to the scientific community as a whole (8).
CURE-net (http://curenet.cns.utexas.edu/), a National Science Foundation-supported Research Coordination Effort, issued a report from a working group with expertise in CURE instruction and assessment. This report lists five essential elements of a CURE: (a) scientific practices are utilized; (b) the outcome of the investigation must be unknown to both the students and the instructor; (c) the work is broadly relevant or important to society; (d) group work is an important pedagogical element; and (e) iteration must be exercised at potentially multiple levels (3). Although proven effective, CUREs remain rare in our nation's undergraduate science labs, due to lack of time for faculty to develop new research experiences (7). One way to decrease time needed to develop an effective CURE is to follow a simple and flexible framework that guides the instructors through the process of designing and implementing their own unique research experiences. We utilized an established four-step pedagogical framework that includes five essential CURE elements and has been developed to simplify and streamline the development and implementation process of a CURE in an introductory biology laboratory setting (9-11).
The four steps of the pedagogical framework scaffold the scientific process, allowing students to progressively gain familiarity and comfort with the essential elements of scientific inquiry (Figure 1).
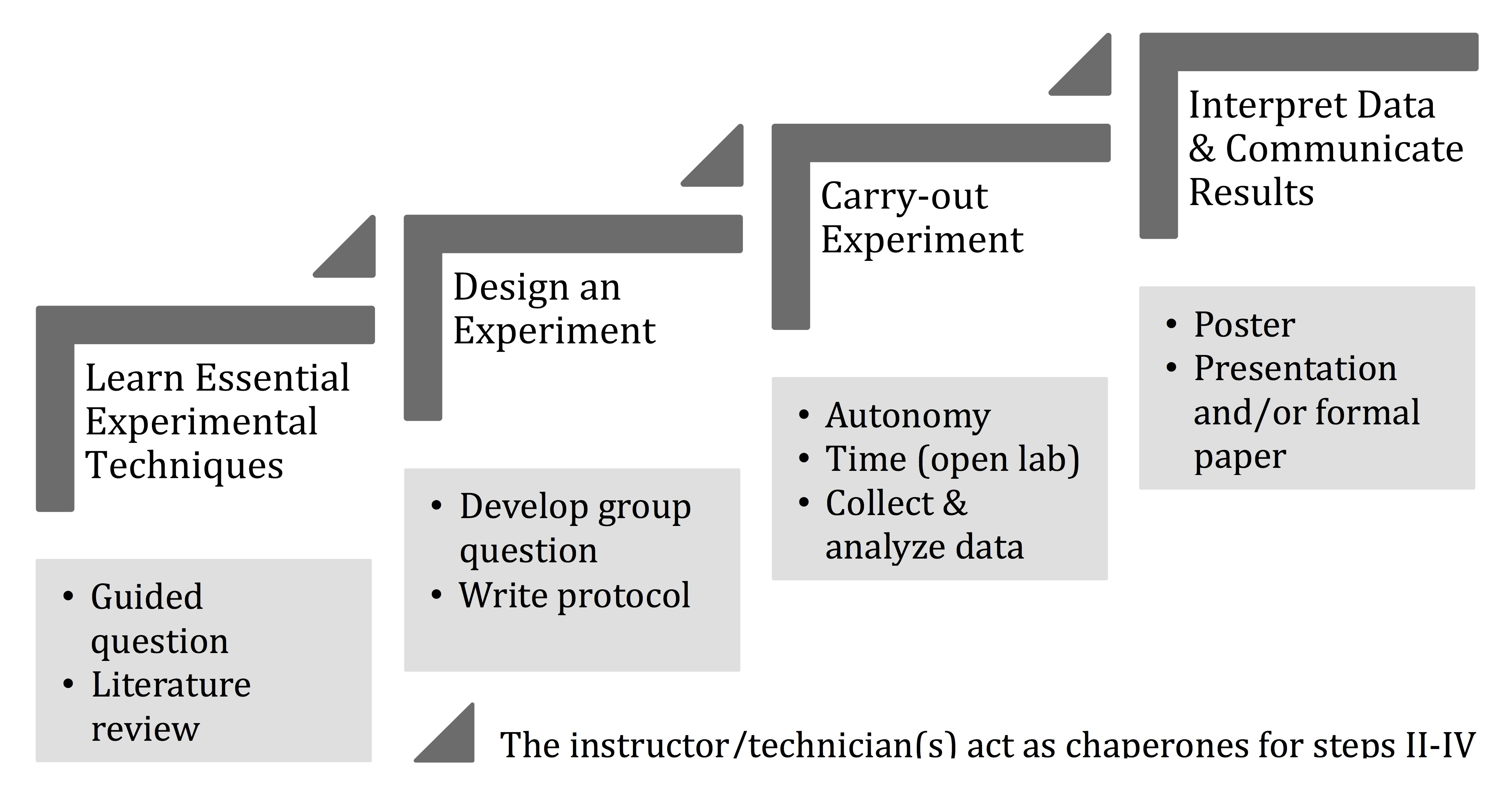
Figure 1. Pedagogical framework used to implement laboratory transformation(9)
During Step One, students learn essential and relevant experimental techniques via pre-lab lectures and hands-on training. This step introduces students to critical elements of the scientific process and allows them to learn how to use scientific equipment and methods necessary for the study system. Students are also introduced to the overall research goal and to its larger societal significance. During Step Two, the instructor asks a large-scale guided question related to the study system. Students then work in small groups to select and read primary literature articles related to the guided question, so that they can devise a specific, self-directed research question, formulate a hypothesis, and design an accompanying experiment to test the hypothesis. In Step Three, students conduct their autonomous experiment while using the techniques they learned in Step One and collect data. Finally, in Step Four, students interpret their data and present the results of their study in a professional scientific manner. Throughout this framework, instructors act as research chaperones, guiding student scientists through each step, providing constant feedback in an environment that allows student self-reflection, collaboration, mistakes, trouble-shooting, iterations, and dialogue over assignments before their final submission for grading (i.e., protocol, notebook, scientific presentation, etc.).
Our laboratory module tasked students with formulating a unique research question, developing and executing a research investigation pertaining to the guided question: How does a specific household chemical affect the growth rate of S. cerevisiae? Students analyzed primary literature about their chemical of interest, and related that analysis to yeast cell biology. In general, determining the response of a eukaryotic model organism to chemical exposure can shed light on how human cells might respond to the same chemical. Looking specifically at mitotic cell division alterations and cell death because of chemical exposure could also help determine if these commonly used chemicals are toxic or carcinogenic at elevated concentrations. For example, a student group investigated the effect of energy drinks on yeast growth. Generally, energy drinks are not regulated by the Food and Drug Administration, and therefore have no implicit guarantees associated with the safety or efficacy of their use. Given that millions consume these drinks daily, a student project that identified a potential hazardous chemical would be of interest to the scientific community and the general public.
INTENDED AUDIENCE
The laboratory module is used in a large enrollment (80 students per semester), mid-level cell biology lab course at a small, selective liberal arts university. The students meet for a common lecture given by a full-time instructor and then are split into four lab sections. Each section is capped at 24 students. A full-time instructor runs all lab sections, but the lab instructor is not always the same instructor for the related lecture course. This laboratory module could readily be adapted for a microbiology course or introductory biology course.
REQUIRED LEARNING TIME
The laboratory module spans six weeks and is implemented in 90-minute lab periods that meet twice a week. Each growth curve requires six hours. Students start growth curves during their assigned laboratory period, then use open lab time to obtain data for all required time points. Students work in groups of three to four to minimize time constraints on individual students and to promote the perception of science as a team effort. Note that this module can also be applied to labs that meet once a week for three hours. In fact, given the extended lab duration this 3-hour format would significantly reduce the amount of outside of the lab the students would need to devote to collecting data.
PRE-REQUISITE STUDENT KNOWLEDGE
This laboratory module is designed for students with minimal background in the subject matter. The module includes all necessary resources to educate/train the students appropriately. The specific knowledge of S. cerevisiae biology needed to complete the module is provided during pre-lab lectures to ensure all students understand the material. Basic lab skills, such as dilution calculations, pipetting, and aseptic technique are required to successfully complete the lab module. Students are required to use word processing, data analysis/graphing software, and presentation software to analyze their data and assemble a final presentation.
PRE-REQUISITE TEACHER KNOWLEDGE
Instructors should have sufficient mastery of molecular cell biology to explain basic features of eukaryotic cell structure, cell signaling, cell division, and aseptic technique. An elementary understanding of S. cerevisiae growth and reproduction is required. If the instructor is not familiar with this model organism, numerous guides are available that explain basic yeast biology and culture methods (12-14). We have provided information about yeast strains and media recipes in Supporting File S1: Yeast Resources.
SCIENTIFIC TEACHING THEMES
ACTIVE LEARNING
This laboratory module incorporates students' autonomy as they pursue their own group projects and therefore encourages active learning. Students must work collaboratively to perform all the following tasks: select their experimental variables, conduct a scientific literature review to determine which variable they choose to investigate, formulate a research question and testable hypothesis, collect time points outside of designated lab periods, analyze and interpret data, and present their findings to the class.
Importantly, students are required to work in self-selected groups of three or four students for this laboratory module. The team aspect of the CURE reflects the landscape of scientific research, which is rarely done in isolation. Group-work also forces students to rely on each other, as a researcher typically relies on colleagues and collaborators to move a project forward. As an added benefit, group work encourages deeper learning and allows students to experience science as a process completed by groups of people (3,15).
ASSESSMENT
Assessment of this laboratory module includes four distinct elements:
- The Team Research Proposal (35% of grade): The first major assessment of the laboratory module is the written proposal. Students must submit a two-page proposal introducing the purpose of their experiment, their hypothesis, a materials list, a brief protocol, a discussion of the variables, and how they plan to interpret them. Correctly cited references are required as per American Psychological Association format. Other reference styles can be substituted based on preference. We prefer to expose the students to multiple reference styles throughout their coursework. The proposal is written and submitted by the research group, and evaluated using the proposal rubric (Supporting File S2: Proposal Rubric). Since it is a collective assignment, all students in a group receive the same grade for this assignment. The proposal assesses Learning Objectives 5 and 6.
- Individual Lab Notebooks (15% of grade): Each student's lab notebook is graded for format, completion, accuracy of information presented, and the student's interpretation of their results. Although we did not utilize a rubric, a rubric that mirrors our expectations and we would consider using in the future can be found here: www.sunyjcc.edu/hurisuri/sites/default/files/Lab_Notebook_Rubric_revised_1.doc. Student lab notebooks are graded individually. The notebook assesses Learning Objectives 2, 3, 4, 6, 7, 8 and 9.
- Individual Lab Quiz (15% of grade): The quiz includes factual recall and application questions (Supporting File S3: Lab Quiz). Quizzes are taken individually. The quiz assesses Learning Objectives 1 and 5.
- The Team Oral Presentation (35% of grade): The last assessment of the laboratory module is a ten minute group presentation to the class. We assess both the quality of the overall presentation (60% of assessment grade) and the quality of individual student speaking performance (20% of assessment grade) (Supporting File S4: Group Project Evaluation Rubric). As part of this assessment, the group members determine an additional 20% of each student's grade for this assessment. This peer evaluation allows the instructor to identify students who are not performing to the level expected in the course and to that of their peers (Supporting File S5: Peer Group Assessment Rubric). The presentation assesses Learning Objective 1 and 10.
INCLUSIVE TEACHING
Students can self-select their partners for the group projects, which typically consist of three or four members. The group is then free to choose the topic they wish to investigate, allowing them to express their individuality and diversity. Alternatively, an instructor can assign students to lab groups, requiring diverse groups of students to interact inside and outside of the laboratory. However, based on personal observation, we prefer to allow students to self-select their groups because students pick other students with whom they feel comfortable working. In addition, self-selected groups tend to mimic real-world working groups more than randomly assigned groups because both self-selected groups and real-world working groups are usually composed of one or two people who know each other plus others who may not (16). Indeed, self-selection appears to add more value to the students' experience when considering communication, enthusiasm, pride, and attitude towards their work (16). The groups are free to divide the work among members based on students' preferences and/or strengths. In addition, this laboratory module includes four different styles of assessment in an attempt to address the diversity of student backgrounds and preferences.
LESSON PLAN
Initial Preparation
This laboratory requires large amounts of yeast liquid media (you should assume ~100 mL per student group per week). This requirement is driven by the need for students to perform their experiments at least twice, as well as account for errors or contamination. We find that many of our student laboratory groups performed their experiments three or more times. To ensure that we have an ample supply of sterile media before the laboratory series begins, we save time and resources by providing the students with 10 mL aliquots of sterile YPD in glass culture tubes with plastic caps. Supplying pre-filled tubes also saves the expense of using sterile serological pipets and diminishes the risk of contamination. Sterile YPD is stable at room temperature for three years. Thus, there is little concern that the quality of the growth media will diminish if prepared several weeks in advance.
Group work is required for this laboratory module. We have students form groups of three to four students during the first meeting of the laboratory. We run this laboratory module during the second half of the semester, so these students have been working with one another for about six weeks. During this time, students are free to switch groups if conflicts arise. If this module is run during the first week of the semester, the instructor may need to plan an ice breaker to allow students to get to know one another prior to choosing their groups. We find this group size is large enough to allow for the scheduling plasticity required to complete this laboratory module. We have students plan out the responsibility for all time points before beginning the experiment. Some groups have all members participate in each time point. In other groups, students with family or other evening commitments take responsibility for the earlier time points while other students perform the later time points. To promote the active involvement of all group members, students assess their own and their peers' participation and quality of experimental work at the end of the laboratory module, using a rubric that was adapted from M. Lombardi (17).
LAB WEEK 1: Introduction to Yeast and Viability Assay (Learning Objectives 1, 2, and 3)
During this lab period, students in each 24-student lab section watch a lecture given by the instructor about yeast biology (Supporting File S6: Introduction to Yeast Biology). They are instructed in aseptic technique appropriate to microbial work and inoculation of yeast into liquid media. Students practice inoculating a single yeast colony into sterile liquid media. Students also use a methylene blue assay to determine percent viability of a prepared yeast culture (Supporting File S7: Yeast Viability Assay Protocol).
Instructor Preparation
At least two days prior to lab, we streak out the yeast strain on solid agar YPD plates, so that individual yeast colonies will be available for students to use in their practice inoculations. Each lab group receives one plate. We also prepare one yeast liquid culture per lab group, by transferring a single colony of the yeast strain to 10 mL of YPD medium in 25 mL glass tubes the night before the laboratory session. These cultures are grown overnight in a 30°C shaking incubator at 180 rpm. We set up microscopes around the lab and provide dropper bottles of methylene blue viability stain (Supporting File S1: Yeast Resources).
Pre-laboratory activity
We start by providing background on S. cerevisiae, including information about the yeast cell cycle, the evolutionary relationships of yeast to plants and animals, and techniques for culturing yeast in the laboratory. We have discovered that students are often familiar with the use of bacteria in the research laboratory, but not yeast. Students often confound bacteria with yeast because of the ability of both organisms to be cultured in liquid media and on agar plates. We emphasize yeast's importance as a eukaryotic model organism and explain to students the value of using model organisms to further research into human biology.
We then demonstrate sterile inoculation technique to the students. Again, many of them have worked with bacterial cultures in past courses. We take care to explain to them that yeast growth media (YPD) does not contain antibiotics. Many bacteria and fungi that enter the tube will be able to grow. As a result, it is critical to maintain high quality, consistent aseptic techniques throughout the experiment. We do not provide students with Bunsen burners during this laboratory as we do not wish the students to use them later on in the experiment when they work unsupervised in the laboratory. We use individually packaged sterile plastic loops for inoculation. A less costly alternative is autoclaved wooden applicator sticks.
Laboratory Activity
Students are provided with sterile loops, 25 mL borosilicate culture tubes with polypropylene caps filled with 10 mL liquid YPD media, and yeast colonies on plates. The students practice inoculating yeast colonies into the tubes of liquid YPD media. They place these cultures in a 30°C shaking incubator at 180 rpm.
Students are provided with pre-grown yeast cultures. They perform a methylene blue yeast viability assay by combining 10 uL of this culture with a small drop of 0.1% methylene blue stain on a microscope slide. In the presence of methylene blue, dead cells appear dark blue. Using a microscope at 100X total magnification, students count 100 cells, identifying each cell as alive (unstained by methylene blue) or dead (stained with methylene blue.) They use those numbers to calculate a percent viability value.
Post-laboratory Instructor Activity
The following day, we remove the student-inoculated liquid cultures from the shaking incubator and place them at 4°C.
LAB WEEK 2: Practicing Growth Curves and Viability Counts (Learning Objectives 2, 3, 4, and 7)
During this lab period, students familiarize themselves with the techniques they will be using during their student-driven experiments. They plot a growth curve of yeast grown in standard YPD media.
Instructor Preparation
We provide culture tubes with 10 mL of sterile YPD medium. We set up 1 microscope and 1 spectrophotometer per group around the room. If this equipment is limited, two groups could share a microscope and a spectrophotometer. We give the students their own culture tubes from Lab Day 1. (Alternatively, we inoculate fresh yeast cultures the night before for student use.) We provide a detailed protocol with data tables for this activity in Supporting File S8: Saccharomyces cerevisiae Growth Preliminary Laboratory Protocol. We have determined that students easily lose track of their data if entering it into blank lab notebook pages. The data table helps keep them organized and on task.
Pre-laboratory Activity
We explain the use of a spectrophotometer to students. We require that each lab group determine the person who is responsible for each time point in advance. Most groups perform the first time point together and then take turns doing the rest. The yeast in this experiment are grown for six hours. The students are required to work independently of the instructor for the second half of the experiment. Good planning and organization is essential.
Laboratory Activity
Students use a spectrophotometer to determine the optical density of their starter yeast cultures at 600 nm of light. They are given a starter culture of yeast grown overnight to stationary phase. They use this culture to make four secondary cultures of yeast at A600 ~0.05-0.1 and place them in the shaking incubator.
Students return every hour to measure cell density and viability over the next six hours. Each time point requires two measurements. The students first measure cell density using the spectrophotometer (optical density at 600 nm). The students next determine percent viability by using the methylene blue viability assay protocol.
Yeast cultures in glass tubes should be collected and disposed of according to your institution's regulations. Yeast is not biohazardous and can be disposed of by washing down the sink or discarding in regular lab waste.
Students generate two graphs of their data using a spreadsheet program such as Microsoft Excel. The first graph shows percent viability at each time point. The second graph is a growth curve that shows cell density at each time point.
LAB WEEK 3: Literature Search and Experimental Design (Learning Objectives 5, 6)
Instructor Preparation
Because this lab requires computers, we instruct the students to bring their laptops to class. Alternatively, we have reserved campus computer laboratories when available.
Pre-laboratory Activity
We give the students a handout about searching biological literature (Supporting File S9: Searching Biological Literature). We give a brief lecture to each lab section on how to conduct a scientific literature search (Supporting File S10: Locating and Using Biological Literature). We demonstrate a typical literature search using Google Scholar, PubMed, and the university library databases. We instruct the students to confer with their lab partners and their literature reviews when choosing a chemical that they reason will affect the growth rate of their yeast and be of relevance to society. We require students to select common, non-toxic chemicals that are not known to be toxic or carcinogenic.
Laboratory Activity
Students search the biological literature for information about possible water-soluble chemicals to study. Typical household chemicals selected by students include caffeine, artificial sweeteners, and athletic supplements.
Students use the biological literature to calculate the amount of chemical that should be included in their growth medium. We instruct the students make their calculations based on the weight of the yeast culture (10 g for 10 mL). Students use the literature to determine the typical dosage/amount of the chemical that is consumed by humans, assuming the average human weighs 70 kg (18). They then calculate the amount of chemical to add to their growth medium. The students often determine that they must make a dilution series to scale down the amount of chemical accurately. As an example, a four cup-a day coffee habit would yield a caffeine dose of 350 mg/day for an average-sized person. To determine the same concentration of caffeine to be applied to a yeast culture, the following equation must be solved for X:
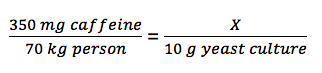
Equation
The equation yields a value of 0.05 mg of caffeine to be added to the yeast culture. This amount is too small to measure on a laboratory digital scale. Thus, the students would need to make a stock solution (typically at 100or 200X final concentration) and add an appropriate amount to their 10ml cultures. The students calculate how to prepare four cultures with a chemical concentration ranging from zero (no added chemical, control) to the "high concentration." Students have previously determined these concentrations through their reviews of the literature and with input from the instructor. Typically, though not necessarily, the high concentration reflects a maximum human dose, a value derived from the literature search. The medium concentration reflects a normal or average human dose. The low concentration reflects a less than average human dose.
Thus, the four cultures will be:
- Control culture (no chemical)
- Low concentration of chemical, 0.1 to 0.5X average human dose
- Medium concentration of chemical, average human dose
- High concentration of chemical, maximum human dose
Each lab group writes their research proposal. The proposal explains the purpose of their experiment, the materials they require, identify the independent and dependent variables, propose how they will interpret the data, and include references (Supporting File S11: Sample Student Proposal).
Post-laboratory Instructor Activity
We use these proposals to order materials for the students, since they are not required to purchase their own chemicals. At this point, we reject chemicals that we consider to be potentially unsafe, unworkable, or illegal. If the chemical is unfamiliar to us, we use the references provided in the proposal to investigate the safety and practicality of its use in this module.
LAB WEEKS 4 & 5: Student-Driven Experiments (Learning Objectives 2, 3, 4, 7, 8, 9)
During these two weeks (four lab periods), students work independently on their projects during their scheduled lab periods and open lab time as needed using the methods they practiced during Lab Day 2 (growth curves and viability counts).
Instructor Preparation
The instructor's role is to answer questions and support the students as they perform their experiments. We maintain an open lab policy during the two weeks of the self-guided experiments. The laboratory is unlocked and well-stocked during normal daytime hours, not including weekends. The instructor is available in the laboratory or is always nearby during these days. Students are free to ask questions or request technical help. We enlist a second faculty member and a laboratory technician as "backups" during these days, in case the instructor has an appointment or becomes busy. Students are free to come and go as they please in the lab, working at their own pace. During all independent laboratory activities, we require students to work in pairs, either with members of their own group or other groups. Students may not perform any part of the experiments alone and are instructed to leave the laboratory if they are unaccompanied. Because the time points are hourly and we have six microscopes and spectrophotometers in our teaching lab, we have not yet had an instance where the space became too crowded for students to work.
Laboratory Activity
Students inoculate their own starter cultures in preparation for these labs. Consequently, they must come in approximately 24 hours before their laboratory period to inoculate a yeast colony into a culture tube containing 10 mL of sterile YPD and place it on the 30°C shaker overnight.
At the beginning of their lab period, students use a spectrophotometer to determine the optical density of their starter (overnight) yeast cultures at 600 nm of light. They use the starter culture to inoculate four new cultures to a final A600 between 0.05 and 0.1. They add the chemical to the experimental cultures, at the amounts calculated in the previous lab.
Over the six hours following inoculation, students take one-hour time points to monitor the growth and viability of the four yeast cultures with differing chemical concentration as determined in Lab Week 3.
Students generate a graph of their cell viability and growth curves for each culture after completing their data collection
Students apply statistical analysis to their data. Our students use the free online statistics program GraphPad Quickcalcs (www.graphpad.com/quickcalcs).
Post-laboratory Instructor Activity
Two lab periods are usually sufficient for students to perform the entire experiment twice. Not surprisingly, students occasionally run into trouble with their experiments. The chemical sometimes causes excessive yeast lethality at even a low concentration. Contamination or student error can ruin the experiment. Students can return to redo their experiments. Mistakes and failures are common in scientific research. Therefore, trouble-shooting is emphasized at these times to promote critical thinking and learning.
To accommodate the increased laboratory demand during Lab Week 4 and Lab Week 5, two instructors and a laboratory technician monitor the lab space multiple times a day to maintain it in good order and replenish supplies of sterile loops, cuvettes, media, etc.
LAB WEEK 6: Formal Presentation of Results (Learning Objective 10)
Instructor Preparation
The students are required to communicate their experimental findings to their lab section, using an oral presentation with slides. We guide students on this step via grading rubrics and a set of detailed group presentation guidelines (Supporting File S12: Group Presentation Guidelines). The students are also required to submit an electronic copy of their presentation slides via email to the instructor before the presentation. The group presentation is assessed based on the use of graphics and visual aids, content, the appropriate use of references, and the length of the presentation. In addition, each individual receives a grade for presentation effectiveness and their contribution to the group work, which is assessed by both the instructor and other members of the group.
Laboratory Activity
The students present their research findings in a professional manner. Each group is allotted ten minutes to present and five minutes for questions from the class.
A teaching timeline can be found in Table 1.
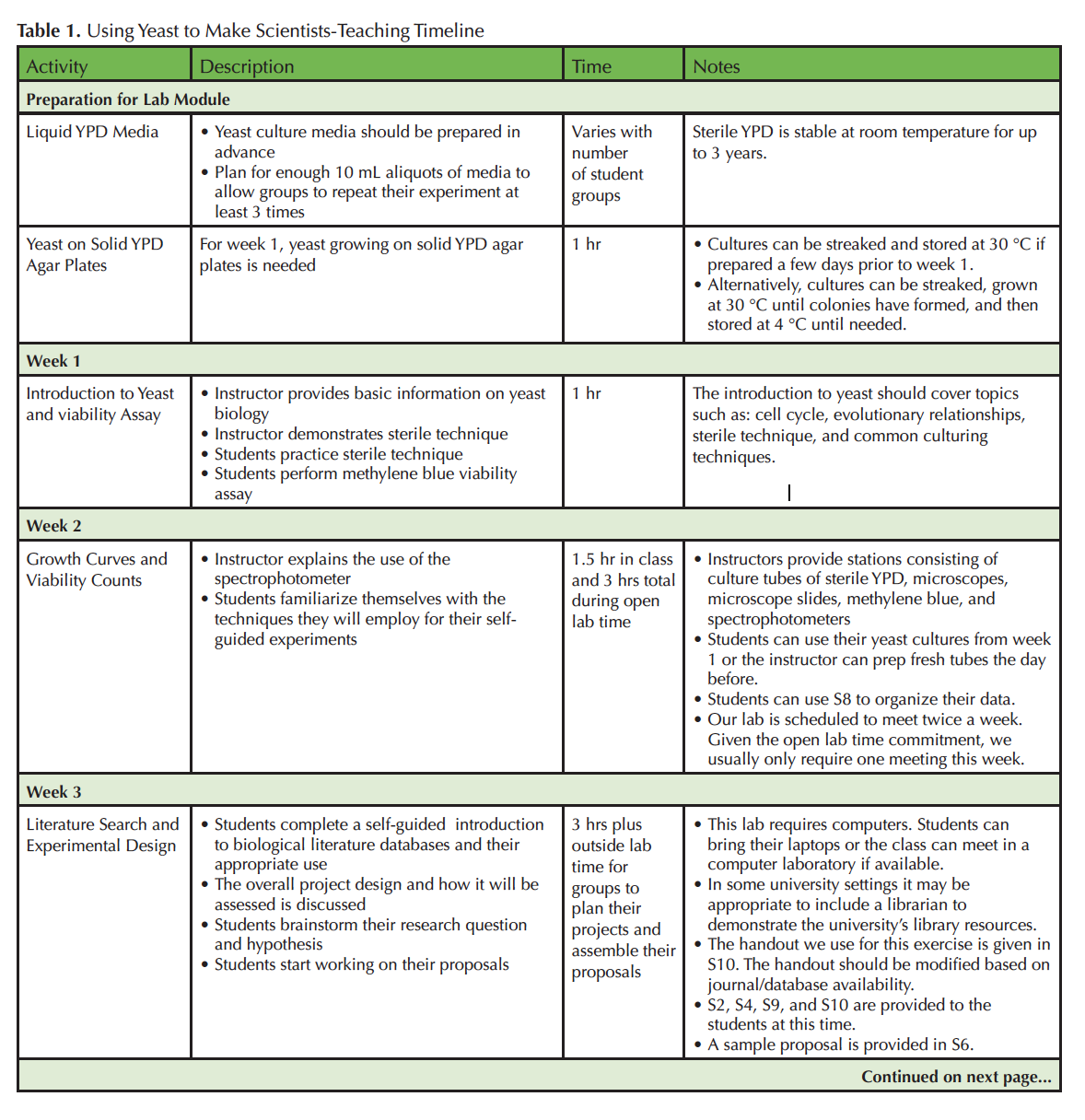
Table 1.1. Using Yeast to Make Scientists-Teaching Timeline Part 1
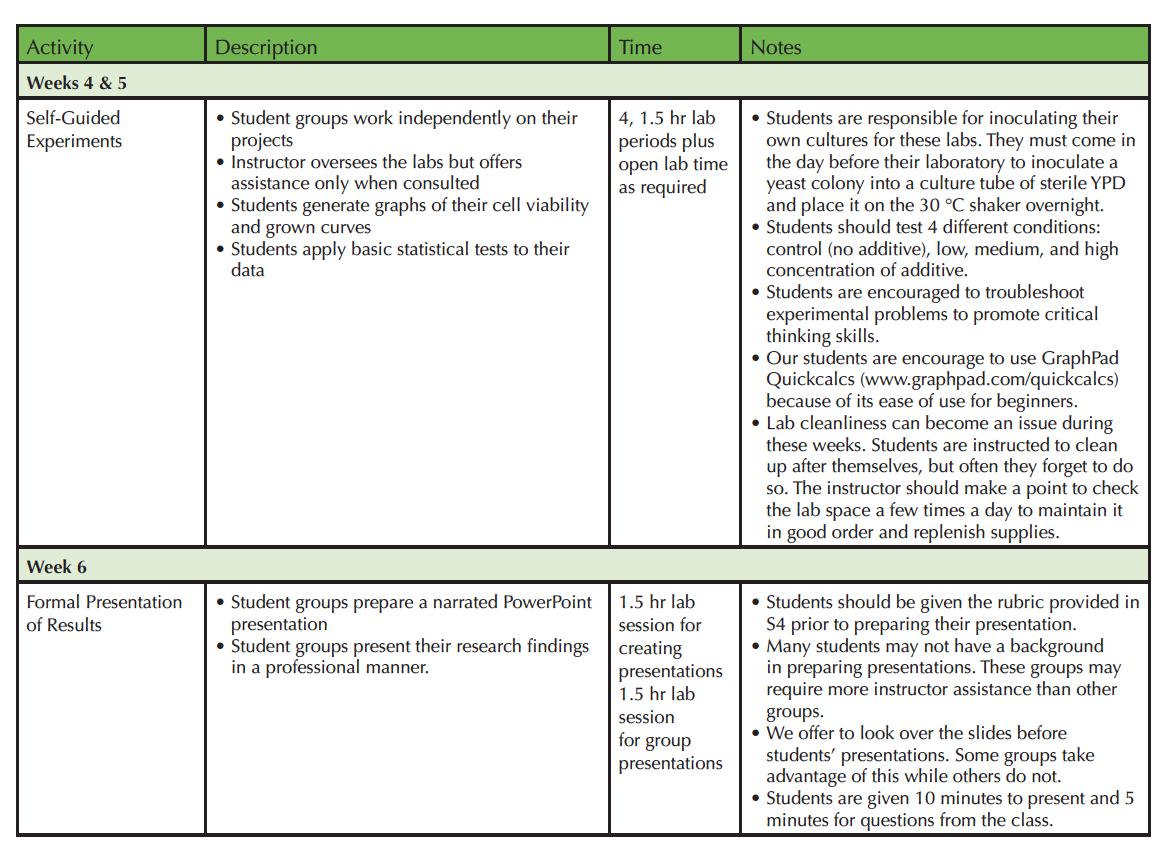
Table 1.2. Using Yeast to Make Scientists-Teaching Timeline Part 2
TEACHING DISCUSSION
This authentic research laboratory allows students to use inquiry-based experiments to test the effect of a chemical on eukaryotic cell proliferation. Students are responsible for selecting their own variables and devising their own hypotheses as to whether their chemical(s) will affect yeast growth. Since this class is a sophomore level course, it is students' first or second attempt at hypothesis generation. Therefore, we permit the students to formulate their hypotheses as a simple predictions such as "yeast will grow more quickly if supplemented with caffeine." In our experience, students struggle with formulating a strong testable hypothesis, so we start with simple predictions that are easily testable. Instructors provide feedback and guidance during this step, but do not dictate the experimental conditions. Chemicals were usually selected from foods or drugs that were familiar to the students. Many chose to use artificial sweeteners, vitamins, or athletic supplements such as creatine, caffeine, or protein-based powders.
In addition to providing a student-centered learning opportunity, we also designed this laboratory series to function as an authentic research experience. A literature search is an important component of any laboratory research experience. Students are required to perform a search of the scientific literature to gather information about the chemical they have selected for their experiment, a process that promotes the development of scientific process skills and improves self-confidence in scientific thinking (19-22). They use this opportunity to determine such things as the concentration of the chemical they should add to their dividing yeast culture and by what mechanism the chemical might affect the yeast cell cycle, augmenting critical thinking and the application of knowledge. Based on our past experiences in other biology classrooms, most students cringe when presented with the need to read scientific papers. The language and structure of journal articles are intimidating to the typical sophomore or junior-level undergraduate. Indeed, others have documented that primary literature may be too difficult for the average undergraduate student (23-25). However, because the literature search for our six-week laboratory series is self-guided, we note that students show less apprehension when attempting to read papers of their own choosing. They often read collaboratively, helping each other puzzle through new or difficult material. During this process, instructors always welcome questions and are happy to assist students in navigating their chosen articles.
Because this laboratory module is an authentic research experience, many students will also experience experimental failure. For example, bacterial contamination was common in our laboratories. In several cases, we found that even very low concentrations of a chemical resulted in excessive cell lethality. In one memorable instance, a group of students added coffee to their cultures and discovered that it has a very high absorbance of light at 600 nm, preventing them from using spectrophotometry to measure growth rates. Groups whose experiments do not yield clear results on the first trial must modify and/or repeat their experiments, in the same way that "real-life" researchers would have to do. We work closely with our students to assist them in reworking their experiments to yield reproducible results. In the case of the coffee group, the students needed to find an alternative method to determine the growth of their cultures since the optical density of the coffee overlapped with the optical density of the yeast cultures. The students, under the guidance of the instructor, decided to use a hemocytometer to determine cell concentration as an alternative assay. The troubleshooting activity is a useful problem solving opportunity for students and instructors alike.
Communication of experimental results to peers is an important facet of scientific research. Authentic research experiences, by definition, must include the presentation of research. We have students participate in group-based, oral presentations of their research findings. In advance of these presentations, we provide rubrics that help students determine which information is appropriate for their presentation and which information is superfluous. In addition, students are required to use presentation software such as PowerPoint and submit their slides to their instructor. Our laboratory groups typically have four members. Equal participation is necessary to ensure equal workloads. To prevent one student from "coasting" on the work of others, we incorporate both group and individual grades into our assessments of the oral presentation. In this way, the instructor can weigh the student feedback of their peers and their own assessment of student participation. The peer assessment of participation is worth 10 points (see the individual assessment portion of the presentation rubric provided in Supporting File S4.) A portion of the individual grade comes from an anonymous assessment of each group member by the other two or three members of the same group. We find that when we suspect one group member of not doing their fair share, the anonymous peer assessment usually confirms this impression.
Others have proposed similar peer assessments of group work, because they tend to improve student involvement and more accurately reflect performance (15,17). In addition, it has been reported that students often feel that it is unfair that all students in a group receive the same grade regardless of their level of participation and performance (26). We feel that without these safeguards in place, many students will just rely on the goodwill of other students to achieve good grade. Therefore, 20% of the student's overall presentation grade is based upon their contribution to the group as assessed by the other group members and the instructor. We justify this proportion of the grade because it only negatively affects students who fail to participate. This strategy seems to encourage students to take an active role in all parts of the project from start to finish. In general, we find that almost all students participated well in their respective groups and their peers rewarded them with very positive feedback. In our iterations of this laboratory module, less than 5% of the class received anything less than full credit for the oral presentation, and most groups received an overall grade of B or better on the presentation. This high performance likely reflects the application of grading rubrics, which spell out exactly what is expected, allowing the students to pre-assess their work based on the rubric requirements.
Our goal was that this laboratory would help our students experience the process of scientific discovery-- from inquiry to experimentation to presentation-- as well as gain factual knowledge and laboratory skills. Our assessments, in particular the laboratory notebook and quiz, demonstrated that the students gained fundamental knowledge about eukaryotic cell proliferation and biology, as well as laboratory techniques and calculations. These observations suggest that our six-week laboratory series accomplished our overarching goal.
The design of the laboratory module lends itself to several possible extensions. In place of, or in addition to, chemicals, students could alter yeast growth conditions such as temperature or gas concentration. Students can assess growth of different yeast strains or mutants, or even isolate wild-type yeast from fruit skins or brewer's yeast from unfiltered beer. An instructor can introduce a cytology component, in which students evaluate whether altered cell growth is reflected in altered cell morphology. This laboratory module is also easily adaptable to the available equipment at a given institution. For example, if spectrophotometers are not available, hemocytometers can be used to assess growth, as our coffee group did. In addition, yeast will grow at room temperature if incubators are not available, or could be grown on solid medium instead of liquid for a longer experimental period (days instead of hours).
We are considering multiple improvements to the laboratory module. First, we chose a 6-hour period for the growth curve to accommodate the constraints of our students' schedules, but this narrow time frame made it difficult for students to see a statistically significant change in the growth rate of their culture. In the future, we would recommend extending the length of this experiment to at least 8 hours, with a possible final time point at 24 hours.
Second, we would recommend devoting additional instructional time to data analysis and statistics. We found that our students lacked the necessary statistical knowledge and skills to tackle the mathematical problems in this laboratory module, despite most of our students having completing a probability and statistics course. Depending on your institution's math requirements, this need for additional statistical analysis instruction may also be required.
Third, we would devote added focus to writing the proposal, which many students admitted was a challenging process. Although most of our students complete two English/writing courses prior to taking this course, we found that most were not proficient in scientific writing, which has a unique style. Given additional time, we would include multiple iterations of the proposal prior to the final submission and grading.
Fourth, we would fine-tune the balance of open-ended and guided inquiry. We likely erred on the side of being too open-ended, allowing the students to pick whatever chemicals they wanted as long as they were deemed safe. A more guided approach might help the students achieve more scientifically valuable results.
Finally, as potential extensions that might be valuable to other instructors, depending on the specific learning outcomes of your course, students might be asked to write a scientific manuscript or create a scientific poster as a means to communicate their science with the class. Perhaps students could "peer review" each other's proposals and decide whether or not the project should be funded based on the information presented. A peer review process could also be applied to a scientific manuscript or poster presentation.
SUPPORTING MATERIALS
- S1. Using Yeast to Make Scientists-Yeast Resources
- S2. Using Yeast to Make Scientists-Proposal Rubric
- S3. Using Yeast to Make Scientists-Lab Quiz
- S4. Using Yeast to Make Scientists-Group Project Evaluation Rubric
- S5. Using Yeast to Make Scientists-Peer Group Assessment Rubric
- S6. Using Yeast to Make Scientists-Introduction to Yeast Biology
- S7. Using Yeast to Make Scientists-Yeast Viability Assay Protocol
- S8. Using Yeast to Make Scientists-Saccharomyces cerevisiae Growth Preliminary Laboratory Protocol
- S9. Using Yeast to Make Scientists-Searching Biological Literature
- S10. Using Yeast to Make Scientists-Locating and Using Biological Literature
- S11. Using Yeast to Make Scientists-Sample Student Proposal
- S12. Using Yeast to Make Scientists-Group Presentation Guidelines
ACKNOWLEDGMENTS
We thank Dr. Julie Himmelberger for her input in assembling the grading rubrics, Fr. Peter Leonard for his insight into the literature search exercise, and Karen Ruggles for her assistance in preparing the figures.
References
- Zhivotovsky B, Orrenius S. 2010. Cell cycle and cell death in disease: past, present and future. J Intern Med 268:395-409.
- Brewer C, Smith D. 2011. Vision and change in undergraduate biology education: a call to actionAmerican Association for the Advancement of Science.
- Auchincloss LC, Laursen SL, Branchaw JL, Eagan K, Graham M, Hanauer DI, Lawrie G, McLinn CM, Pelaez N, Rowland S, Towns M, Trautmann NM, Varma-Nelson P, Weston TJ, Dolan EL. 2014. Assessment of course-based undergraduate research experiences: a meeting report. CBE Life Sci Educ 13:29-40.
- Bangera G, Brownell SE. 2014. Course-based undergraduate research experiences can make scientific research more inclusive. CBE Life Sci Educ 13:602-6.
- Seago , James L. 1992. The Role of Research in Undergraduate Instruction. Am Biol Teach 54.
- Lopatto D. 2003. The Essential Features of Undergraduate Research. CUR Q 139-142.
- Spell RM, Guinan JA, Miller KR, Beck CW. 2014. Redefining authentic research experiences in introductory biology laboratories and barriers to their implementation. CBE Life Sci Educ 13:102-110.
- Alkaher I. Integrating Research into Undergraduate Courses: Current Practices and Future Directions. Sunal, D, Sunal, C, Wright, E, Mason, C, Zollman, D (2014), Res based Undergrad Sci teaching Charlotte, NC Inf Age Pub.
- McLaughlin JS, Coyle MS. 2016. Increasing Authenticity & Inquiry in the Cell & Molecular Biology Laboratory. Am Biol Teach 78.
- Goedhart CM, McLaughlin JS. 2016. Student Scientists: Transforming the Undergraduate Biology Lab into a Research Experience. Am Biol Teach 78.
- McLaughlin, J. S., Favre, D. E., Weinstein, S., & Goedhart CM. 2017. The impact of a four-step laboratory pedagogical framework on biology students' perceptions of laboratory skills, knowledge and interest in research. J Coll Sci Teach In press.
- Manney T. 1996. A Classroom Guide to Yeast Experiments. Kansas State University.
- Sherman F. 2002. Getting started with yeast. Methods Enzymol 350:3-41.
- Sherman F, Fink GR, Hicks JB. 1987. Laboratory course manual for Methods in yeast genetics. Cold Spring Harbor Laboratory.
- Freeman M. 1995. Peer Assessment by Groups of Group Work. Assess Eval High Educ 20:289-300.
- Chapman KJ, Meuter M, Toy D, Wright L. 2006. Can't We Pick our Own Groups? The Influence of Group Selection Method on Group Dynamics and Outcomes. J Manag Educ 30:557-569.
- Lombardi MM. 2008. Making the Grade: The Role of Assessment in Authentic Learning.
- Munro HN, Eaton JC, Glen A. 1949. Survey of a Scottish diabetic clinic: a study of the etiology of diabetes mellitus. J Clin Endocrinol 9:48-78.
- Gillen CM. 2006. Criticism and interpretation: teaching the persuasive aspects of research articles. CBE Life Sci Educ 5:34-8.
- Kozeracki CA, Carey MF, Colicelli J, Levis-Fitzgerald M, Grossel M. 2006. An intensive primary-literature-based teaching program directly benefits undergraduate science majors and facilitates their transition to doctoral programs. CBE Life Sci Educ 5:340-7.
- Levine E. 2001. Reading Your Way to Scientific Literacy. J Coll Sci Teach 31:122-25.
- Mulnix AB. 2003. Investigations of Protein Structure and Function Using the Scientific Literature: An Assignment for an Undergraduate Cell Physiology Course. Cell Biol Educ 2:248-255.
- Muench SB. 2000. Choosing Primary Literature in Biology To Achieve Specific Educational Goals. J Coll Sci Teach 29:255-60.
- Porter JR. 2005. Information Literacy in Biology Education: An Example from an Advanced Cell Biology Course. Cell Biol Educ 4:335-343.
- Smith GR. 2001. Guided Literature Explorations: Introducing Students to the Primary Literature. J Coll Sci Teach 30:465-69.
- Conway R, Kember D, Sivan A, WU M. 1993. Peer Assessment of an Individual 's Contribution to a Group Project. Assess Eval High Educ 18:45-56.
Article Files
Login to access supporting documents
Using Yeast to Make Scientists: A Six-Week Student-Driven Research Project for the Cell Biology Laboratory(PDF | 477 KB)
S1. Using Yeast to Make Scientists-Yeast Resources .docx(DOCX | 16 KB)
S2. Using Yeast to Make Scientists-Proposal Rubric .docx(DOCX | 15 KB)
S3. Using Yeast to Make Scientists-Lab Quiz .docx(DOCX | 20 KB)
S4. Using Yeast to Make Scientists-Group Project Evaluation Rubric .docx(DOCX | 19 KB)
S5. Using Yeast to Make Scientists-Peer Group Assessment Rubric .docx(DOCX | 19 KB)
S6. Using Yeast to Make Scientists-Introduction to Yeast Biology .pptx(PPTX | 2 MB)
S7. Using Yeast to Make Scientists-Yeast Viability Assay Protocol .docx(DOCX | 76 KB)
S8. Using Yeast to Make Scientists-Saccharomyces cerevisiae Growth Preliminary Laboratory Protocol _0.docx(DOCX | 352 KB)
S9. Using Yeast to Make Scientists-Searching Biological Literature .docx(DOCX | 16 KB)
S10. Using Yeast to Make Scientists-Locating and Using Biological Literature .pptx(PPTX | 174 KB)
S11. Using Yeast to Make Scientists-Sample Student Proposal .docx(DOCX | 22 KB)
S12. Using Yeast to Make Scientists-Group Presentation Guidelines .docx(DOCX | 19 KB)
- License terms

Comments
Comments
There are no comments on this resource.