The impact of diet and antibiotics on the gut microbiome
Published online:
Abstract
The goal of this article is to describe an active learning exercise that can be used in a variety of advanced microbiology courses, including bacterial physiology, ecology, or systems biology. The gut microbiome is a multi-species bacterial community that is impacted by outside factors, such as the food we consume or treatments with antibiotics, and impacts our health. In this active learning experience; students start with a simple 'pasta' simulation of a gut microbiome, adapted from a previously published lesson, where different types of pasta in a plastic bag simulate different bacteria in the gut and the composition of the pasta types is representative of diet related differences in the microbiome. Students will then mimic an antibiotic treatment by removing certain pasta/bacteria and replacing them with beans/different bacteria. Next, students will analyze the gut microbiome at the level of phylum, genus, or species. With the help of assigned scientific literature, students will learn how the composition of the gut microbiome responds to diet, a process that is accompanied by the synthesis of bacterial fermentation and other bacterial metabolic products that elicit a molecular response in the host intestinal cells. Students will gain an initial understanding of how these changes impact human health. Through this experience, students will increase their knowledge of bacterial metabolic pathways and products, improve their understanding of the complex community that constitutes the microbiome, analyze the microbiome at multiple systematic levels, and apply their knowledge in a context that is relevant to human health.
Citation
Pruess BM. 2019. The impact of diet and antibiotics on the gut microbiome. CourseSource. https://doi.org/10.24918/cs.2019.28Society Learning Goals
Microbiology
- Metabolism
- How does the survival and growth of any microorganism in a given environment depend on its metabolic characteristics?
- Systems
- How do microorganisms, cellular and viral, interact with both human and non-human hosts in beneficial, neutral, or detrimental ways?
- How do microorganisms interact with their environment and modify each other?
Lesson Learning Goals
In a unique active learning experience, students will use a simple simulation to analyze the gut microbiome at multiple systematic levels (e.g. phylum, genus, species) and apply the newly gained knowledge in a context that is relevant to human health. They will identify metabolic pathways and products that contribute to the microbiome as a system and learn about the impact of microorganisms on their bodies. The learning objectives are in alignment with the Microbiology Learning Framework from the American Society for Microbiology. The mimicking of the antibiotic treatment is an example of Choose a perturbation to a novel environment, and predict the change to the resident microbial community. under Systems. The determination of the bacterial metabolic byproducts that the bacteria excrete falls under How does the survival and growth of any microorganism in a given environment depend on its metabolic characteristics? under Metabolism. The investigation of the human host response to a certain diet covers Describe how the human biome influences the host human organism., also under Systems.Lesson Learning Objectives
After completing the exercise, students will be able to:- Identify several of the nine phyla that contribute to the gut microbiome and name the two predominant ones;
- Describe how diet impacts the gut microbiome and compare the composition of the gut microbiome between different diets;
- Describe how antibiotic treatment impacts the gut microbiome and understand how this leads to infection, for example by Clostridium difficile;
- Trace the response to a change in diet, starting with i) changes in the composition of the microbiome, followed by ii) changes in the bacterial metabolic pathways and the respective excreted metabolic products, resulting in iii) a molecular response in the host intestinal cells, and eventually iv) resulting in human disease;
- Improve their ability to read scientific literature;
- Express themselves orally and in writing;
- Develop team working skill
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
The human microbiome is a community of bacteria and other microorganisms that lives in or on our human bodies and impacts our health. While an unborn child is almost sterile, the number of bacteria that call our adult bodies their home is approximately 10 times larger than the human cells that comprise our body. Bacteria live on our skin, in our mouth, nose, gut, vagina, and just about every other place they can access. These respective microbiomes are now referred to as skin microbiome, oral microbiome, gut microbiome, etc. The Human Microbiome Project (http://commonfund.nih.gov/hmp) is a governmental initiative investigating these microbiomes.
The gut microbiome includes bacteria from nine phyla; Firmicutes, Bacteriodetes, Actinobacteria, Proteobacteria, Fusobacteria, Tenericutes, Spirochaetes, Cyanobacteria, and Verrucomicrobia; Firmicutes and Bacteriodetes are the predominant ones (1). Each of these phyla are represented by many genera and species. While the microbiome of adult humans is reasonably stable, changes in the environment (e.g. food, antibiotics) may alter the composition of the microbiome, which has the potential of triggering the immune system to cause chronic inflammation (2). Generally stated, the pathway that starts with a change in diet involves the following steps: i) changes in the bacterial composition of the microbiome, ii) excretion of bacterial metabolic products into the intestine, iii) molecular response in the host intestinal cells, iv) altered host status, including possible human disease. Among the metabolic products that bacteria excrete in the response to a high fiber diet, short chain fatty acids (SCFA) exert multiple positive impacts on human health through very specific molecular pathways in the intestinal cells. As some examples, propionate reduces cholesterol and hepatic triglycerides (3,4) and butyrate increases the endogenous production of the glucagon-like peptide-2 that improves the mucosal barrier function (5). Possible mechanism by which SCFA exert their anti-inflammatory activity include an inhibition of histone deacetylase in the intestinal cells, which impacts gene expression in colon cancer cells (6). Diseases resulting from imbalances of the gut microbiome include type I diabetes (7), cardiovascular disease (8), colon cancer (9), and pancreatic disorders (10).
Beyond diet, another environmental change that can impact the composition of the gut microbiome is antibiotic treatment. In particular, recurring infection with Clostridium difficile has been recognized for a long time as a consequence of antibiotic treatment (11) and is still of continued concern today (12). Intriguingly, these infections can be reduced by fecal transplantation (13), where the patient receives a fecal sample from a healthy person with the ultimate goal to modify the patient's gut microbiome for an improvement of health.
The active learning exercise that is described in this article builds on a 'pasta' simulation by Anne Estes that was developed for an undergraduate General Microbiology class (14). The original simulation used pasta bags to represent the composition of the gut microbiome under the influence of different diets and bean bags to mimic an antibiotic treatment that will lead to infection by Clostridium difficile for some of the groups. To adjust the original exercise for suitability in a 400 level Bacterial Physiology course, several review articles are provided to the students (15,16), as well as information on the Human Microbiome Project (1). In particular, the review article by Portune and coworkers (15) is used to guide students through the alignment of pasta types with specific types of bacteria that are characteristic for each diet (15). To facilitate this analysis, the types of diet had to be modified from the Estes paper (14) to match the Portune paper (15).
The instructor will prepare two different plastic bags for each student group; a pasta bag and a bean bag (Figure 1). The pasta bag -human gut- contains different types of pasta; each pasta type simulates a type of bacterium and the composition of the pasta in the bag simulates the composition of the bacteria in the gut. Different mixtures of pasta simulate differences in the gut microbiome under the influence of the diet that is written on the bag. A total of six different diets (pasta bags) are distributed to the groups of students, with each student group receiving one bag (diet). The second bag -external environment- contains a random mix of dried beans, simulating an assortment of environmental bacteria. Some of the student groups will receive lentils simulating bacterial pathogens of the species Clostridium difficile in their bean bag. A distinction will be made between toxin producing and non-toxin producing strains of C. difficile, with green lentils resembling toxin producing and yellow lentils representing non-toxin producing C. difficile.
The original pasta simulation (14) was expanded to make it suitable for junior and senior college students, as well as first year graduate students. Gut microbiome information is provided to the students in the form of two review articles through an online Learning Management System (15,16). With the help of these articles, students identify bacteria that are characteristic for their assigned diet (pasta bag), determine bacterial metabolic byproducts that these bacteria excrete, and investigate the resulting human host response to a certain diet.
In addition to the content objectives, this active learning activity encourages students to read scientific literature, to express themselves orally and in writing, and to develop their team working skills. Such skills have been described as high impact educational practices, in particular "collaborative assignments and projects, where students learn to work and solve problems in groups" and "writing intensive courses, where students will produce various forms of writing" (17,18). Student engagement, as well as collaborative learning are also part of the definition of active learning where 'active learning is generally defined as any instructional method that engages students in the learning process' (19). The described exercise engages students in their own learning, while providing an opportunity for collaborative learning. It will help students improve their oral and written communication skills through in class discussion and presentations, as well as written assignments.
INTENDED AUDIENCE
The targeted audience is microbiology majors and students from related disciplines with an interest in microbiology at the junior or senior level. First year graduate students can be included. The exercise was incorporated in the author's 400/600 level Bacterial Physiology course. The microbiome section of this course includes four lectures that are not included in this lecture plan and precede this exercise. The simulation is designed for six groups of students, where each group can contain three to five students. For different class sizes, the exercise can be adapted by modifying the number of students per group or the number of groups.
REQUIRED LEARNING TIME
The learning time for the students is about one or two hours to read the Portune review article (15) that will be provided to them ahead of class through a learning management system, 50 minutes class time, and another one or two hours outside class to complete the follow up paper assignment. Instructor time includes about 20 min of shopping and another hour of assembling the pasta and bean bags, 50 min of class time, and grading of the follow up assignment.
PREREQUISITE STUDENT KNOWLEDGE
Since the students are expected to name bacteria phyla, genera, and species, they will need knowledge of bacterial nomenclature of the kind that is taught in General Microbiology courses (pre-requisite for Bacterial Physiology in NDSU's Microbiology program) or covered in General Microbiology text books. They will also need to have a general concept of what constitutes the microbiome and how bacterial metabolic pathways contribute to the health of the total microbiome, as well as that of the host. It will be beneficial to the students if they already know about bacterial fermentation pathways. This information can be acquired in Biochemistry courses (co-requisite for Bacterial Physiology at NDSU) or Biochemistry text books. In addition, there many relevant videos on YouTube (www.youtube.com). Examples of this are 'Microbiology: Glycolysis, Fermentation, Respiration' by Amanda Doig (https://www.youtube.com/watch?v=2b6ArLmPMwA) or 'Anaerobic Respiration-Fermentation' by J. Cauthers (https://www.youtube.com/watch?v=U5BpXEvOMrg).
PREREQUISITE TEACHER KNOWLEDGE
Content knowledge includes general microbiology, bacterial physiology, and ecology. Information can be found in textbooks and an article on the Human Microbiome Project (1). The exercise involves diverse aspects and a thorough knowledge of microbiology and the microbiome.
SCIENTIFIC TEACHING THEMES
ACTIVE LEARNING
This active learning exercise engages students through multiple activities, including an in class simulation. Students will individually prepare for the class by reading an assigned review article in a professional journal (15). During class time, students will work in small groups to discover how the different forms and shapes of pasta from the simulation exercise connect to the information that is provided in the review article. Individual students may find additional sources of information provided online. Students then compile their results. Since every group received a pasta bag simulating a different diet, each group reports on their respective diet and students learn about all diets through reciprocal teaching. The instructor uses this information to compile a total picture of the gut microbiome in the form of a Formative Assessment Table (Table 3). To deepen their understanding of the molecular changes in the host intestinal cells that are caused by the changes in the microbiome and underly the health impact of diet, the follow up assignment is a two pages essay that the students will write in the same groups during the three days that follow the class exercise.
ASSESSMENT
Assessment includes a pre-test that students take online the day before the class session. The pre-test explores students' understanding of the Portune paper (15) and includes three questions, examples of correct answers are given in Table 1. The pre-test is designed to encourage students to read the paper (15), and the questions contain information that the students will need during the class session. Students receive one point per correct answer. The test can be administered through an online Learning Management System to save in class time.
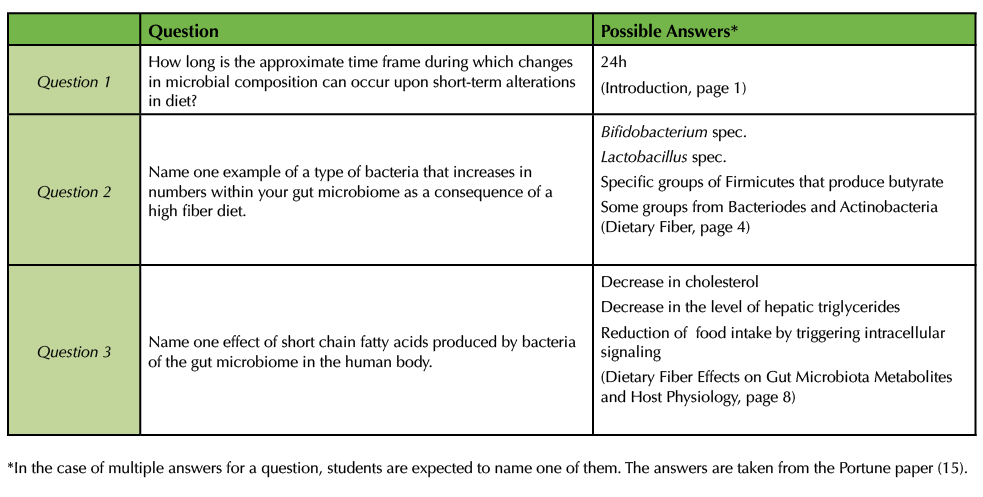
Table 1. Pre-test. This table lists questions for a pre-test with examples of correct answers.
The instructor measures learning formatively during the class activity by listening and responding to the student's report of their findings. By constructing a Formative Assessment Table (Table 3) on the board, instructors can provide feedback on the information that the students are providing. This kind of assessment gives students the opportunity to compare their understanding with what the instructor identifies as both important and correct. The follow-up assignment is summative, in which students return to the Portune paper (15) and write about the molecular response to changes in diet in the host intestinal cells.
INCLUSIVE TEACHING
The MICR480/680 Bacterial Physiology course enrolls students from different majors and academic levels. The course is required for Microbiology majors, but students from majors such as Biology, Soil Sciences, Pre-Pharmacy, Pre-Veterinary Medicine, Medical Lab Sciences, Genomics and Bioinformatics, and several Engineering disciplines routinely attend. Likewise, the academic level is usually junior or senior, but first year graduate students at MS or Ph.D. level are included. In addition to the diversity of fields of study and academic level, students vary in age, race, national background and possibly other characteristics that may not be visible to the instructor.
The activity involves individual and group tasks. Small group tasks are considered cooperative learning techniques and have been described as active learning exercises that foster student learning (20). Furthermore, high impact active learning practices have been recognized to serve the improvement of attrition and retention of minority students (18). The major benefit is that students are actively engaged in their own learning and learn from each other, not just from their instructor.
LESSON PLAN
BACKGROUND
The connection between the gut microbiome and human health has been the topic of intense research for at least the past 10 years. To help students handle the vast amount of research, this learning module relies heavily on review articles. In particular the articles by Koenig et al (16) and Portune and coworkers (15) are a good fit with the pasta simulation that was modified from Estes (14) to suit an upper level Bacterial Physiology course. There are many other review articles that could be useful, such as Pothuraju and Sharma, which emphasizes the role of the gut microbiome in obesity (21). Other articles that could benefit the students (and instructors) are by Petrof (22) and Bhat and Kapila (23) who describe the bacterial metabolites that have impact on human health and are relevant in the context of a Bacterial Physiology course. A good source for general information of the human microbiome can be found in an article on the Human Microbiome Project (1).
LESSON: OVERVIEW
The complete pasta simulation consists of a pre-test, pre-class preparation for instructor and students, 50 min in class time, and a written follow up assignment. Table 2 guides instructors through the timeline for pre-class and in class activities.
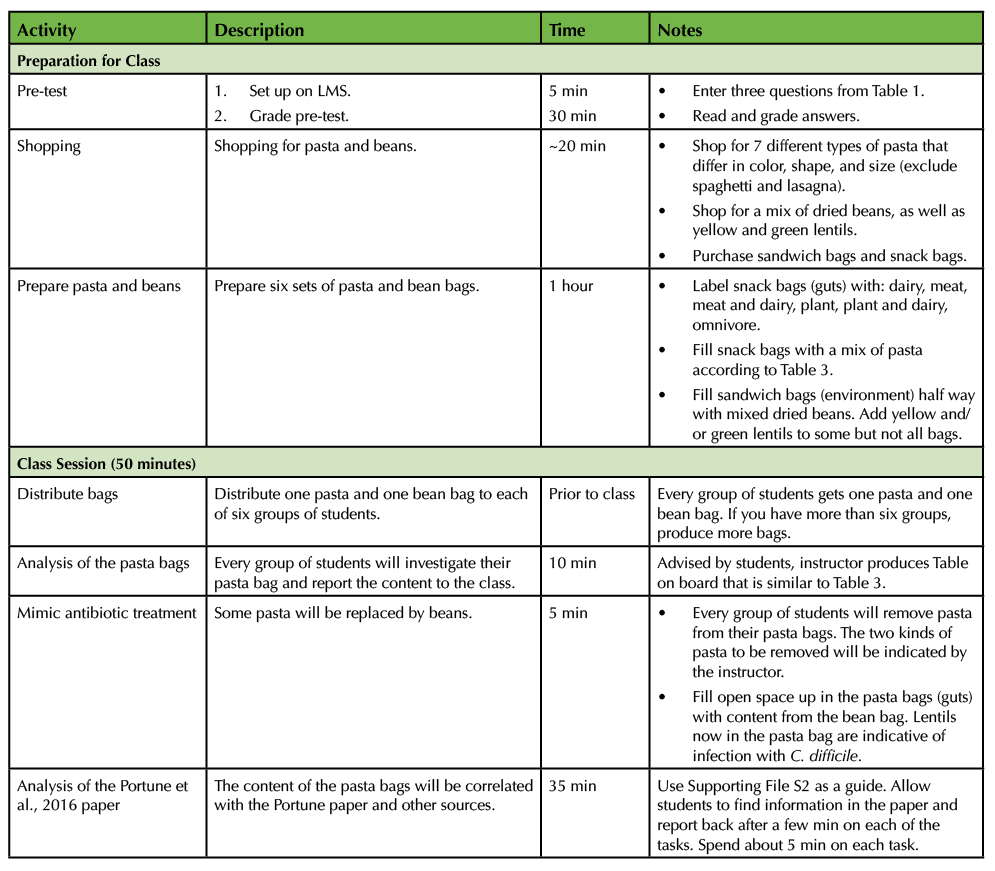
Table 2. Lesson Plan Timeline. This table summarizes the activity, description, estimated time and notes for the class preparation and class section for the instructor.
PRE-CLASS PREPARATION
The pre-class preparation for the instructor can be done at any time before the class (Figure 1 and Table 3). The instructor will need two types of bags, one for the pasta and one for the beans. Small snack bags for the pasta (gut) and larger sandwich bags for the beans (environment) are suitable. The filled pasta and bean bags can be re-used, so this initial preparation can be used for multiple classes. It is recommended to check the composition of the pasta bags prior to each course, because pasta may get mixed up during the activity. For the pasta, the instructor will need to purchase each of seven different types of pasta to a total of about 2 pounds. Examples are included in Table 3 and Figure 1, but other combinations will work as well. For the beans, the instructor will need one to two pounds of random dried beans, including a small number of green and yellow lentils.
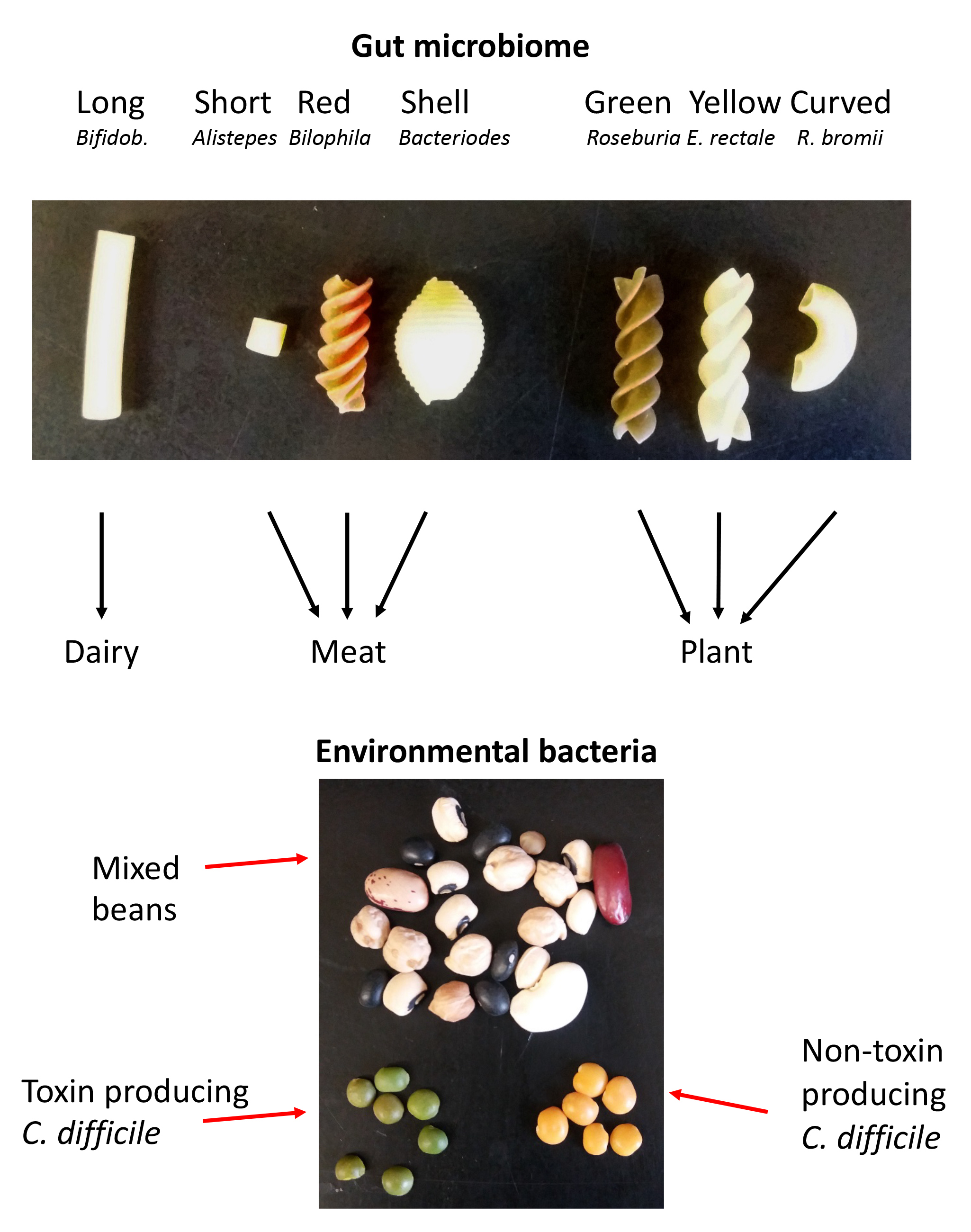
Figure 1. A schematic of the relationship between the different types of pasta or beans and the respective gut and environmental bacteria. C. difficile = Clostridium difficile. The pasta types have been modified from Estes (14) to suit the advanced topic of the Bacterial Physiology course.
The instructor will fill the pasta into the smaller snack bags according to Table 3, filling the bags all the way up. Bags will be labelled with the respective diets as indicated in Table 3. The filled pasta bags are the bacteria filled gut, with each pasta simulating different types (e.g. phyla, genera, species) of bacteria. As one example, the bag labelled 'meat diet' will receive short pasta, red spirals, and shells, simulating Alistipes, Bilophila, and Bacteriodes. Larger bags will be filled to about half with the mix of dried beans. Five to twenty yellow and/or orange lentils will be added to some of the bags. The lentils represent C. difficile; the green lentils simulate the toxin producing bacteria and the yellow ones the non-toxin producing C. difficile. Keep in mind that Table 3 is set up for six groups of students. If you have more groups, you can duplicate some bags. It is important to have different bags because each bag represents a different diet and different gut microbiome.
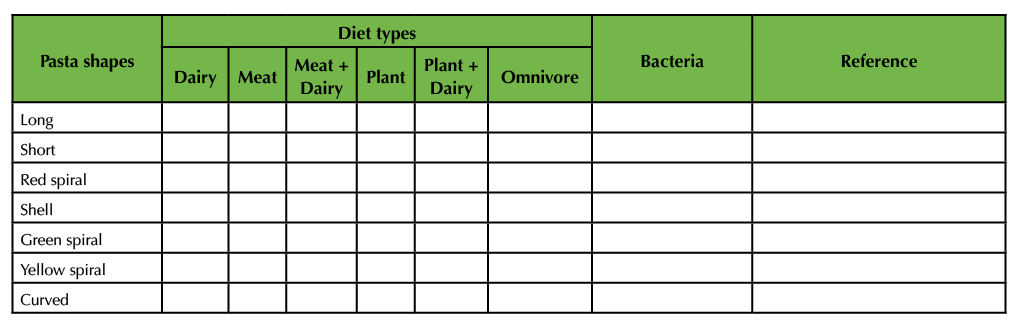
Table 3A. Formative Assessment. This table is an example of the compilation of student information received by the instructor that can be assembled on the board. It connects information from the Koenig (16) and Portune (15) papers with the different types of pasta. Table 3A is the version of the Table that the instructor will initially construct on the board.
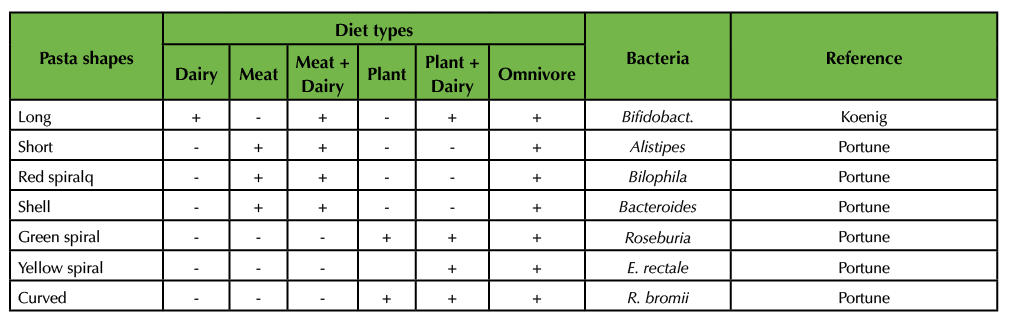
Table 3B. Formative Assessment. This table is an example of the compilation of student information received by the instructor that can be assembled on the board. It connects information from the Koenig (16) and Portune (15) papers with the different types of pasta. Table 3B is the final version after students helped the instructor fill in the blanks.
With the help of the students, the instructor fills in the blanks of the Table. Specifically, the different types of pasta (e.g. long) represent different types of bacteria (e.g. Bifidobacteria). Since differences in diet (e.g. dairy) give rise to the occurrence (indicated by a ‘+’) of specific types of bacteria (e.g. Bifidobacteria), each pasta bag is assembled in a way that only pasta types that belong to the respective diet (as indicated by +) are included in the bag. A (-) indicates that these types of pasta are not to be added to the bags and that these types of bacteria are not predominant under the respective diet. Mark the bags with the diet (e.g. dairy). Note, that this Table can also be used to guide the instructor through the assembly of the bags prior to class.
CLASS SESSION (50 MINUTES)
Distribution of the pasta bags. Every group of students receives one pasta and one bean bag prior to beginning of class.
Analysis of the pasta bags (10 minutes). Students will analyze the content of the pasta bag, sorting the pasta by color and shape. Each group selects a reporter who will report out each group's findings. While the students sort the pasta, the instructor will write the early form of the Assessment Table on the board that is described in Table 3A. The instructor will then use the student's reports to fill in the blanks. The final version of the Table is described in Table 3B.
Mimic antibiotic treatment (5 minutes). The antibiotic treatment that was first described by Estes (14) will take no more than 5 minutes. Since broad spectrum antibiotics typically get rid of more bacteria than just the targeted pathogen, long and curved pasta will be removed from those of the bags that contain these types of pasta. This reflects how an antibiotic can impact multiple types of bacteria. Students who received the dairy diet resembling bag of pasta will now have an empty bag and students who received the omnivore diet resembling pasta bag will still have a reasonably full bag of pasta (Figure 2). The instructor should guide discussion so students notice that some bags are emptier than others and that the number of different types of pasta has decreased in all bags except the meat diet resembling bag. These observations simulate a decrease in the biodiversity of the gut microbiome after antibiotic treatment, accompanied by empty niches in which new bacteria can colonize. Students then fill up these empty spaces in the pasta bags with the beans that simulate the environmental bacteria (Figure 2). Upon close inspection, some student groups will notice the presence of yellow or green lentils in their pasta bags, resembling infection with C. difficile. Note that only student groups who received green lentils which are indicative of toxin-producing C. difficile will 'develop symptoms.'
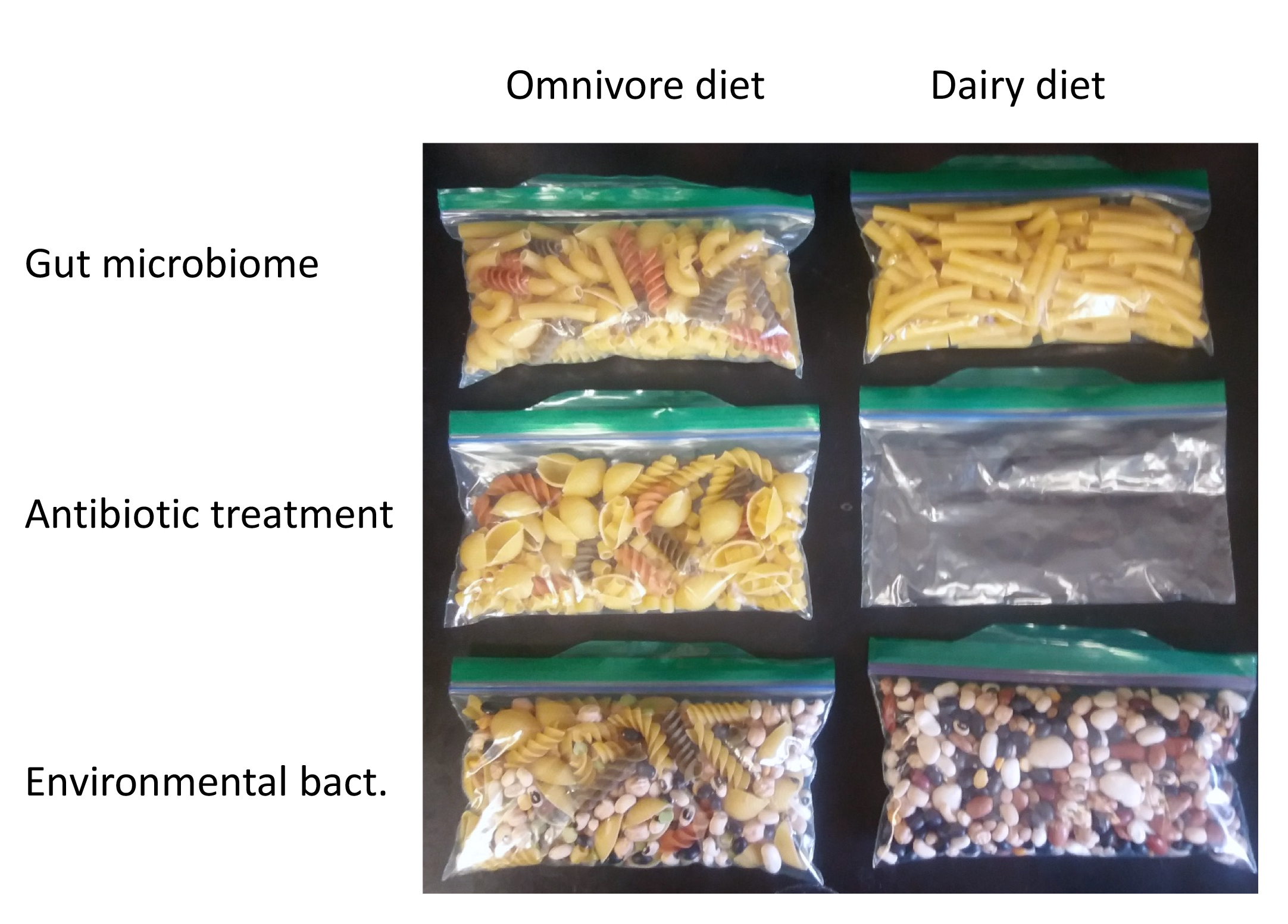
Figure 2. An example of the antibiotic treatment. On the left side, students received a pasta bag that resembles an omnivore diet and contains seven different types of past. On the right side, the pasta bag is representative of a dairy diet and contains only long pasta. The first row is the original pasta bag that students received. The second row shows the respective pasta bags after long and curved pasta were removed in the simulation of antibiotic treatment that reduces different types of bacteria, but not all types. In the third row, students filled up the now empty spaces with beans from the bean bag. Beans resemble environmental bacteria that move into niches in the gut that the antibiotic treatment cleared out. A small number of these beans are lentils which represent C. difficile.
Mapping the in class simulation to research on the gut microbiome (35 minutes). The advanced part of the exercise will be to identify specific microbes, as well as their metabolic products in dependence on the diet. Students will perform seven tasks, each of which should take approximately 5 minutes. The first task is to use the Nature paper on the Human Microbiome Project (1) to identify the nine phyla of bacteria that contribute to the gut microbiome. These are Firmicutes, Bacteriodetes, Actinobacteria, Proteobacteria, Fusobacteria, Tenericutes, Spirochaetes, Cyanobacteria, and Verrucomicrobia. Among these, Firmicutes and Bacteroides are predominant.
Second, students will determine the predominant bacteria in the gut microbiome of a person who lives on a dairy diet. Typically, newborn babies live on a diet that consists exclusively of milk. Students will be referred to Koenig and coworkers (16) who investigated the changes in the growing infant's gut microbiome that accompany the transition from a dairy diet to adult food. The information that is pertinent to this pasta simulation can be found in the first paragraph of the introduction. While the gut of the newborn baby is almost sterile, the vaginal microbiome of the mother serves as the first source of bacteria, mainly of the genus Bifidobacteria. The growth of these bacteria is further supported by the milk diet, as Bifidobacteria metabolize and degrade oligosaccharides that are provided with the milk and cannot be degraded by the human intestine. Bacteroidetes are also found in breast milk and help the baby transition to the adult gut microbiome and a more diverse diet. Students will report Bifidobacteria as an indicator of a dairy diet and the instructor will include this information in the Formative Assessment Table (Table 3) on the board in column 8 (Bacteria). Students will note the Koenig reference in column 9 (Reference). For the student's information, Bifidobacteria belong to the phylum Actinobacteria.
The third task is to find information in the Portune paper (15) on the predominant bacteria in adult humans who live on a diet that consists predominantly of meat. According to Table 1 of this paper (15), people with this diet have a gut microbiome that includes bacteria of the genera Alistipes, Bilophila, and Bacteriodes (24). Students will find these in the Table and the instructor will add the bacteria to Table 3 on the board, column 8.
Task 4 is to identify the bacteria that are predominant in people on a plant diet. These too are listed in Table 1 (15) and include Roseburia, Eubacterium rectale, and Ruminoccus bromii, all from the phylum Firmicutes. These bacteria will go in Table 3, column 8. By this time, the students will have assigned specific bacteria to the different types of pasta in their snack bags and the instructor will have constructed Table 3B, Formative Assessment on the board. There should be about 15 min left of class time.
The remaining three tasks focus on how changes in the diet to high fat, high fiber, or high protein impact the gut microbiome and specific bacterial metabolic pathways that will lead to the respective metabolic products excreted into the human intestine. This is the next step in the signal transduction pathway from the diet to the ultimate impact on human health and will transition students for the follow up homework assignment. The relevant information is listed in Tables 1, 2, and 3 of the Portune paper. To complete the in class part of the exercise, students will need to identify metabolic products that the bacteria secrete and that contribute to human health. As one example, high fiber diet leads to an increase in Bifidobacteria and Lactobacilli, which are considered probiotics and produce short chain fatty acids including acetate, propionate, and butyrate (27). While it is not fully understood which metabolic products these bacteria produce, long chain fatty acids themselves can reduce short chain fatty acids in the gut, which favors a pro-inflammatory environment (28). Diseases that result from this include obesity, type 2 diabetes, and colon cancer.
FOLLOW UP ASSIGNMENT
This active learning activity will conclude with a follow up homework assignment. So far, students have studied the composition of the gut microbiome in response to the respective diets and identified metabolic byproducts that some of these bacteria produce and excrete into the host intestine. During the three days following the in class time, students will get together in their group and concentrate on the part of the Portune paper (15) that focuses on the link between changes in the gut microbiome/bacterial metabolic products and the resulting diseases, as well as the molecular response in the host intestinal cells that contributes to chronic inflammation. To limit the scope of the paper, students will focus on a high fat diet (section 2). Students will summarize their findings in a short paper of 1-2 pages of text including a figure. Assignment instructions to be given to the students are provided as Supporting File S1.
The microbe/host response to a change towards a high fat diet includes an increase in the lipopolysaccharide (LPS) production by the bacteria (e.g. Firmicutes) and an increase in the permeability of the intestinal barrier by the host. The latter may be due to a decreased integrity of tight junction proteins (29). Students are given the assignment to summarize the effect of the high fat diet through the bacterial responses on the host system. Students will use references from the Portune paper (15) and PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) to find additional literature and go into details with their chosen mechanism.
For the final paper, it is important that students get to the level of individual molecules that are impacted in the human host cells, as well as the function of these molecules and their possible interactions (e.g. LPS binds to its receptor on the host cell). It needs to be clear what the described molecules contribute to the signal transduction chain from the change in diet to the bacteria and ultimately the host response. A sample assignment is provided as Supporting File S2, the grading rubric as Supporting File S3. Students are free to select any three molecules that contribute to the signal transduction of their choice and go into the details of their function. 20 points are dedicated to this assignment by the author of this manuscript on a scale of 350 to 400 points for the course.
TEACHING DISCUSSION
This activity engages students to explore the role of diet on the gut microbiome, the differences in bacterial metabolism, the molecular response in the human intestinal cells, and the resulting impact on human health.
Students typically have no difficulty identifying the nine phyla that contribute to the gut microbiome. The expectation is not that they will remember all nine. However, phyla such as Firmicutes or Bacteroidetes have been discussed in the lectures that preceded this exercise. Students are expected to recognize and remember these two predominant phyla. The most effective part of the pasta exercise may be the short demonstration of an infection by C. difficile in response to antibiotic treatment. The empty pasta bags that now contain a smaller number of different pasta types are an effective simulation. When students 'fill up' their gut with new bacteria, some of which may not be beneficial to human health, students often experience a light-bulb moment. This response is consistent with the student responses that were described by Estes (14).
Junior and senior Microbiology students, as well as graduate students should have heard of C. difficile infection during previous classes. Likewise, they should have knowledge about chronic inflammatory diseases. However, this exercise is very practical and provides a real life scenario, which makes it more interesting for the students. It is likely that this activity makes ideas and knowledge more tangible for the learner and promotes long-term retention of the content. Although all students participated in the group discussions, to promote inclusiveness, I would advise instructors to have different students report from the groups throughout the class.
This lesson can be scaled to accommodate differently sized classes. The activity was designed for six groups of three to five students. If the class size is smaller, the number of students per group can simply be reduced. It is critical to have different diets and gut microbiomes, which makes it essential to have at least six bags. If the class size is larger, some of the pasta bags can be duplicated, rather than increasing the number of students per group; larger groups may result in a smaller percentage of students participating.
The exercise can be adjusted for lower academic levels, such as for precollege students, by going back to the original Estes exercise (14) and doing the pasta/bean work without asking students to relate information on actual bacteria to the pasta and beans. Stressing the antibiotic mimicry by providing students with additional information on C. difficile and other antibiotic related infections will increase the practical relevance of this aspect of the exercise and make it suitable for 100 or 200 level classes.
Finally, the module may be applied for a course in health-related professional programs (e.g. medicine, pharmacy) by asking students to predict whether people with a variety of diets would be more or less susceptible to different diseases based upon their diet dependent gut microbiome. Students would have to do their own literature search to determine if evidence exists to support hypotheses that they themselves formulate. This task could either be an in class activity for a second class period or an additional assignment.
SUPPORTING MATERIALS
- S1. Impact of Diet and Antibiotics - Assignment instructions that instructors will give to students for the follow up assignment.
- S2. Impact of Diet and Antibiotics - Sample follow up assignment for instructors.
- S3. Impact of Diet and Antibiotics - Grading rubric for the follow up assignment.
ACKNOWLEDGMENTS
The author thanks the NDSU Gateways ND program for introducing her to the concept of active learning and Dr. Jennifer Momsen (Biological Sciences Department, NDSU) for critically reading the manuscript multiple times.
References
- Human Microbiome Project C. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486:207-214.
- Ercolini D, Fogliano V. 2018. Food design to feed the human gut microbiota. J Agric Food Chem 66:3754-3758.
- den Besten G, Havinga R, Bleeker A, Rao S, Gerding A, van Eunen K, Groen AK, Reijngoud DJ, Bakker BM. 2014. The short-chain fatty acid uptake fluxes by mice on a guar gum supplemented diet associate with amelioration of major biomarkers of the metabolic syndrome. PLoS One 9:e107392.
- Lin HV, Frassetto A, Kowalik EJ, Jr., Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ. 2012. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 7:e35240.
- Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. 2009. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58:1091-1103.
- Kiefer J, Beyer-Sehlmeyer G, Pool-Zobel BL. 2006. Mixtures of SCFA, composed according to physiologically available concentrations in the gut lumen, modulate histone acetylation in human HT29 colon cancer cells. Br J Nutr 96:803-810.
- Pahwa R, Balderas M, Jialal I, Chen X, Luna RA, Devaraj S. 2017. Gut microbiome and inflammation: a study of diabetic inflammasome-knockout mice. J Diabetes Res 2017:6519785.
- Kasselman LJ, Vernice NA, DeLeon J, Reiss AB. 2018. The gut microbiome and elevated cardiovascular risk in obesity and autoimmunity. Atherosclerosis 271:203-213.
- Zou S, Fang L, Lee MH. 2018. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol Rep (Oxf) 6:1-12.
- Pagliari D, Saviano A, Newton EE, Serricchio ML, Dal Lago AA, Gasbarrini A, Cianci R. 2018. Gut microbiota-immune system crosstalk and pancreatic disorders. Mediators Inflamm 2018:7946431.
- Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. 1978. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med 298:531-534.
- Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. 2012. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol 107:1011-1019.
- Tariq R, Pardi DS, Tosh PK, Walker RC, Razonable RR, Khanna S. 2017. Fecal microbiota transplantation for recurrent Clostridium difficile infection reduces recurrent urinary tract infection frequency. Clin Infect Dis 65:1745-1747.
- Estes AM. 2015. Modeling the dynamic digestive system microbiome. J Microbiol Biol Educ 16:271-273.
- Portune KJ, Benitez-Paez A, Del Pulgar EM, Cerrudo V, Sanz Y. 2017. Gut microbiota, diet, and obesity-related disorders-the good, the bad, and the future challenges. Mol Nutr Food Res 61:1600252.
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. 2011. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 108:4578-4585.
- Kuh GD. 2008. High-impact educational practices. Association of American Colleges and Universities.
- Sweat JJ, G.; Han, S.; Wolfgram, S.M. 2013. How does high impact practice predict student engagement? A comparison of white and minority students. Internat J Scholarship Teach Learn 7:17.
- Prince M. 2013. Does active learning work? A review of the research. J Eng Educ 93:223-231.
- Froyd JE. 2007. Evidence for the efficacy of student-active learning pedagogies.
- Pothuraju R, Sharma RK. 2018. Interplay of gut microbiota, probiotics in obesity: a review. Endocr Metab Immune Disord Drug Targets 18:212-220.
- Petrof EO. 2009. Probiotics and gastrointestinal disease: clinical evidence and basic science. Antiinflamm Antiallergy Agents Med Chem 8:260-269.
- Bhat MI, Kapila R. 2017. Dietary metabolites derived from gut microbiota: critical modulators of epigenetic changes in mammals. Nutr Rev 75:374-389.
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559-563.
- Costabile A, Klinder A, Fava F, Napolitano A, Fogliano V, Leonard C, Gibson GR, Tuohy KM. 2008. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. Br J Nutr 99:110-120.
- Wright RS, Anderson JW, Bridges SR. 1990. Propionate inhibits hepatocyte lipid synthesis. Proc Soc Exp Biol Med 195:26-29.
- Zhang C, Zhang M, Pang X, Zhao Y, Wang L, Zhao L. 2012. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J 6:1848-1857.
- Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, Balogh A, Ostermann AI, Schebb NH, Akkad DA, Grohme DA, Kleinewietfeld M, Kempa S, Thone J, Demir S, Muller DN, Gold R, Linker RA. 2015. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 43:817-829.
- Erridge C, Attina T, Spickett CM, Webb DJ. 2007. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr 86:1286-1292.
Article Files
Login to access supporting documents
The impact of diet and antibiotics on the gut microbiome(PDF | 458 KB)
S1.Impact of diet and antibiotics-Assignment instructions.docx(DOCX | 15 KB)
S2.Impact of diet and antibiotics-Sample follow up assignment.docx(DOCX | 58 KB)
S3.Impact of diet and antibiotics-Grading rubric.docx(DOCX | 19 KB)
- License terms

Comments
Comments
There are no comments on this resource.