The Avocado Lab: An Inquiry-Driven Exploration of an Enzymatic Browning Reaction
Published online:
Abstract
Typical biochemistry labs exploring basic enzyme activity rely on costly, time-consuming protein purification and rarely explore enzyme function in situ. Further, complex purification procedures leave little room for novelty in experimental design. Here we present an inquiry-driven laboratory exercise for biochemistry undergraduates and adaptations for a general education science course. Each student designs a unique experiment to test their hypothesis regarding the nature of avocado browning in a three-hour span. In the presence of oxygen, polyphenol oxidases (PPO) catalyze oxidation of phenolic compounds into quinones, the polymerization of which creates the visible browning of many cut fruits. Avocado fruit, a source of both enzyme and substrate, is a safe, low-cost vehicle for semi-quantitative experimentation. During the incubation, biochemistry students use the Protein Data Bank and primary literature to understand the structure-function relationship of PPO and other molecular components of the avocado. Non-major students discuss how pH, temperature, and substrate availability affect PPO. Visible browning pigments appear on a controllable time scale. Students can photograph results to create a figure to accompany semi-quantitative analysis of experimental results in a single lab period. Since avocados are familiar foods and select test reagents are generally recognized as safe, the optimal protocol investigated in the lab can be further applied to best practices in the kitchen in everyday life, promoting the transfer of knowledge learned in the classroom to practical environments.
Citation
Peres ST, Oonk KA, Riley KJ. 2019. The Avocado Lab: An Inquiry-Driven Exploration of an Enzymatic Browning Reaction. CourseSource. https://doi.org/10.24918/cs.2019.38Society Learning Goals
Biochemistry and Molecular Biology
- Energy is required and transformed in biological systems
- How do enzymes catalyze biological reactions?
- Macromolecular Structure Determines Function and Regulation
- How are structure and function related?
- How is the biological activity of macromolecules regulated?
Lesson Learning Goals
From Biochemistry and Molecular Biology Learning Framework:- "How do enzymes catalyze biological reactions?"
- Students will be able to explain the role of multiple conditions (e.g. reducing reagents, pH, chelators, temperature, etc.) on an oxidation reaction.
- "How is the biological activity of macromolecules regulated?"
- Students will understand how substrate and cofactor availability affect enzymatic reactions.
- Students will be able to explain how subcellular organization restricts enzyme activity.
- "How are structure and function related?"
- Students will be able to explain the importance of optimal temperature and pH for enzyme function.
- Students will know that metal ions are critical in metalloprotein catalysis.
Lesson Learning Objectives
Students will be able to:- develop a testable research question and supportive hypothesis regarding the browning of damaged avocado flesh caused by the activity of avocado polyphenol oxidase (aPPO).
- design and execute a well-controlled experiment to test aPPO hypotheses.
- evaluate qualitative enzyme activity data.
- create a figure and legend to present qualitative data that tests multiple hypotheses and variables.
- search for and correctly cite primary literature to support or refute hypotheses.
- know the role of reducing reagents, pH, chelators, and temperature in reactions catalyzed by aPPO.
- explain why the effects of salt and detergent differ for aPPO experiments conducted in situ
- (in mashed avocado flesh) as compared to in vitro (on purified protein).
- discuss how substrate and cofactor availability affect aPPO reactions.
- describe how endogenous subcellular organization restricts aPPO reactions in a healthy avocado.
- evaluate food handling practices for fruits expressing PPO.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
Origin of the Lesson
We created this lab to introduce biochemistry students to experimental design, data interpretation, and the limitations of a given experimental approach, all of which are critical skills for biochemistry and molecular biology undergraduates (1). We selected a study of enzyme activity for this foray into experimental design because it is understood by most students early in the first semester of biochemistry and is of central disciplinary importance. Polyphenol oxidase (PPO) is a good model enzyme because it is highly active in many plants and fungi and reliably produces a visible product on a reasonable time scale (2). Several available exercises exploring enzymatic browning couple protein purification from fruits such as grapes, apples, or bananas with kinetic analyses using a variety of synthetic substrates and/or inhibitors (3-5). While such labs are excellent for teaching technical biochemistry skills and demonstrating basic principles of enzymology, they leave little or no room for inquiry-driven experimentation, a key recommendation of the Vision and Change report (6).
Inquiry-driven laboratory experiences can be broadly categorized as either "guided" or "open" based on the extent to which the instructor knowingly controls the experimental design and outcome (7). A guided inquiry approach expects students to follow prescribed experimental directions, gather their own data, and analyze their results to establish relationships between variables. In open inquiry, students use provided background to design their own procedures to precede their analysis and conclusions. Ultimately, research scientists engage in authentic inquiry, developing every element of the scientific method (8). Early in a 200- or 300-level biochemistry course, we expect to see students begin tackling the challenges of open inquiry as they develop into independent thinkers. Because of the inherent technical complexities of biochemistry experimentation, most biochemistry lab exercises are multi-week, semester-long, or year-long projects, often as part of a course-based undergraduate research (CURE) format (9-11). More recently published exercises on PPO browning have simplified the enzyme extraction protocol but still require the use of added substrates and would be considered more "guided inquiry" (12-14).
In order to introduce open-inquiry biochemistry experimentation in a one-week, standalone format, we provide a protocol to use unpurified avocado pulp as a relatively cheap, broadly available source of both the enzyme and substrate. Students employ either common household items or laboratory reagents to systematically test one of several different variables that affect avocado browning on a roughly two-hour time scale. All required reagents can be purchased at a grocery store. Laboratory facilities and equipment are not required. Students are assessed with an in-lab presentation and with a figure coupled to a publication-style "Results & Discussion" section. Enzyme activity and inhibition in situ is relevant to the food industry (15,16), so conclusions can be related back to handling of fruit. The goal of our open inquiry exercise is to relieve students of technical complexities and give them an opportunity to focus on quality experimental design in a qualitative exploration of enzyme catalysis.
Background on Avocado Polyphenol Oxidase
The avocado tree (Persea americana) produces edible fruits marketed worldwide. Since the 1970s, the primary global variety is the "Hass" avocado, rich in diverse lipids, minerals, vitamins, and phytochemicals (17). Avocados are difficult to preserve and process due to a tendency to quickly turn brown when cut or damaged (15). Avocado browning is a multi-step process initiated by the enzyme avocado polyphenol oxidase (aPPO). The literature discussing browning reactions employs aliases for the enzymes that drive the process. PPO is a general term for oxidoreductases that employ a dinuclear copper center to catalyze two types of reactions: the insertion of oxygen in a position ortho- to an existing hydroxyl group in an aromatic ring (tyrosinase or monophenol oxidase activity; EC 1.14.18.1) and the oxidation of o-diphenols (tyrosinase and catechol-oxidase activities; EC 1.14.18.1 and EC 1.10.3.2) [Figure 1; (2,18)]. While enzyme nomenclature differentiates between the two activities, it appears that aPPO has dual activity in catalyzing the oxidation of monophenols and polyphenols in the presence of oxygen (19). Resulting o-quinone products are subsequently converted irreversibly into brown polymeric pigments (melanins) in a series of non-enzymatic reactions. Kinetics of Hass avocado puree browning and aPPO inhibition follow first-order models (20). PPOs are responsible for browning in many fruits and vegetables as well as diverse species including animals and fungi (21).
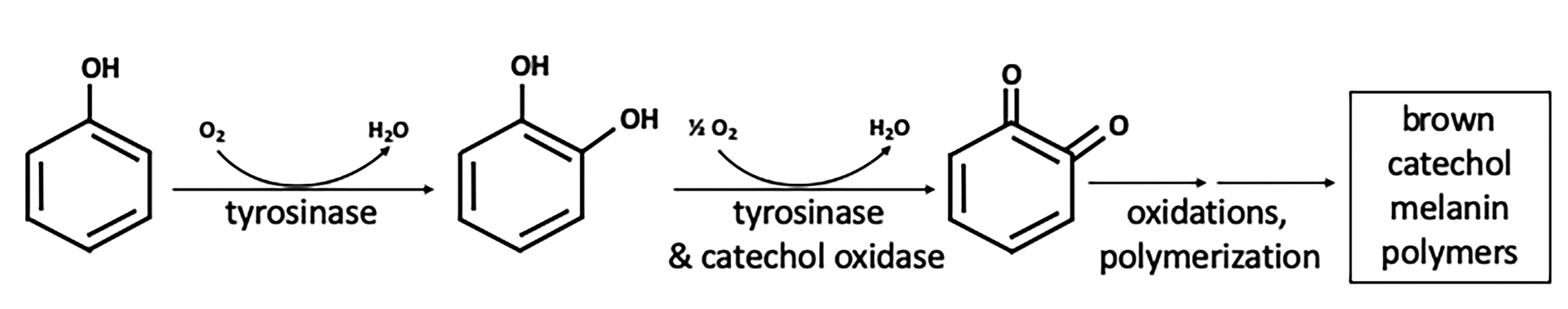
Figure 1. Reaction pathway in the browning of Hass avocado by polyphenol oxidase. The first two steps, monooxygenation and oxidation, are catalyzed by dual enzyme activities of aPPO in the presence of oxygen. Non-enzymatic polymerization reactions occur in multiple subsequent steps. The brown polymers are visible to the naked eye.
The structure of aPPO is not yet published, though the Hass genome (GenBank: NXHZ01000005.1) and transcriptome (22) have been sequenced. The 3D crystal structures of diverse plant PPOs (Figure 2A) depict their universal classification as type-3 copper proteins with structural diversity outside of the active site (23-29). The type-3 copper protein family is defined by a binuclear active site comprised of two copper ions, each coordinated by three conserved histidine residues (Figure 2B-D). Outside of their similar active sites and the ability to bind molecular oxygen, type-3 copper proteins differ in sequence and structure, including substrate-binding pockets (30).
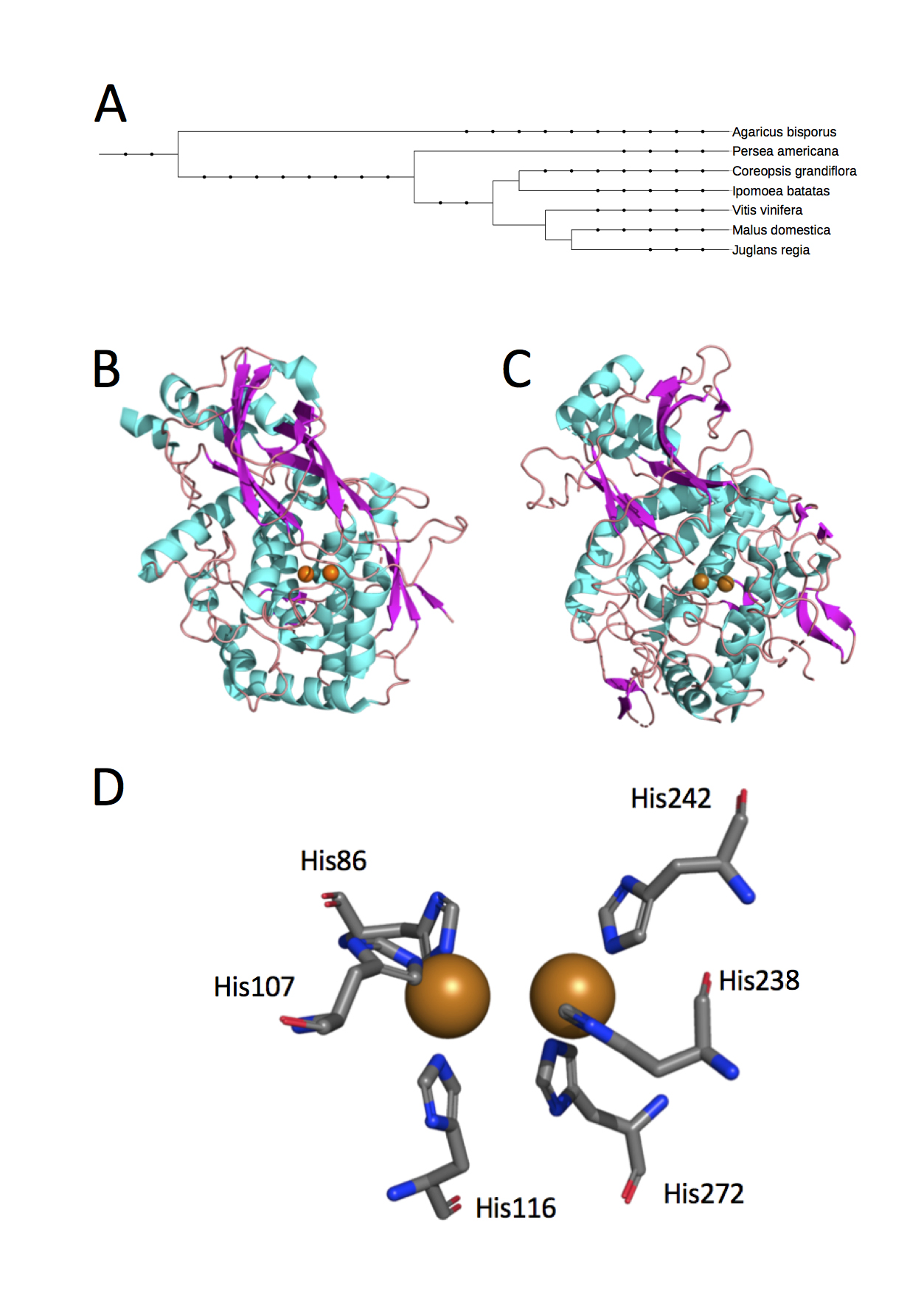
Figure 2. The 3D structures of PPO from plant species reveal type-3 copper protein classification. (A) While the structure of aPPO has not yet been published, the Protein Data Bank (PDB) includes crystal structures from six unique species of somewhat diverse phylogenetic classification that have homologous PPO (A. bisporus is an edible fungus; the others are plants). Phylogenetic relationships were generated by the Interactive Tree of Life (44). The dicopper active site is similarly placed within apple (B) and mushroom (C) PPO [PDB IDs 6ELS and 5M6B; (24, 27)]. Clusters of helice (cyan) create hydrophobic pockets for substrate binding. (D) Two copper ions are each coordinated with three critical histidine residues in apple and other PPOs (PDB ID 6ELS). Protein structures are depicted using The PyMOL Molecular Graphics System, Version 2.2.3, Schrodinger, LLC (licensed to K. Riley).
Latent aPPO accumulates embedded in thylakoid membranes in avocado parenchyma cells, and its manifold phenolic substrates are separately sequestered in cell vacuoles [Figure 3; (31,32)]. Thus, interaction between aPPO, phenolic substrates, and oxygen occurs only after cell disruption and exposure to air, which occur during cutting and mashing (33). Both aPPO (34) and its phenolic substrates (35) vary in identity and quantity amongst avocado varieties. The brown melanins are thought to confer resistance against herbivory in some plants, though the in vivo function of aPPO has not been thoroughly studied (33). In contrast, inhibition of fruit browning is heavily studied because it adversely affects food marketability. Efforts to control avocado browning date back to 1937 (36). Purified aPPO is active over a wide range of pH values (4-8), with a maximum around pH 7. Thermal denaturation occurs around 60-65˚C (37). Relative to other fruits, purified aPPO is highly active and resistant to inhibition (34). Enzyme activity and response to inhibitors differs for purified aPPO versus that which is present in situ within the context of mashed avocado fruit pulp (19), illustrating the influence of environment on enzyme activity.
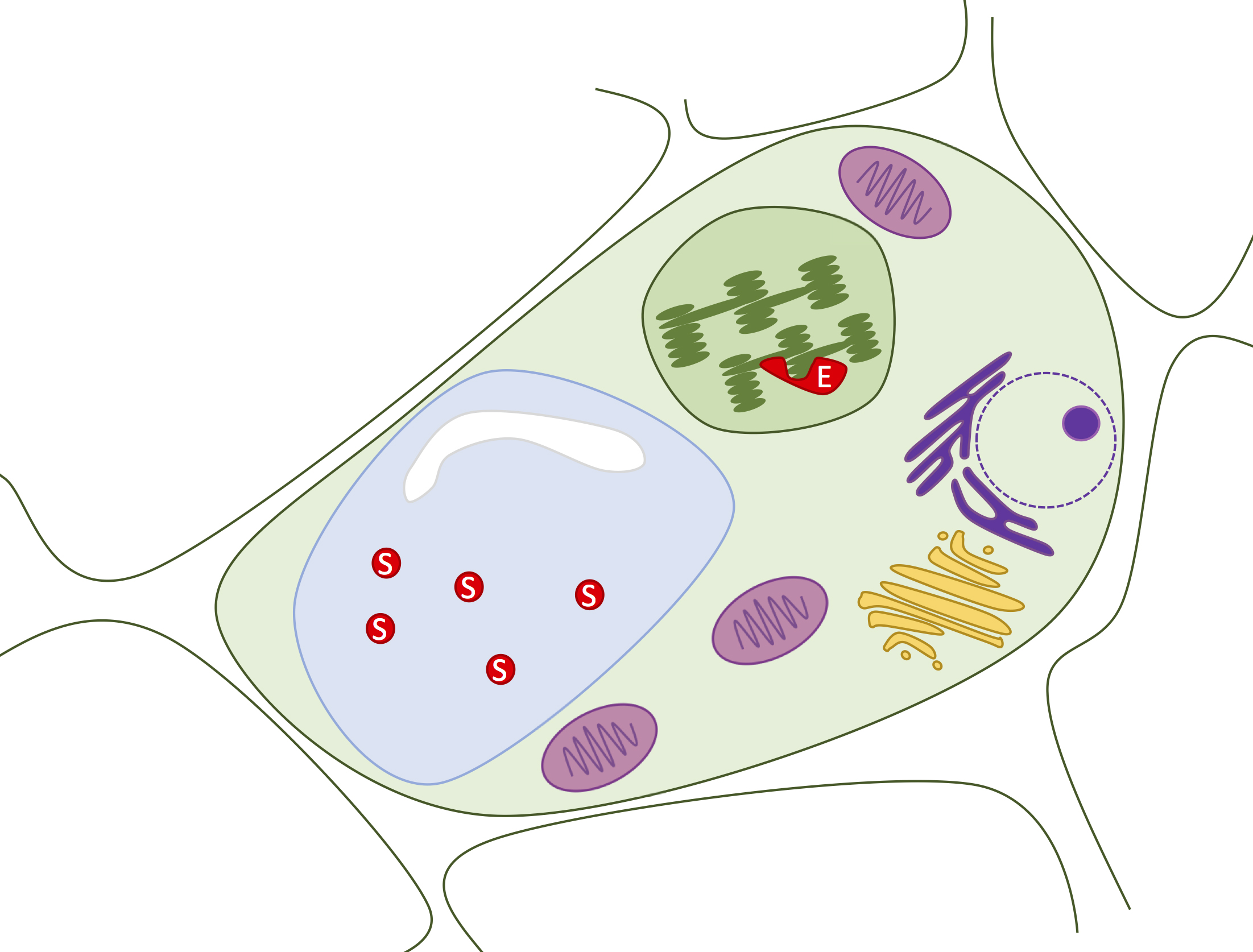
Figure 3. Phenolic substrates and aPPO are physically separated in avocado parenchyma cells. A variety of phenolic substrates (S; red) accumulate in the vacuole (blue). The aPPO enzyme (E; red) is embedded in thylakoid membranes within the avocado plastids (green). Enzyme can meet substrate only upon physical disruption of the many membrane layers that lie between.
Intended Audience
This experiment is intended for delivery early in the semester of an upper-level undergraduate biochemistry lab for students majoring in biochemistry or a related field. A simplified version, described in the discussion, is modified for non-science majors in a kitchen chemistry course and could also be deployed in a general biology classroom. It has been tested at a small liberal arts college during 10 semesters with 6-29 students per biochemistry lab section and two semesters with 12-16 non-majors per lab section.
Required Learning Time
Independent student preparation time is approximately one hour in advance of the lab. This entire exercise can be completed in a single lab session of three hours. A detailed timeline with typical progress is provided (Table 1).
Prerequisite Student Knowledge
For 300-level biochemistry students, we expect a basic understanding of the scientific method, organic chemistry, redox reactions, protein structure/function, and enzymes. For general education students, minimal prerequisite knowledge is required beyond basic high school science content.
Prerequisite Teacher Knowledge
Whether the course is being taught at the introductory or advanced level, the instructor should be well versed in the characteristics of enzyme-driven reactions and enzyme inhibition. Minimally, a biochemistry instructor should become familiar with the role of polyphenol oxidases (PPOs) in enzymatic browning. Ideally, the instructor should know how to search the Protein Data Bank (PDB) to find protein crystal structures. A biochemistry textbook should provide a starting point for general background. In addition to the introduction to this article, the review by Toledo and coauthors (19) provides a thorough discussion of enzymatic browning in avocados. The PDB has an educational website with extensive free resources (38).
SCIENTIFIC TEACHING THEMES
Active Learning
Outside of class, students are expected to prepare by reading the lab exercise handout. Advanced students must independently investigate published literature to develop their own testable hypotheses in advance of the inquiry-driven lab. Five-minute oral presentations by student pairs help strengthen communication skills. Each student physically performs all parts of this exercise, drawing their own conclusions from experimental evidence. A think-pair-share discussion closes the exercise in a manner that engages the entire class (see Table 1 for details).
Assessment
The final assessment linked to this lab exercise is an individual partial lab report: a figure with legend and a results & discussion section, written in the format of the ACS journal Biochemistry (a style guide is available online: https://pubs.acs.org/journal/bichaw). Lab exercises in our 300-level biochemistry course systematically introduce students to writing in the discipline by scaffolding partial lab reports, leading up to a final full-length report. Prior to this lab, students have received feedback on two figures and legends. The avocado lab is the first opportunity in our biochemistry course for students to write a results & discussion section. Oral presentations during lab give students a chance to demonstrate and teach core knowledge relevant to the exercise.
Inclusive Teaching
Each student tests a different hypothesis, and the entire class must share data at the end to complete a picture, making each student's contribution equally important. For the five minute, in-class oral presentation, students work in pairs to alleviate presentation anxiety. The time allotted for in-class preparation gives equivalent time and resources to all students. Because this lab does not require any special equipment or technical skills, students with physical disabilities are inherently accommodated.
LESSON PLAN
Pre-Laboratory Preparation
Materials Purchasing and Setup
The avocado experiment requires no specialized laboratory equipment or facilities, and it can be adapted to test a wide range of potential enzyme inhibitors and chemicals present in standard undergraduate chemical stockrooms. With advance permission, we allow students to bring in their own test reagents from the kitchen. A full list of materials and suggested sources that we have tested is provided (Supporting File S1: The Avocado Lab-Reagents Used). It takes us roughly 15 minutes to set up the materials in advance of the lab.
Prior to lab, the avocados (roughly one per student) must be ripened properly to produce soft but previously undamaged pulp critical for analysis. Hass avocados ripen in response to hormonal ethylene signaling after harvest (39). We recommend purchasing unripened (firm, bright green) Hass avocados approximately one week before the exercise so the ripening can be controlled (see Table 1 for detailed instructions).
Student Preparation
We post the laboratory handout (Supporting File S2: The Avocado Lab-300-Level Student Handout) at least a week ahead of the exercise. Students are required to read the handout in its entirety in advance of the class. It contains background on aPPO and, most importantly, instructions on how the students should develop their own experimental question, hypotheses, and experimental design starting from information available in published literature. It is critical that students at least preliminarily design their experiments before coming to lab, or they will run out of time to complete the experiment.
Laboratory Exercise
A detailed lesson plan timeline breaks the three-hour exercise into four components (Table 1). The pre-experiment discussion should give each student the opportunity to publicly state their variable of interest, discuss proper controls, and time to amend any mistakes in their design. In particular, all students should consider whether they plan to have their samples exposed to air and/or light. It is also helpful to holistically look at the experiments to confirm that all experiments are unique and complementary.
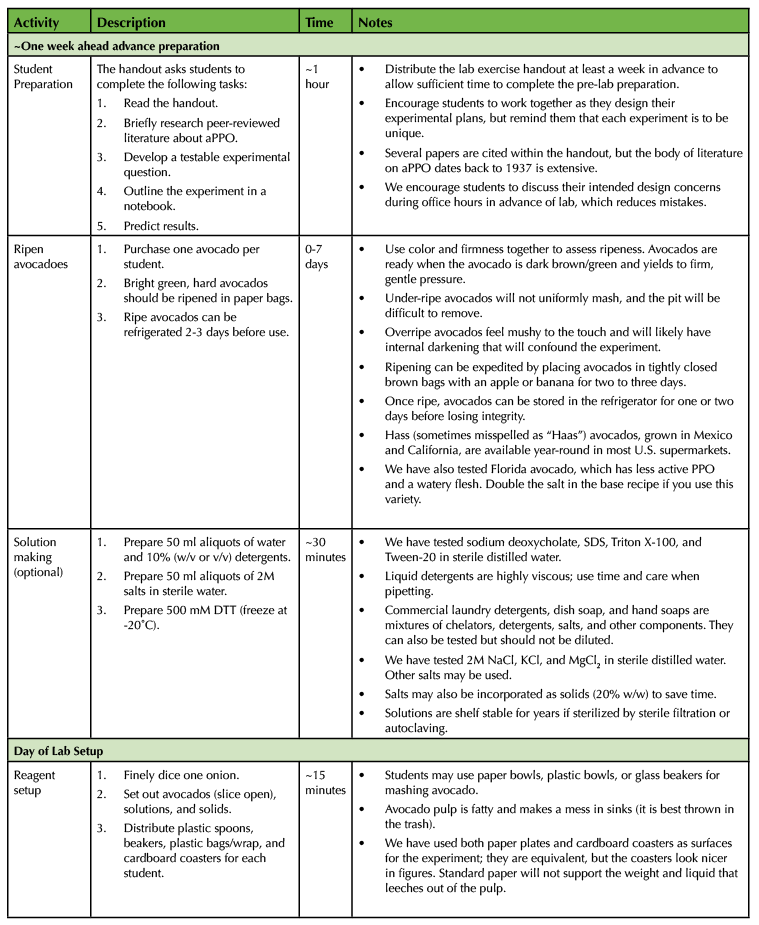
Table 1. The Avocado Lab - 300-Level Biochemistry Lesson Plan Timeline
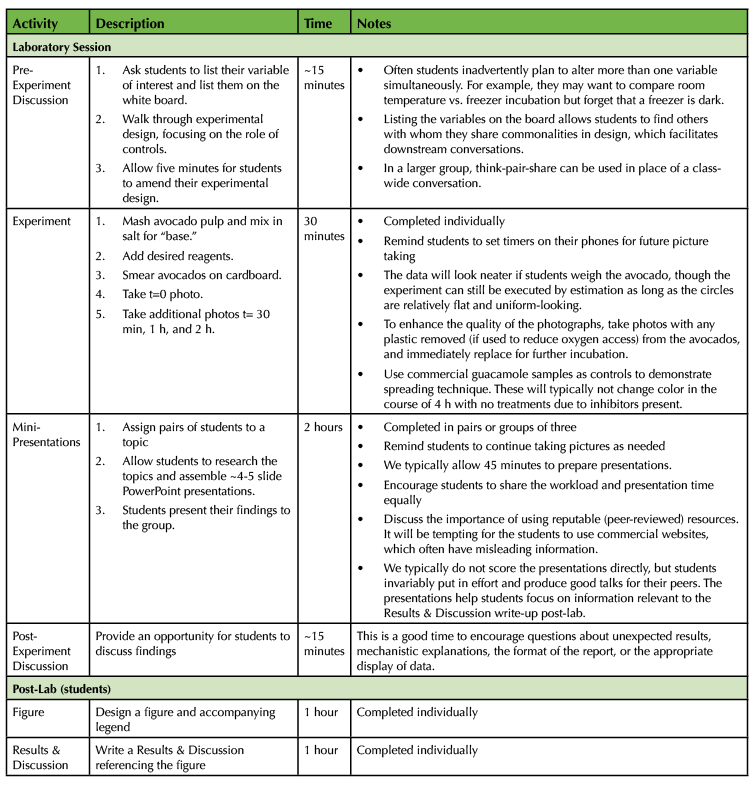
Table 1. The Avocado Lab - 300-Level Biochemistry Lesson Plan Timeline (continued)
It takes roughly half an hour to set up the experiment, which simply consists of preparing avocado mash and smearing it on cardboard coasters. Before students begin, we demonstrate the process of properly labeling the cardboard coasters, mashing the avocado to homogeneity, and spreading even circles on the coaster. We ask students to label cardboard coasters as shown (Figure 4). Pre-labeling the samples is another opportunity for students to catch errors in design, including missing controls.
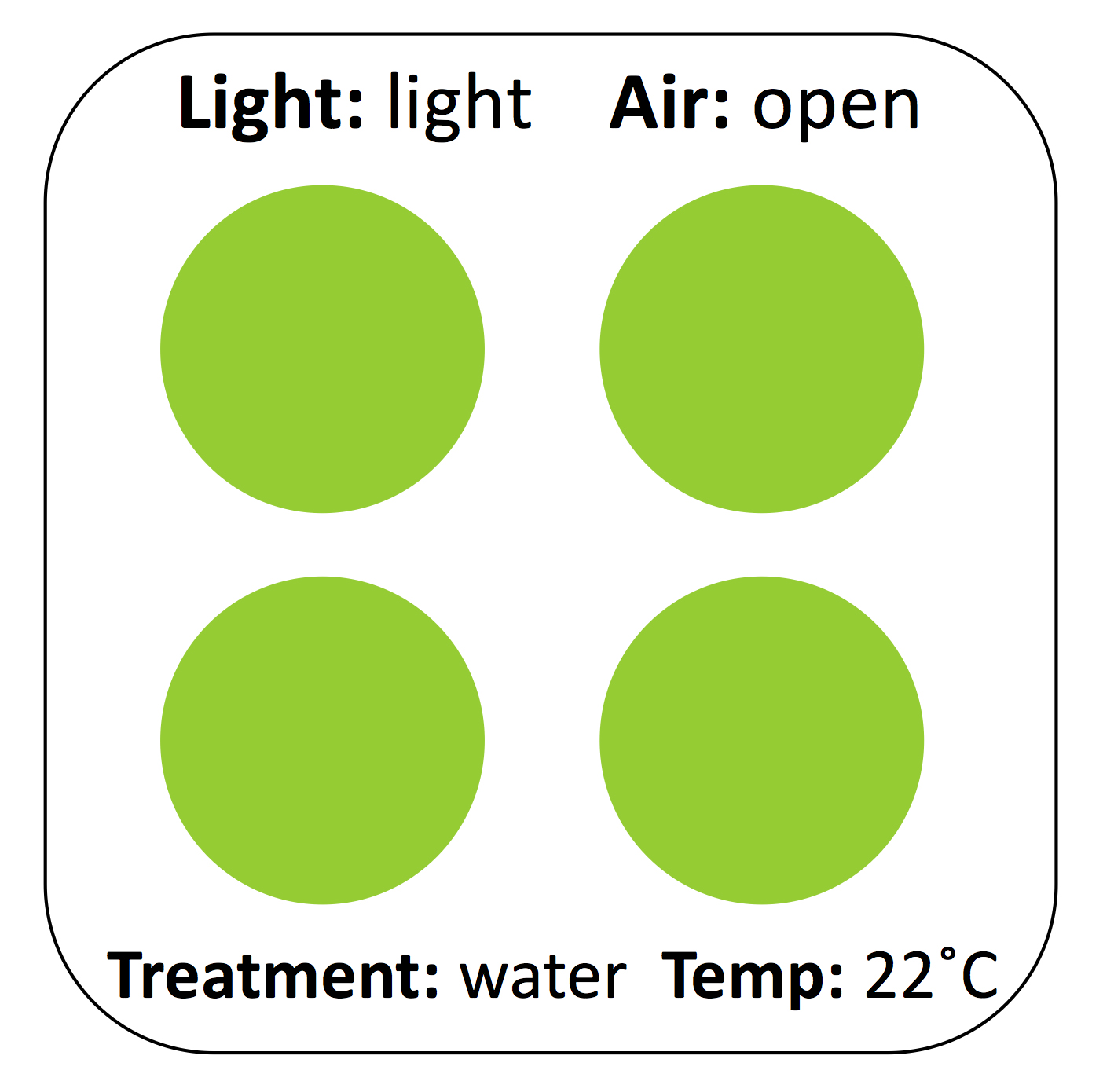
Figure 4. Schematic of experimental setup. Four experimental replicates, approx. 1” each, can fit on a 4” x 4” cardboard coaster or small paper plate. Labeling the cardboard clearly and consistently before applying avocado is critical to the eventual generation of an interpretable figure.
The most critical step in experimental setup is the correct preparation of the avocado mash. Freshly cut, unsalted Hass avocado stored uncovered at room temperature takes roughly 4-6 hours to turn brown, which is not an ideal timeline for teaching lab exercises. The addition of a limited amount of salt to create what we call the "base" avocado pulp speeds up the reaction, likely by increasing enzyme-substrate interactions. This adjusts the exercise to fit into a timeline that better suits a standard three-hour lab period. For a three or four-hour lab exercise, we add 1/2 teaspoon (roughly 2.5 g on a kitchen scale or lab balance) of table salt per avocado at "time = 0." Each student can mash their avocado using a bowl and spoon, though we have also mashed all the avocados for a lab en masse to save time. Students who are only varying environmental conditions (temperature, light, air exposure) can begin smearing avocado onto the cardboard. Roughly four-gram aliquots of avocado should be smeared in one-inch circles on a pre-labeled cardboard coaster in four experimental replicates (a total of ~16 g per experimental condition; Figure 4). Students who are varying chemical conditions (chelators, detergents, salts, pH, reducing reagents) must thoroughly mix in their chosen chemicals before smearing the avocado. If a student is testing for the effect of sodium chloride on avocado browning, remind that student to take a "no salt" sample (if needed) before salt is added to the base. Addition of extra salt (we have tested NaCl, MgCl2, or KCl) to the base will speed it up further. Remind students to take a "time = 0" photo of all of their avocado coasters prior to placing them wherever they will incubate. Start a timer and suggest that students photograph their samples at 30 min, 1 h, and 2 h after starting the experiment.
We recommend using one of four different activities to engage the students during the incubation time. While the reaction is proceeding, we ask students to form pairs or groups of three to create a five-minute mini-presentation using four or five PowerPoint slides. We assign a different topic to each student group from the provided list, which focuses on topics tangentially relevant to discussion of their experimental results (Supporting File S3: The Avocado Lab-Talk Topics). There are several easy-to-read articles that can get students pointed in the right direction in their brief time for research (15,17,19,27,28,40). Alternatively, teams of students can get a sense of the diversity of PPO structures by exploring different crystal structures of a plant or fungal PPO using the Protein Data Bank (PDB; Table 2). Students can construct and present an image of the protein structure directly from the PDB, a publication, or using PyMol, if this is taught previously in the course (Figure 2). The presentations themselves are limited to approximately five minutes per team, depending on how much time is available. Presentations may be left un-graded or can be scored according to the provided rubric (Supporting File S4: The Avocado Lab-Presentation Rubric). In lower-level courses, we use the incubation time to lead a discussion relevant to the exercise (details are below). Finally, either during the incubation time or after the lab, we lead an optional guacamole-making competition in a food-safe room. Students bring in secret ingredients and are offered a variety of standard guacamole ingredients. We bring in outside students and faculty to conduct blind taste testing before the class enjoys a snack. Regardless of how incubation time is used, we remind the students to set timers and regularly check in on/photograph their experimental results.
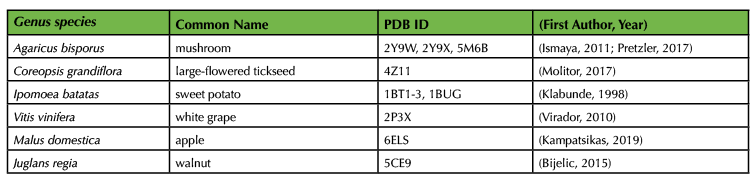
Table 2. Plant PPO crystal structures in the Protein Data Bank (PDB)
Before the end of the lab period, all students take a photo at a final timepoint (usually about 2 h of incubation). The students take five minutes to look over their photos to draw conclusions and record them in their notebook. We summarize class-wide results on the whiteboard and discuss any confusing results before the students depart.
Expected Results and Potential Pitfalls
We have taught this exercise with varying levels of control over the students' experimental approach. We recommend taking a guided inquiry approach (8), where the instructor restricts the reagents used to a subset of the possibilities, pH for example (Supporting File S1: The Avocado Lab- Reagents Used), if the class is large (over 12 students) and/or the students have had little previous experience with independent experimental design. Conversely, if students have engaged in course-based or independent research experiences, a more open inquiry approach broadens their possibilities for complexity in experimental design. In either case, 300-level students should be able to independently develop testable hypotheses.
Every student will see a change in the quantity of visible brown pigment that develops over time. As mentioned before, untreated avocado pulp turns dark brown after 4-6 hours of exposure to air at room temperature (Figure 5, column 1), so it will be clear if a student skips this step. If students add half a teaspoon of table salt to their avocado to create the "avocado base" mixture, browning will be visible within 30 minutes and complete after roughly two hours of air exposure at room temperature (Figure 5, column 2). Some reagents, such as additional sodium chloride, will dramatically speed up the browning process. Other reagents, such as lemon juice, inhibit browning (Figure 5, columns 3-4). When aqueous reagents are added to the avocado pulp, it will seep into the cardboard over time, but it does not appear to affect the browning process.

Figure 5. Student-generated experimental results of the avocado browning experiment. All samples were incubated at room temperature. Photos were taken on a student cell phone at the noted time increments (left). Untreated avocado pulp turns dark brown after about four hours (column 1). Columns 2-4 include 2.5 g NaCl per avocado (avocado base) to expedite the reaction. The base avocado, when treated with extra NaCl, becomes dark brown within two hours (column 3). Lemon juice inhibits browning by lowering the pH to 2 (column 4). For each condition, a single replicate is depicted for clarity.
There are two overall comparisons to make for each experiment: time and treatment. Students should semi-quantitatively score each photo on a scale of 1-5, with 1 being totally bright green and 5 being totally dark brown. All samples should be bright green (score = 1) at t = 0 hours, and all students should have at least one condition that turns brown within two hours. First, within a given treatment group, a comparison between the color at t = 0 hours and t = 2 hours. Secondly, different treatment conditions should be compared at some time point after incubation. For example, at t = 1h, untreated avocado pulp is bright green (score = 1), avocado base is a medium brown (score = 3), the base with additional sodium chloride is darker brown (score = 4), and the base with lemon juice is bright green (score = 1) (Figure 5). To facilitate scoring, a color key is provided in the handout (Supporting File S2: The Avocado Lab 300-Level Student Handout).
Poor mashing or mixing will be evident in heterogeneous results within the replicates for a single condition. Students often mistakenly assume that findings regarding purified PPO will translate to results observed from their in-situ experiment. While pH, temperature, light, reducing reagents, oxygen availability, and chelators influence in situ and purified aPPO activity similarly and predictably, salts and detergents differentially affect in situ and purified aPPO. All tested salts (iodized and kosher table salt, NaCl, MgCl2, or KCl) dramatically increase the rate of in situ browning. Laboratory detergents (e.g. Triton X-100, SDS, sodium deoxycholate) accelerate browning. Some commercial detergents accelerate browning, and others inhibit it, likely owing to the variations in their compositions (which include varied amounts of water, detergents, salts, and chelators).
Conclusions
As with any lab exercise, one should expect variation in the quality of the students' final reports (assessed by Supporting File S5: The Avocado Lab-Grading Score Card). At the 300 level, all students are able to generate a figure from the avocado images, but the quality and layout of the pictures varies, as does the labeling of the samples. Most students realize that the hand-labeled cardboard is for in-lab use, and a well written report requires detail in the figure legend. The images should clearly show differences in color, and many reports will include a key. In our experience, the "Results" section is organized and complete, while the "Discussion" section is most lacking. Students take the conversation in many different directions and to varying depths, largely depending on what types of primary literature they select for reference and how much effort they invest into the final report. Suggested future experiments range from things that are predictable or already published to truly novel and relevant to the food industry.
Students should depart the classroom having clear experimental data to support or refute the hypotheses they came with. We allow students approximately two days to write up their results and figure. A good discussion section must reflect an understanding of in situ enzyme function, including substrate availability and ideal enzyme conditions, and they must fit their results into the context of published literature. To create a multi-day exercise, this lesson can be followed with an experiment to explore purified aPPO (32).
TEACHING DISCUSSION
While we initially created this lab to introduce biochemistry students to experimental design, we have discovered a variety of additional benefits. Because the body of literature on fruit PPO is relatively small and easily understood by undergraduates, students have an opportunity to gain confidence in independently navigating primary literature as they prepare for lab and write up their results. The PDB has many available structures for PPO, so students can learn about the mechanism of this class of enzymes and protein homology while exploring this database for the first time. Brief, low-stakes presentations with peers help break the ice for longer independent presentations required later in the semester. Students mention this lab exercise as a favorite every semester we have used it as an introduction to experimental design in 300-level biochemistry. They have noted relief at its simplicity and find it to be a morale-booster in an otherwise technically demanding course. The technical ease of the experimental setup, which may not even require the use of pipettes, enables the students to focus on an increased quality and complexity of experimental design.
This single-week exercise can serve the first in a line of avocado-themed labs. We are piloting an inquiry-driven exercise where students will partially extract aPPO to compare kinetic parameters to a commercial source, similar to an experiment proposed by Murthy and colleagues (41). Other unique extensions could explore lipid content or soap production from avocado (42,43).
Modifications for Non-Science Major Students
100-Level Class Context
Prior to this exercise, our students understand the pH scale, the definition of ionic compounds (salts), water, and the basic theory of how enzymes work to catalyze reactions. Students have conducted several basic experiments with enzymes, including mozzarella making (rennet) and digestion (salivary amylase). This is the students' first introduction to self-directed experimental design. This exercise could also be used to introduce students to the basics of enzyme function with the addition of a brief pre-lab lecture defining enzymes as biological catalysts, substrates, products, active sites, and inhibitors.
100-Level Pre-Laboratory Preparation
We provide students with a lab handout (Supporting File S6: The Avocado Lab-100-Level Student Handout) that provides background information, experimental questions, and lists relevant variables for the basic procedure. The handout also includes a results and discussion worksheet, which is turned in in lieu of a report. Students are expected to have read over the entire document and come prepared with questions.
Modifications to the 300-Level Laboratory Experiment
We begin class by assigning one test condition to each student and designing our experiments together in class, which takes roughly 30 minutes. For non-science majors, we do not provide reducing reagents or chelators. In lieu of salt or detergent solutions, we employ salts and soaps purchased at a local grocery store. Instead of in-class presentations, we use an instructor-led discussion about avocado composition and classification, PPO, enzyme structures, the relationship between pH and temperature and enzyme activity, and enzyme inactivation. Before taking our final images, we predict outcomes for each variable tested as a group on the white board.
SUPPORTING MATERIALS
Supporting Files for the Avocado Lab:
- S1. The Avocado Lab-Reagents Used
- S2. The Avocado Lab-300-Level Student Handout
- S3. The Avocado Lab-Talk Topics
- S4. The Avocado Lab-Presentation Rubric
- S5: The Avocado Lab-Grading Score Card
- S6: The Avocado Lab-100-Level Student Handout
ACKNOWLEDGMENTS
We would like to thank the students who have engaged in this lab experience over the past seven years and Dr. James Patrone for additional implementation, testing, and feedback. We thank D. and S. Riley for critical commentary on the manuscript. This work was supported by the Rollins College Chemistry Department.
References
- Voet JG, Bell E, Boyer R, Boyle J, O'Leary M, Zimmerman JK. 2003. Recommended curriculum for a program in biochemistry and molecular biology. Biochem. Mol. Biol. Educ. 31:161-162. doi:10.1002/bmb.2003.494031030223.
- Mayer AM. 2006. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 67:2318-2331. doi:10.1016/j.phytochem.2006.08.006.
- Archer MC, Palmer JK. 1975. An Experiment in Enzyme Characterization: Banana Polyphenoloxidase. Biochem. Educ. 3:50-52.
- Sanchez-Ferrer A, Garcia-Caromona F. 1992. A Simple Procedure for Purifying Polyphenol Oxidase for a Practical Biochemistry Course. Biochem. Educ. 20:178-179.
- Correa S, Aires B. 1994. Polyphenoloxidase: An Enzyme Widespread in Fruits. Biochem. 343Educ. 22:152-153.
- AAAS. 2011. Vision and Change in Undergraduate Biology Education: A Call to Action. Washington, DC. www.visionandchange.org.
- Chatteriee S, Williamson VM, McCann K, Peck ML. 2009. Surveying students' attitudes and perceptions toward guided-inquiry and open-inquiry laboratories. J. Chem. Educ. 86:1427- 3471432.
- Buck LB, Bretz SL, Towns MH. 2008. Characterizing the level of inquiry in the undergraduate laboratory: discrepancies abound in use of the word "inquiry." We propose a quantitative rubric to characterize inquiry in undergraduate laboratories. J. Coll. Sci. Teach. 38:52.
- Johanson KE, Watt TJ. 2015. Inquiry-based experiments for large-scale introduction to PCR and restriction enzyme digests. Biochem. Mol. Biol. Educ. 43:441-448.
- Oelkers PM. 2017. Semester-long inquiry-based molecular biology laboratory: Transcriptional regulation in yeast. Biochem. Mol. Biol. Educ. 45:145-151.
- Knutson K, Smith J, Wallert MA, Provost JJ. 2010. Bringing the excitement and motivation of research to students; Using inquiry and research-based learning in a year-long biochemistry laboratory: Part I-guided inquiry-purification and characterization of a fusion protein: Histidine tag, malate dehydrogen. Biochem. Mol. Biol. Educ. 38:317-323.
- Busch JM. 1999. Enzymic browning in potatoes: A simple assay for a polyphenol oxidase catalysed reaction. Biochem. Educ. 27:171-173.
- Melberg AR, Flurkey WH, Inlow JK. 2009. Tissue printing to visualize polyphenol oxidase and peroxidase in vegetables, fruits, and mushrooms. Biochem. Mol. Biol. Educ. 37:92-98.
- Deutch CE. 2018. Browning in apples: Exploring the biochemical basis of an easily-observable phenotype. Biochem. Mol. Biol. Educ. 46:76-82.
- Kassim A, Workneh TS, Bezuidenhout CN. 2013. A review on postharvest handling of avocado fruit. African J. Agric. Res. 8:2385-2402.
- Laurila E, Kervinen R, Ahvenainen R. 1998. The inhibition of enzymatic browning in minimally processed vegetables and fruits. Postharvest News Info. 9:53N-66N.
- Dreher ML, Davenport AJ. 2013. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 53:738-50.
- Gerdemann C, Eicken C, Krebs B. 2002. The crystal structure of catechol oxidase: New insight into the function of type-3 copper proteins. Acc. Chem. Res. 35:183-191.
- Toledo L, Aguirre C. 2017. Enzymatic browning in avocado (Persea americana) revisited: History, advances, and future perspectives. Crit Rev Food Sci Nutr 57:3860-3872.
- Soliva-Fortuny RC, Elez-Martínez P, Sebastián-Calderó M, Martín-Belloso O. 2002. Kinetics of polyphenol oxidase activity inhibition and browning of avocado purée preserved by combined methods. J Food Eng 55:131-137.
- Pilizota V, Subaric D. 1998. Control of Enzymatic Browing of Foods. Food Technol Biotechnol.
- Ibarra-Laclette E, Méndez-Bravo A, Pérez-Torres CA, Albert VA, Mockaitis K, Kilaru A, López-Gómez R, Cervantes-Luevano JI, Herrera-Estrella L. 2015. Deep sequencing of the Mexican avocado transcriptome, an ancient angiosperm with a high content of fatty acids. BMC Genomics 16:599.
- Ismaya WT, Rozeboom HJ, Weijn A, Mes JJ, Fusetti F, Wichers HJ, Dijkstra BW. 2011. Crystal Structure of Agaricus bisporus Mushroom Tyrosinase: Identity of the Tetramer Subunits and Interaction with Tropolone. Biochemistry 50:5477-5486.
- Pretzler M, Bijelic A, Rompel A. 2017. Heterologous expression and characterization of functional mushroom tyrosinase (AbPPO4). Sci Rep 7:1810.
- Klabunde T, Eicken C, Sacchettini JC, Krebs B. 1998. Crystal structure of a plant catechol oxidase containing a dicopper center. Nat Struct Biol 5:1084-1090.
- Virador VM, Reyes Grajeda JP, Blanco-Labra A, Mendiola-Olaya E, Smith GM, Moreno A, Whitaker JR. 2010. Cloning, Sequencing, Purification, and Crystal Structure of Grenache (Vitis vinifera ) Polyphenol Oxidase. J Agric Food Chem 58:1189-1201.
- Kampatsikas I, Bijelic A, Pretzler M, Rompel A. 2019. A Peptide-Induced Self-Cleavage Reaction Initiates the Activation of Tyrosinase. Angew Chemie - Int Ed 58:7475-7479.
- Bijelic A, Pretzler M, Molitor C, Zekiri F, Rompel A. 2015. The Structure of a Plant Tyrosinase from Walnut Leaves Reveals the Importance of "Substrate-Guiding Residues" for Enzymatic Specificity. Angew Chemie Int Ed 54:14677-14680.
- Molitor C, Mauracher SG, Rompel A. 2016. Aurone synthase is a catechol oxidase with hydroxylase activity and provides insights into the mechanism of plant polyphenol oxidases. Proc Natl Acad Sci U S A 113:E1806-15.
- Aguilera F, McDougall C, Degnan BM. 2013. Origin, evolution and classification of type-3 copper proteins: lineage-specific gene expansions and losses across the Metazoa. BMC Evol Biol 13:96.
- Engelbrecht AHP. 1982. Intracellular localization of Poly-phenoloxidase in avocado fruit. South African Avocado Grow Assoc Yearb 5:30-31.
- Medina MA, Garcia de Veas R, Garcia-Hermoso A, Nunez de Castro I. 1991. An experiment in membrane enzyme purification with Triton X-114: avocado pear polyphenoloxidase. Biochem Educ 19:210-211.
- Constabel CP, Barbehenn R. 2008. Defensive Roles of Polyphenol Oxidase in Plants, p. 253-269. In Induced Plant Resistance to Herbivory.
- Weemaes CA, Ludikhuyze LR, Van den Broeck I, Hendrickx ME, Tobback PP. 1998. Activity, Electrophoretic Characteristics and Heat Inactivation of Polyphenoloxidases from Apples, Avocados, Grapes, Pears and Plums. LWT - Food Sci Technol 31:44-49.
- Rodríguez-Carpena JG, Morcuende D, Andrade MJ, Kylli P, Estevez M. 2011. Avocado (Persea americana Mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. J Agric Food Chem 59:5625-5635.
- Samish R. 1937. Contribution to the knowledge of plant phenolases. Plant Physiol 12:499-508.
- Van den Broeck I, Tobback PP, Weemaes CA, Ludikhuyze LR, Hendrickx ME. 2002. Activity, electrophoretic characteristics and heat inactivation of polyphenoloxidases from apples, avocados, grapes, pears and plums. LWT - Food Sci Technol 31:44-49.
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. 2000. The Protein Data Bank. Nucleic Acids Res 28:235-242.
- Vergara-Pulgar C, Rothkegel K, González-Agüero M, Pedreschi R, Campos-Vargas R, Defilippi BG, Meneses C. 2019. De novo assembly of Persea americana cv. 'Hass' transcriptome during fruit development. BMC Genomics 20:108.
- Chanderbali AS, Albert VA, Ashworth VETM, Clegg MT, Litz RE, Soltis DE, Soltis PS. 2008. Persea americana (avocado): Bringing ancient flowers to fruit in the genomics era. BioEssays 30:386-396.
- Murthy PPN, Thompson M, Hungwe K. 2014. Development of a Semester-Long, Inquiry- Based Laboratory Course in Upper-Level Biochemistry and Molecular Biology. J Chem Educ 91:1909-1917.
- Bendinskas K, Weber B, Nsouli T, Nguyen H V., Joyce C, Niri V, Jaskolla TW. 2014. A teaching laboratory for comprehensive lipid characterization from food samples. J Chem Educ 91:1697-1701
- Sutheimer S, Caster JM, Smith SH. 2015. Green Soap: An Extraction and Saponification of Avocado Oil. J Chem Educ 92:1763-1765.
- Letunic I, Bork P. 2019. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res 47:W256-W259.
Article Files
Login to access supporting documents
The Avocado Lab - Inquiry-Driven Exploration of an Enzymatic Browning Reaction(PDF | 794 KB)
S1. The Avocado Lab-Reagents Used.docx(DOCX | 51 KB)
S2. The Avocado Lab-300 Level Student Handout.docx(DOCX | 923 KB)
S3. The Avocado Lab-Talk topics.docx(DOCX | 7 KB)
S4. The Avocado Lab-Presentation Rubric.docx(DOCX | 7 KB)
S5. The Avocado Lab-Grading Score Card.docx(DOCX | 18 KB)
S6. The Avocado Lab-100 Level Student Handout.docx(DOCX | 406 KB)
- License terms

Comments
Comments
There are no comments on this resource.