Necessary and Sufficient? Solving the Mystery of the Mitochondrial Pyruvate Transporter
Editor: Leocadia V. Paliulis
Published online:
Abstract
While there are several available lessons for teaching introductory biology students about diffusion, facilitated diffusion, and active transport, fewer materials exist to support upper-division students' understanding of the proteins that mediate these forms of transport. In the 1970s, mitochondrial pyruvate carrier (MPC) proteins were predicted to import pyruvate from the cytoplasm into mitochondria for cellular respiration. Yet it was not until 2012 that the identity of the proteins responsible for this transport was confirmed in two seminal publications. In this Lesson, students will use their background knowledge of transport mechanisms to analyze data from those papers to determine which of the predicted MPC proteins are actually part of the mitochondrial pyruvate transporter. Student will also learn how scientists test whether a protein is necessary and sufficient. The Lesson is written in the style of process-oriented guided inquiry learning (POGIL). POGIL is a teaching approach that requires students to work collaboratively in small groups to answer a set of questions based on scientific data. Questions in the POGIL activity, called the problem set, are structured so that each question leads to the next, helping to guide students to a deeper understanding of the content. During this Lesson, the instructor acts as a facilitator to guide student learning. Several forms of assessment are included within the Lesson, allowing instructors to assess learning gains. This Lesson has been used multiple times by over 10 faculty in an upper-division Cell Biology course and can also be used in other upper-division biology courses.
Citation
Pfeifer MA, Stanton JD. 2020. Necessary and sufficient? Solving the mystery of the mitochondrial pyruvate transporter. CourseSource. https://doi.org/10.24918/cs.2020.11Society Learning Goals
Cell Biology
- Membrane Structure and Function
- How do solutes and other materials move across membranes?
- Methods & Tools of Cell Biology
- How do the methods and tools of cell biology enable and limit our understanding of the cell?
Lesson Learning Goals
Students will:- understand how scientists identify proteins responsible for transport across membranes.
- be able to analyze data from primary literature to draw conclusions about transport mechanisms within the cell.
Lesson Learning Objectives
After completing the Lesson, students will be able to:- Differentiate between types of transport across membranes (diffusion, facilitated diffusion, and active transport)
- Determine if proteins are necessary or sufficient for transport of pyruvate across a membrane based on experimental data
- Interpret data obtained from pyruvate transport mutants
- Design an experiment to test a specific hypothesis related to transport across membranes
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
The cell's ability to selectively transport molecules across its membranes is critical for its survival. Whether transport of molecules occurs across the cell membrane or the membranes of organelles, regulation of molecular gradients is important for proper cellular function. Thus, life science students need a broad understanding of transport mechanisms such as diffusion, facilitated diffusion, and active transport, as well as the proteins that mediate these mechanisms.
Origin and Rationale for the Lesson
Lessons exist for helping introductory biology students understand the basics of membrane transport. These lessons rely on role playing (1-3), computer simulations (4), case studies (5), and analysis of classic papers in the primary literature (6). Our lesson differs from existing lessons in three major ways. First, our lesson was specifically developed for upper-division biology students rather than introductory biology students. We wanted students in our upper-division Cell Biology course to use their basic understanding of membrane transport concepts to gain a deeper understanding of the mechanisms involved. Second, we designed our lesson so that students would learn how scientists identify the proteins that are responsible for transport of a molecule using biochemical and genetic experiments. We also wanted students to understand how scientists use results from more than one organism to confirm the identity of transport proteins. Third, we created a lesson that draws on an approach for small-group learning called Process-Oriented Guided Inquiry Learning (POGIL) (7).
We used two seminal studies published back-to-back in Science that revealed the identity of the long unknown mitochondrial pyruvate carrier (MPC), a transporter that brings pyruvate into the mitochondrial matrix from the cytoplasm (8,9). We selected key figures from the papers to give students practice analyzing real data while drawing on their pre-requisite knowledge from biochemistry and genetics. The studies from the papers serve as models to help students understand how to design experiments for confirming whether other proteins serve as transporters for particular molecules. We created a Lesson in the spirit of POGIL, similar to our previous POGIL-inspired Lesson on protein localization methods (10).
Intended Audience
The Lesson is intended for senior-level life science majors who are taking an upper-division Cell Biology course. This Lesson has been used in an upper-division Cell Biology course at a large research university in classes ranging from 40 to 135 students. Depending on the background of the students, this Lesson can also be used in an upper-division Biochemistry or Genetics course.
Required Learning Time
The Lesson is designed for a 75-minute class session. We have also taught this Lesson in a 50-minute class session with some modifications (see Suggestions for Possible Adaptations).
Prerequisite Student Knowledge
Background Content Knowledge
- Introductory Biology: mitochondria structure and function, cellular respiration, role of pyruvate, differences between prokaryotic and eukaryotic cells, differences in cellular transport mechanisms (i.e., diffusion, facilitated diffusion, and active transport)
- Genetics: conserved genes, wild-type, single mutant, double mutant, pedigree analysis
- Biochemistry: properties of amino acid side chains
Background Skills:
- Ability to interpret diagrams of molecules
- Ability to interpret basic graphs
Prerequisite Instructor Knowledge
Instructors should be familiar with the concepts and skills included in the Prerequisite Student Knowledge section. We recommend that instructors review the two Science papers covered in the activity (8,9). For more information, Bender and Martinou's 2016 review of mitochondrial pyruvate carriers (MPC) provides a general overview and brief summary of the discovery of MPC (11).
SCIENTIFIC TEACHING THEMES
Active Learning
Students engage in active learning by working collaboratively in small groups during the Lesson. Students must use their pre-existing knowledge to answer questions posed in the problem set. Students will collaborate to analyze data, form conclusions, and propose experimental designs to test hypotheses. At the end of the class, students lead a group discussion in which the instructor acts only as the facilitator. During discussion, students share their answers to the most difficult questions while displaying their problem sets on a document camera.
Assessment
Formative Assessment
Ongoing formative assessment occurs as the instructor circulates through the room during the Lesson, answering student questions. Formative assessment also occurs during the group discussion at the end of the Lesson. Student groups will share their answers in front of the class, including their experimental designs. The group discussion permits the instructor to provide clarification and feedback to the whole class.
Each group will turn in one copy of the problem set for feedback. Students earn full credit if they make reasonable progress on the problem set and offer thoughtful answers. We give full credit for "good faith effort" because we want the Lesson to be a low-stakes assessment. Detailed written feedback is provided by either the instructor or the teaching assistant. We encourage students to take pictures of the written feedback when it is returned to the group, to ensure all students have a record of our comments. In the following class period, frequently-missed questions or common points of confusion are addressed with the whole class. Finally, during the next class session, students are directed to review their graded problems sets and indicate whether they understand the feedback they received. Students are also asked to write down any remaining questions they have about the topic. This presents another avenue for instructors to formatively assess student learning.
Summative Assessment
Summative assessment occurs using matched-pair exam or isomorphic questions (12). On the corresponding exam, students are asked similar but not identical questions based on the problem set (Supporting File S3: MPC – Matched-Pair Exam Questions). The goal of using matched-pair exam questions is to provide students the opportunity to transfer the knowledge they have gained from the Lesson to novel contexts.
Inclusive Teaching
Students work in groups of three and each student is assigned a rotating role: Manager, Recorder, and Presenter. See Lesson Plan below for a full description of each role. These randomly-assigned roles promote inclusivity by allowing all students the chance to contribute in the classroom. Additionally, the collaborative nature of the problem set gives students the chance to share their own ideas about data analysis and experimental design.
LESSON PLAN
Before the Lesson
Student Preparation.
Student pre-reading should be assigned no more than a week in advance of the Lesson. The pre-reading assignment should provide a review of cellular transport mechanisms (diffusion, facilitated diffusion, and active transport). These topics are covered in Alberts' Molecular Biology of the Cell (13) and Lodish's Molecular Cell Biology (14). The National Center for Biotechnology Information Bookshelf has free older versions of both textbooks (https://www.ncbi.nlm.nih.gov/books/NBK21054/?term=alberts; https://www.ncbi.nlm.nih.gov/books/NBK21475/?term=molecular%20cell%20biology). Depending upon the background knowledge of the students, it may be beneficial to provide additional reading on topics students are already expected to know (see Student Prerequisite Knowledge). Pre-reading will allow students to complete the activity within the allotted class time.
Instructor Preparation.
The instructor should prepare to teach the Lesson by reviewing the questions in the problem set carefully (Supporting File S2: MPC – Problem Set Instructor Version). Some students may not be familiar with some of the terminology in the questions. For example, the instructor should be prepared to clarify the difference between a gene that is necessary versus a gene that is sufficient. The instructor should be prepared to explain to students that the drug UK5099 inhibits mitochondrial pyruvate transporters. Instructors may also need to clarify question 4 by describing to students what it means to biochemically reconstitute a protein. For further discussion of each problem, see Problem Set below.
Classroom Environment.
This Lesson works well in a SCALE-UP (Student-Centered Active Learning Environment with Upside-down Pedagogies) classroom (15), but it has also been taught in traditional classrooms. Students will need to work collaboratively in groups of three. As such, it may be necessary to arrange the classroom seating to best facilitate small group discussion. We teach the Lesson in a SCALE-UP classroom where each group of three students has access to one computer. Students can use a personal electronic device to look up terms if they do not have access to a computer. At the end of the Lesson, students share their experimental design with the class on whiteboards or large writing pads. As each group completes their problem set, we ask the Presenters to share their answer to question 4b on whiteboards around the room. It is helpful to use a document camera to best facilitate the group discussion at the end of the Lesson. Students can place their problem sets under the document camera for the entire class to view as they present their answers.
Problem Set
Introduction.
Once all the students have entered the classroom assign students to groups. We prefer to assign groups rather than allow students to choose their own groups so that no student is left out. We randomly assign groups by asking students to count off 1-15 (for a class of 45 students). Each group will have a Manager, Recorder, and Presenter. We assign roles within each group randomly based on birthdays. The Manager is the student with the birthday closest to the date of the class, the Recorder is the student with the birthday next closest, and the Presenter is the student with birthday farthest away. The Manager will keep track of time and ensure everyone contributes to the problem set. The Recorder will write the group's answers on the official copy of the problem set that is submitted at the end of the period for a grade. The Presenter will share their group's answers with the class during the class discussion at the end of the Lesson. We give students copies of our published descriptions of these roles, see Supporting Materials from our previous CourseSource paper (10).
Once group roles have been assigned, introduce the problem set. The problem set contains five questions that ask students to use the results of previous scientific studies to draw conclusions and to design their own experiments to test specific hypotheses. The questions are written so that each group should not need to use the internet to answer the question, although this depends on the background of the students. We ask students not to use their phones during class because phones distract members from interacting as a group. In our experience, even if a student is looking up something related to the problem set, phone usage promotes "parallel play" rather than collaboration because it is difficult for all group members to see the small screen. Give the class approximately 45-50 minutes to complete the problem set. Write the time that students need to be finished on the board. Remind students that the when their group is finished the Presenter should write their answer to Question 4b on a whiteboard.
Problem Set.
Students will begin working on the questions after the introduction (Supporting File S1: MPC – Problem Set Student Version). Distribute copies of the problem set to each student. Although only one group copy will be submitted for grading at the end of the Lesson, all students should write answers on their own copy of the problem set during collaboration so they stay actively involved. Circulate throughout the room to answer any questions the student groups may have. We use questions to help guide students to the correct answer as opposed to telling them answers directly. It is also helpful to provide students with time updates to keep groups on-task and on-time for the group discussion.
- Question 1 introduces the context for the problem set by providing a summary of how mitochondrial pyruvate carrier (MPC) proteins were discovered. The terms integral membrane proteins, inner mitochondrial membrane, and the basics of methodologies such as purification and mass spectrometry should be familiar to upper-division cell biology students. We encourage students who need a refresher to look up terms they do not remember on the group computer rather than on a personal electronic device. The question then asks students to recall what types of molecules require a transport protein to cross a membrane. Students should notice that pyruvate is relatively large and carries a charge, which makes it unlikely to pass through a membrane without a transport protein.
- Question 2 requires student to interpret data from MPC single and double mutant yeast strains and compare it to data from a wild-type yeast strain to determine which MPC proteins are necessary for pyruvate uptake. Background information about what it means for a protein to be necessary and sufficient in cell biology is provided in a textbox. To answer Question 2a, students may need to make notes on the graph about which symbol represents which mutant. We want them to see that data published in top journals like Science may have graphs that are not easy to read, but that they can still extract information from them. In response to Question 2b, some students will assume that conclusions about sufficiency can be made from these data. We ask them what the textbox says about testing for sufficiency, and, if needed, remind them that sufficiency cannot be tested in a system that already has the function of interest. An experiment to test sufficiency of MPC proteins is presented in Question 3.
- Question 3 asks students to interpret pyruvate import data from bacteria expressing mouse MPC genes. Students should recall key fundamental differences between eukaryotes and prokaryotes, especially that prokaryotes lack membrane-bound organelles. Question 3a requires students to explain why researchers expressed MPC genes in prokaryotic cells and not eukaryotic cells. Using bacteria allows the researchers to make conclusions about sufficiency because prokaryotic cells do not have mitochondria, and they do not have pyruvate mitochondrial transporters. In Question 3b, students analyze pyruvate import data to see if expression of mouse MPC1 and/or mouse MPC2 is sufficient for high levels of pyruvate uptake into bacterial cells. Question 3c explains what the effect of the inhibitor UK5099 is on cells and asks students what conclusions can be made knowing the effect of the inhibitor in eukaryotes, and the observed effect in the bacterial cells expressing mouse MPC proteins. Students may need help realizing the positive control demonstrates that the pyruvate transport in bacteria is functioning similarly to what is seen in eukaryotic cells. Question 3d asks students to explain why pyruvate import increased in the prokaryotic cells when the extracellular pH dropped from 7.2 to 6.2. This question requires students to connect their pre-existing knowledge about transport mechanisms across membranes to experimental data. Students should recall that a lower extracellular pH translates to increased H+ ions outside the cell. The increase in [H+] outside the cell could help power transport across the cell membrane through a variety of possible mechanisms such as symport of [H+] with pyruvate. Some students will hypothesize that pyruvate is protonated under these conditions, in which case we tell them that the pKa of pyruvate is much lower than pH 6.2.
- Question 4 provides a basic experimental system to test different types of transport across membranes and asks students to design their own experiment. Question 4a asks students to compare and contrast diffusion, facilitated diffusion, and active transport. This is a review based on the pre-reading assignment. Question 4b requires students to design an experiment using the context presented in the question to test whether diffusion, facilitated diffusion, or active transport is occurring in the described system. Sometimes students are unsure about what it means to "biochemically reconstitute" proteins. In this question, it means that the MPC proteins, which are integral membrane proteins, are incorporated into the bilayer of the lipid vesicles (or liposomes). We remind students to design an experiment that makes sense given what they know about biology. The Presenter from each group should use a white board to share the group answer to Question 4b.
- Question 5 transitions from model systems to mitochondrial disease in humans. Students will learn about a disease resulting from defects in mitochondrial pyruvate oxidation linked to mutations in the human MPC1 gene. Pedigrees from three different families affected by this disease are presented, along with descriptions of the mutations in the MPC1 gene. Question 5a asks students to determine which human MPC1 mutation is most deleterious. A key is provided to help students interpret the pedigree symbols. Question 5b asks students to explain why one type of mutation can be more detrimental to normal protein function than another type of mutation. Question 5c asks students to conclude whether the data presented in the pedigrees supports the hypothesis, "MPC1 encodes a gene involved in pyruvate import in mitochondria." To answer this question, students may need to recall that pyruvate oxidation occurs inside the mitochondria. Finally, Question 5d asks students to briefly design an experiment to test the hypothesis that human MPC1 encodes a protein involved in pyruvate transport. Some students may be unsure about how to design an experiment to test this hypothesis. In those cases, we suggest that students review the experiments they have already read about in the problem set for ideas. (The experimental design used in Question 3 would work well.)
Group Discussion
A few minutes before the time deadline, ask students who have not posted their answers on the whiteboard to Question 4b to do so. Once the deadline has been reached, you can ask for Presenters to volunteer to share their groups' answer for the more challenging questions. Depending on the amount of time, we ask for one or two Presenters to report on Question 2, Question 3, and Question 4b. Not all groups will finish Question 5, in which case, we ask them to do this as homework. We use a random number generator to select a group, then that group's Presenter shares the group's answers to a specific question. Presenters may use a document camera to project their group's answers if available. After students share their group answers, the instructor asks other groups to add or edit the first student's response until consensus is reached. The instructor should take opportunities to mention key components of the scientific process for each question. Once the questions have been discussed as a class, you can revisit the learning objectives of the Lesson to provide a conclusion to the Lesson if time allows. Collect the Recorders' copies of the completed problem set for assessment.
Next Class Period
During the next session of the class, students will answer two debriefing questions after receiving feedback on their problem set answers (Supporting File S1: MPC – Problem Set Student Version). These questions encourage students to read the feedback and identify any areas of confusion from the Lesson. This is another opportunity to collect formative assessment regarding the activity.
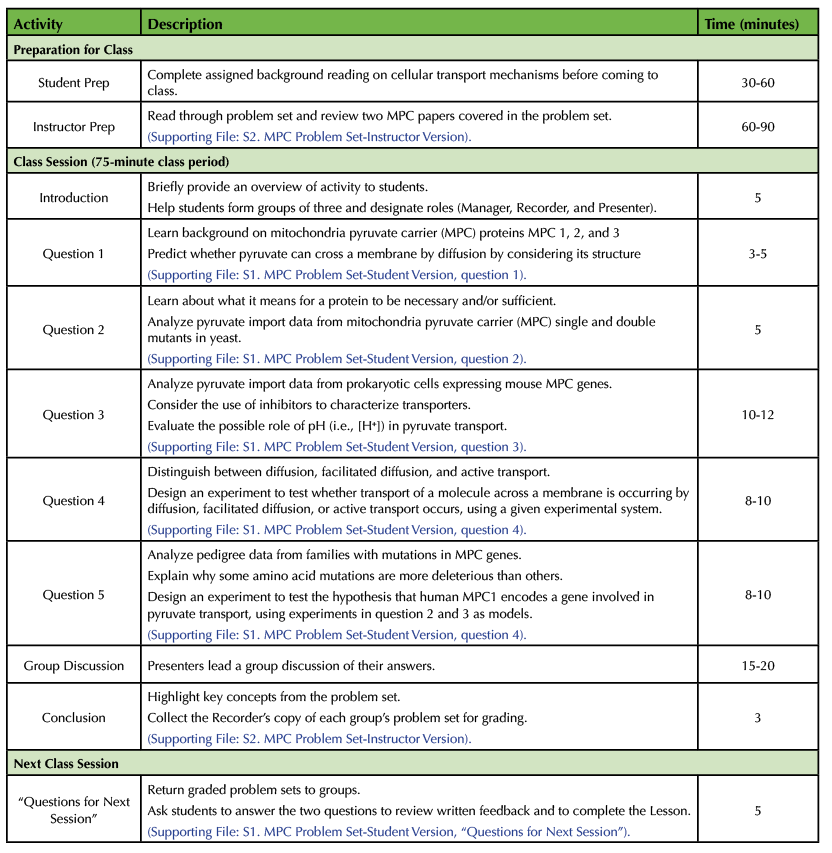
Table 1. Lesson Plan and Teaching Timeline for “Necessary and Sufficient? Solving the Mystery of the Mitochondrial Pyruvate Transporter”
TEACHING DISCUSSION
Effectiveness in Achieving Learning Goals and Objectives
The activity effectively meets the stated learning goals and objectives. Students perform well on summative assessment related to this Lesson. When we have given matched-pair questions on the corresponding unit exam, nearly all students are able to correctly interpret graphs with data from transport mutants. Most students are able to correctly determine whether proteins are necessary or sufficient for transport. The majority of students can also apply their understanding of pyruvate transport to a more challenging question that asks them to explain the effect of an ionophore on pyruvate transport. Student have also performed well when we have given matched-pair questions on a cumulative final exam or an in-class assessment three months after completing the Lesson. Yet some student confusion is revealed about what type of experiment allows a scientist to make conclusions about sufficiency of a protein or proteins for transport. This suggests that time should be spent revisiting the concept of how to test for sufficiency in order to promote long-term understanding.
Student and Instructor Reactions to the Lesson
Students respond positively to the Lesson based on classroom observation by multiple instructors and student participation during class discussion. Instructors who have taught the Lesson note that students struggle at first to understand the difference between necessity and sufficiency, but they become more comfortable after answering the questions and hearing the class discussion. Students seem to be pleased when these concepts finally "click." Students also appear excited to analyze the pedigree data and some students have explained that this is because they appreciate the connection between pyruvate transport and human health. Students seem to enjoy applying their prior knowledge of pedigree analysis, which they learned in the pre-requisite Genetics course, during the Lesson. When we've taught the Lesson in our Cell Biology course, our students readily volunteer to share their answers with the class.
Colleagues who've taught this Lesson report their satisfaction with it as well. They mention their appreciation of the way the questions build from simple to complex concepts, which helps students work in groups without much facilitation and with less frustration. They note that the scaffolding is especially important when the Lesson is being taught by only one instructor without the help of a teaching assistant. Colleagues also appreciated the way questions give students multiple opportunities to practice analyzing real data and considering experimental design. They note that their students can design experiments to test a specific hypothesis related to transport once they understand the way scientists have tested similar hypotheses. Nearly all the colleagues who have taught the Lesson once continue to use it in their subsequent Cell Biology courses.
Suggestions for Possible Adaptations
The Lesson can be adapted for other classes and groups of students. The Lesson could be used in a genetics or biochemistry course if cellular transport is covered. Different levels of students may also find this Lesson effective. Students without a background in genetics and biochemistry may need help with some key concepts used in this activity. For example, students need to be familiar with wild type versus mutant genes and recessive versus dominant traits. Students must also be familiar with the potential impacts amino acid mutations have upon protein function. Finally, students need to be aware of key differences between prokaryotic and eukaryotic cells. If using this Lesson in a class without this background knowledge, it may be helpful to assign additional pre-reading, increase the time students have to complete the activity, and encourage them to use outside resources to look up terms, like the internet or textbooks.
The Lesson can be adapted for larger or smaller groups of students. POGIL is designed for use in large lecture formats and this particular activity has been used in a class as large as 135. It is more difficult for the instructor to interact with individual groups in larger classes, but the questions are written so that each question builds on the knowledge from the question before it. It would be appropriate in such a setting to ensure that students were still able to share their answers during a group discussion. Instead of using white boards, large poster boards could be used. It is also possible to complete this Lesson in 50 minutes instead of 75 minutes. This can be done by assigning Questions 1 and 4a as homework to complete before arriving to the class session.
SUPPORTING MATERIALS
- S1. MPC – Problem Set Student Version. A copy of the student version of the problem set is given to all students to work on during the Lesson.
- S2. MPC – Problem Set Instructor Version. The instructor version of the problem set contains a key with possible answers to all questions.
- S3. MPC – Matched-Pair Exam Questions. These questions are similar to but different than the problem set questions and can be used for summative assessment.
ACKNOWLEDGMENTS
We thank Dr. Marcus Fechheimer for suggesting the two seminal 2012 Science papers for incorporation into a Cell Biology lesson and for providing feedback on the first draft of this problem set. We thank Dr. Janet Iwasa for generously giving us written permission to use her mitochondrion illustration for this paper.
References
- Harrison E. 2018. Role-Playing Activity to Demonstrate Diffusion Across a Cell Membrane. Journal of Microbiology & Biology Education 19.
- McDonald KK, Gnagy SR. 2015. Lights, Camera, Acting Transport! Using role-play to teach membrane transport. CourseSource. https://doi.org/10.24918/cs.2015.12.
- York DW. 2011. Newsflash! Transport Proteins on Strike! National Center for Case Study Teaching in Science.
- Leone FA, Furriel RP, McNamara JC, Horisberger JD, Borin IA. 2010. Cation transport coupled to ATP hydrolysis by the (Na, K)-ATPase: An integrated, animated model. Biochemistry and Molecular Biology Education 38:276-279.
- Armstrong N. 2010. Agony and Ecstasy: A Case Study on Cell Membrane Structure and Function. National Center for Case Study Teaching in Science.
- Braga VA. 2011. Teaching the renal tubular reabsorption of glucose using two classic papers by Shannon et al. Advances in physiology education 35:114-116.
- Moog RS, Spencer JN, Straumanis AR. 2006. Process-oriented guided inquiry learning: POGIL and the POGIL project. Metropolitan Universities 17:41-52.
- Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen Y-C, Cox JE, Cardon CM, Van Vranken JG, Dephoure N. 2012. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 337:96-100.
- Herzig S, Raemy E, Montessuit S, Veuthey J-L, Zamboni N, Westermann B, Kunji ER, Martinou J-C. 2012. Identification and functional expression of the mitochondrial pyruvate carrier. Science 337:93-96.
- Stanton JD, Dye KM. 2017. Investigating the Function of a Transport Protein: Where is ABCB6 Located in Human Cells. CourseSource. https://doi.org/10.24918/cs.2017.19.
- Bender T, Martinou J-C. 2016. The mitochondrial pyruvate carrier in health and disease: to carry or not to carry? Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1863:2436-2442.
- Smith MK, Wood WB, Adams WK, Wieman C, Knight JK, Guild N, Su TT. 2009. Why peer discussion improves student performance on in-class concept questions. Science 323:122-124.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. 2002. Molecular biology of the cell. new york: Garland science; 2002. Classic textbook now in its 5th Edition.
- Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. 2000. Molecular cell biology 4th edition. National Center for Biotechnology Information Bookshelf.
- Beichner RJ, Saul JM, Abbott DS, Morse JJ, Deardorff D, Allain RJ, Bonham SW, Dancy MH, Risley JS. 2007. The student-centered activities for large enrollment undergraduate programs (SCALE-UP) project. Research-based reform of university physics 1:2-39.
Article Files
Login to access supporting documents
Necessary and Sufficient? Solving the Mystery of the Mitochondrial Pyruvate Transporter(PDF | 2 B )
S1 MPC Problem Set Student Version Final_SE JDS 051420.docx(DOCX | 42 KB)
S2 MPC Problem Set Instructor Version Final_SE JDS 051420.docx(DOCX | 46 KB)
S3. MPC Matched-Pair Exam Questions.docx(DOCX | 69 KB)
- License terms

Comments
Comments
There are no comments on this resource.