Using Immunocytochemistry and Fluorescence Microscopy Imaging to Explore the Mechanism of Action of Anti-Cancer Drugs on the Cell Cycle
Editor: Stanley Lo
Published online:
Abstract
Understanding and visualizing the cell cycle and mitosis is an essential concept of cell biology. This multi-week lab module has students investigate the mechanism of action of anti-cancer drugs that disrupt activities associated with mitosis and the cell cycle. Students use modern cell biological techniques such as immunocytochemistry and fluorescence microscopy imaging to visualize the inner workings of mammalian cells and determine the mechanism of drug action. Instructors culture and fix PtK2 Potorous tridactylis (rat kangaroo) kidney epithelial cells in 4-well chamber slides. Two wells serve as untreated controls and the other two wells are treated with the same anti-cancer drug: vinblastine or taxol. We do not tell students which drug their cells were treated with or its mechanisms of action. Student groups stain the cells in all four wells with fluorescence markers for chromosomes and two types of cytoskeletal proteins: actin and tubulin. Each group has access to a fluorescence microscope for at least one hour for cell imaging and data collection. Data is analyzed to compare the following in control and drug-treated wells: 1) the numbers of cells in various mitotic stages, 2) the number of apoptotic cells, 3) the drug's effects on tubulin and actin organization. In an oral presentation, each group uses their data to justify their proposed mechanism of action of their drug. We used a pre/post-test to assess the impact of this lab module on students' attitudes as well as their understanding of basic light and fluorescence microscopy, mitosis, and immunocytochemistry.
Citation
D’Costa AR, Barnes DW, Barrera A, Hurst-Kennedy J, Hammonds-Odie L. 2020. Using immunocytochemistry and fluorescence microscopy imaging to explore the mechanism of action of anti-cancer drugs on the cell cycle. CourseSource. https://doi.org/10.24918/cs.2020.12
Society Learning Goals
Cell Biology
- Cytoskeleton Structure and Function
- How do the different components of the cytoskeleton support a variety of cell functions, such as cell shape, division, movement, sensing the environment, and cell-cell communication?
- Cell Cycle and Cell Division
- How do cells conduct, coordinate, and regulate nuclear and cell division?
- Methods & Tools of Cell Biology
- How do the methods and tools of cell biology enable and limit our understanding of the cell?
Lesson Learning Goals
Learning goals based on ASCB Cell Biology Learning Framework.
Topic: Cytoskeleton Structure and Function
I) Learning Goal: How do the different components of the cytoskeleton support a variety of cell functions, such as cell shape, division, movement, sensing the environment, and cell-cell communication?
Topic: Cell cycle and cell division
II) Learning Goal: How do cells conduct, coordinate, and regulate nuclear and cell division?
Topic: Methods & Tools of Cell Biology
III) Learning Goal: How do the methods and tools of cell biology enable and limit our understanding of the cell?
Lesson Learning Objectives
Students will:
- name and describe the changes to chromosomes and cytoskeleton during each stage of mitosis.
- compare the usefulness and limitations of information obtained by light microscopy and fluorescence microscopy.
- quantify, analyze and summarize their data on the prevalence of cells at different stages of cell division in randomly sampled cell populations.
- describe how cell imaging is used to collect and analyze data on dynamic cellular events.
- present their scientific data in an appropriate and accurate way to an audience.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
The field of Cell Biology focuses on the structure and inner workings of cells. Mitosis, the process by which the nucleus of somatic cells divide, is an essential topic in Cell Biology, and is fundamental to growth, development and tissue repair. Students are introduced to mitosis in introductory biology courses, where they learn the phases and the reorganization of chromosomes and cytoskeleton during each phase. As they move to upper-level Cell Biology courses, the curriculum focuses more in depth on the regulation of cell division and how defects in regulation may lead to cancer.
In most traditional undergraduate labs, mitosis is studied by having students use compound light microscopy to view prepared slides of onion root and fish blastula. The cells are usually stained with a single dye to highlight the chromosomes, devaluing the role of other necessary components such as the cytoskeleton. More advanced labs are inquiry-driven and involve students treating cultured mammalian cells with various chemicals, house-hold agents or anti-cancer drugs. Students determine the effect of these agents on cell proliferation using cell viability assays with hemocytometers, microplate readers and photomicroscopy methods using Motic software (1-4).
Fluorescence microscopy and fluorescent markers have enabled cell biologists to tag and illuminate the chromosomes, microtubules, and actin filaments of dividing cells, producing images with high contrast and fine detail. Virtual labs that use research-quality digital images from free public databases have been developed to help students visualize the dynamic process of mitosis and to teach them how data on mitotic cells can be collected and analyzed (5). Students, however, have no idea how these images were obtained, the difficulties with imaging cells, nor the process of staining cells.
Very few cell biology labs provide undergraduates with the opportunity to capture and analyze their own stained cells with a fluorescence microscope. One of most likely reasons being the cost of these microscopes (see Discussion). In an advanced cell biology course, students stained normal and anti-cancer drug-treated cultured Chinook salmon embryo cells (CHSE-12 cells) for DNA, actin, and tubulin. They then used research-grade fluorescence microscopes to capture and analyze their own images to determine the effect of the treatments on the cytoskeleton (6). Another cell biology course had students dissect, fix and stain Drosophila embryos with fluorescent markers for actin and DNA (7).
The goal of this multi-week lab module is to excite students about the field of cell biology by helping them understand how modern cell biological techniques can be used to investigate the effect of anti-cancer drugs on cultured mammalian cells. Cancer is a topic that impacts the lives of most people either directly or indirectly; therefore, this investigation of drugs used in chemotherapy is very engaging to students (www.chemocare.com). In this lab, students use immunocytochemistry to stain cells with fluorescent markers for chromosomes and cytoskeletal elements. Next, they use a fluorescence microscope to visualize the dynamic changes as the cells go through the stages of mitosis, and capture their own images. Finally, they determine the mechanism by which their anti-cancer drug blocks cell division by comparing the number of mitotic and apoptotic cells, and the organization of the cytoskeleton in normal and drug-treated cells.
Two specific tools make these labs student-friendly and suitable for the cell biology teaching lab: the mammalian cell line used are kidney epithelial cells derived from an Australian rat kangaroo, Potorous tridactylus. PtK2 cells were the first marsupial cell line and have a small number of large chromosomes that can easily be visualized. This helps students identify and count the number of cells in various mitotic phases. In addition, these cells undergo mitosis while adhered to the glass slide so preparation for immunocytochemistry and analysis is easier. Inverted fluorescence microscopes of any type can be used for these labs, however, a student-friendly EVOS cell imaging system (Thermo Fisher Scientific) was purchased. Unlike most fluorescence microscopes that must be placed in a dark room, this three-channel microscope uses a novel, mercury-free, short light path LED illumination design that allows it to be placed in a cell biology teaching lab. And, the conventional oculars are replaced by a large viewing screen on the EVOS, so images are accessible to multiple students simultaneously (Figure 1).

Figure 1: The EVOS inverted fluorescence microscope. Note the oculars are replaced by a large computer screen which enables multiple students to view the image.
In preparation for this lab, PtK2 cells are cultured and fixed on the bottom of a 4-well chamber slide (Figure 2) by the course instructors. Two wells are treated with the same anti-cancer drug (taxol or vinblastine), and the other two wells serve as untreated controls. Each group of four students gets one 4-well slide with a drug treatment unknown to them. The students stain the cells in all 4 wells with fluorescent markers for DNA, tubulin and actin. DAPI (4′,6-diamidino-2-phenylindole), is a blue fluorescent stain that stains DNA in interphase and mitotic chromosomes. Tubulin, a component of microtubules that make up the mitotic spindle is stained green by a fluorophore-conjugated anti-tubulin antibody. Actin filaments are stained red with phalloidin, a fluorophore-conjugated fungal toxin that binds actin filaments.
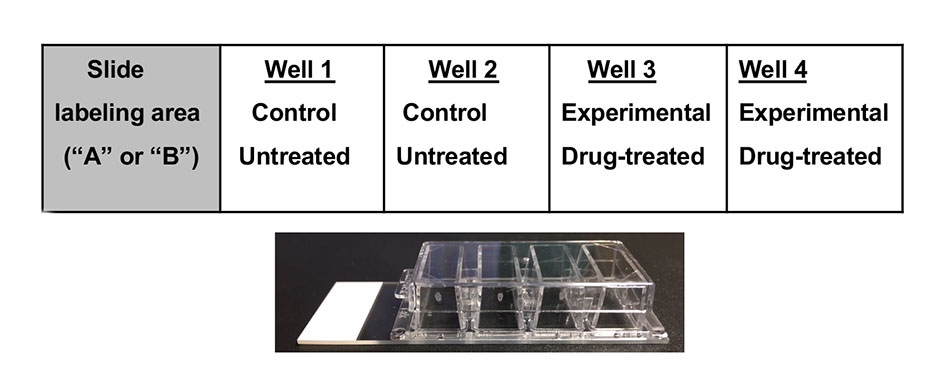
Figure 2: Experimental set up in the 4- well chamber slide. Slides labelled “A” treated with taxol, slides labelled “B” treated with vinblastine.
Following staining, each group gets at least one hour at the EVOS to examine and capture images of cells in untreated and drug-treated wells. The stains can be viewed individually, or together using the "overlay" function on the EVOS. By focusing through the depth of the cell, students can appreciate the three-dimensionality of cells. Students are able to visualize the organization of actin, microtubules and chromosomes in interphase, and see how they change as the cell progresses through each mitotic phase.
Students use their captured images to count the number of mitotic cells and apoptotic cells and to observe the effects of the drug on the organization of microtubules and actin. Students use critical thinking and their knowledge of cell biology to look for differences in staining patterns and devise hypotheses for the mechanism of action of their drug on the PtK2 cells.
Intended Audience
This lab is designed for a sophomore/junior-level cell biology course, required for all biology majors. This module has been tested over eight semesters each with 2-6 course sections of Cell Biology with Lab (BIOL3400K) at Georgia Gwinnett College, and apprioximately 660 students have done this lab.
Required Learning Time
This 6-week module requires one 2.45-hour lab session per week (Table 1). If there are at least three fluorescence microscopes for the students to use, the time for the module can be reduced to five weeks by reducing the data acquisition lab periods from two to one, or by asking students to complete the data analysis as homework.
Prerequisite Student Knowledge
Students must have an introductory college biology course-level knowledge about the cell cycle, mitosis and light microscopy.
Prerequisite Teacher Knowledge
The teacher should have knowledge of the cell cycle and stages of mitosis, including details of structural changes to chromosomes and the cytoskeleton. The teacher should have experience in mammalian cell culture, immunocytochemistry, fluorescence microscopy, and cell imaging techniques. Moreover, the teacher should have access to a cell culture facility. If there is a lack of staff to maintain cell cultures and prepare reagents, then the teacher can train upper-level students to assist in the preparation of the fixed cells on the 4-well chamber slides. (S1. Using Immunocytochemistry – Instructor prep slide and reagents)
SCIENTIFIC TEACHING THEMES
Active Learning
Students are actively learning in group discussions as they brainstorm to remember and draw the stages of mitosis, compare light versus fluorescence microscopy, and develop hypotheses. Students learn hands-on techniques like immunocytochemistry, and how to use a fluorescence microscope to collect and analyze images of cells. In a final oral presentation, students must then use their knowledge of cell biology and their data to explain how their findings support their hypotheses on the mechanism by which their drug is blocking cell division.
Assessment
The major assessment for this lab module is a group oral presentation. Depending on instructor preference, the oral presentation counts for 20-30% of their total lab grade. (Rubric found in S6. Using Immunocytochemistry – Lab Sessions 3-6 Student protocol). Minor assessments include lab quizzes (each 3-4% of lab grade) and a "Participation" grade (10% of lab grade) for attendance and teamwork. Some faculty assess teamwork by asking students to complete a "Peer Review" sheet at the end of the semester. (S11. Using Immunocytochemistry – Peer Review).
A pre/post assessment survey with institutional IRB approval is administered (S9. Using Immunocytochemistry – Assessment Questions Microscopy and Attitudes; Georgia Gwinnett College IRB #110068). The survey addresses content and skills in conventional and fluorescence microscopy (17 questions), and student attitudes (10 questions). The questions ask students to record their perception of their abilities to understand the concepts and implement the techniques to which they were exposed, and their confidence in their ability to use this knowledge in addressing research-based questions. They were asked to give their impressions of the value of inclusion of the lab module in the Cell biology labs in particular, and its contribution to their college experience, in general.
Inclusive Teaching
Students work in groups of 3-4 for the entire 6 weeks. Depending on the instructor, students can self-select their groups, be randomly placed, or assigned groups to include students from diverse (race, gender, and ethnicity, completed courses) backgrounds. The design of this module facilitates that each group member must contribute and acquire skills to work effectively in a team and accomplish the set tasks within a defined time period. They learn to support each other and work as a team while brainstorming, designing experiments, during the hands-on parts of the lab and for their oral presentation. As the instructor, we ensure that every group member is engaged – in discussing, micropipetting, capturing images, analyzing data, and preparing the final presentation – by monitoring student interactions. We discourage student isolation.
During class discussions, some students might wonder why cancer cells are not being used. One strategy is to inform students about HeLa, an immortalized cell line, derived from the cervical cancer of an African-American woman, Henrietta Lacks. HeLa cells are used for biomedical research world-wide and have been taken into space. Students can be encouraged to read "The Immortal Life of Henrietta Lacks" by Rebecca Skloot (8). HeLa cells would have been ideal for this module. However, during our initial testing, we found the HeLa cells would detach from the slide as they divided. Thus, only a few cells in mitosis are observed. In contrast, the PtK2 cells remain attached during cell division and all mitotic stages can be observed.
LESSON PLAN
Lab Session 1: Introduction to Microscopy
Introduction and mitosis review:
Begin the lab session by reviewing the phases of the cell cycle with students (S2. Using Immunocytochemistry – Lab Session 1 Lecture slides) One option is to ask students what they remember about cell division and mitosis. Another option is to direct them to form groups of 3-4 students and sketch a cell undergoing mitosis on small whiteboards. The student groups would eventually be allowed to compare their drawings to the images in their textbook and/or on websites.
Then, the discussion would transition to incorporate prior knowledge of chemotherapeutic drugs. Question prompts that may be asked are: Why do we use chemotherapeutic drugs to fight cancer? How do these drugs work? How exactly, or by what molecular mechanism do these drugs stop cell division? The instructor would then explain that: Cancer is the result of uncontrolled cell division. Cancer treatment options include targeted radiation and a cocktail of chemotherapeutic drugs. The goal of both treatments is to block cell division.
Then using a large display of the cell cycle, ask the student groups to hypothesize how the drugs could work to stop cell division. End the opening discussion by pointing to various points along the cell division pathway where a drug could act to arrest cell division.
Experimental design:
Inform students that each lab group will receive cells treated with a different anti-cancer drug. Ask each group to discuss how they will design a controlled experiment to determine whether their drug blocks cell division. They must define their experimental (drug-treated) and control (untreated) groups, the independent (drug), and dependent (how they will observe or measure the effect of drug treatment), and controlled (same amount of cells/same growing conditions for both experimental and control groups) variables.
Focus the discussion on how the students will measure the effect of the drug in stopping cell division. At this point, inform students that they will use microscopy to compare the cells in both groups. Will there be a difference in the number of mitotic cells, or apoptotic cells (cells undergoing programmed cell death)? Now that several dependent variables have been defined, ask them to develop a hypothesis and prediction. An example hypothesis could be "Cells treated with a chemotherapeutic drug will undergo less cell division." An example prediction could be "Fewer cells will be in mitotic stages when the cells are treated with a chemotherapeutic drug than when the cells are not treated."
Basic microscopy:
MicroscopyU (https://www.microscopyu.com/microscopy-basics) is a good website for review of basic concepts in microscopy. If available, use compound light microscopes and prepared mitosis slides of onion root and fish blastula. Show images of cells taken using a compound and fluorescence microscope and ask students to compare them. Students should notice that the fluorescence images are brighter and show the precise location of cell components. Because the background is black, there is a greater contrast between the fluorescently labeled structure and background, making it possible to see structures that appear faint when using regular light optics.
Principles of fluorescence microscopy:
Information for this section is found in S3. Using Immunocytochemistry – Lab Session 1 Fuorescence microscopy introduction. Fluorescent molecules (termed "fluorophores' or "fluorochromes") absorb light of one particular wavelength (the excitation wavelength) and then re-emit light at a longer, lower-energy wavelength (the emission wavelength). By using filters specific for the excitation and emission wavelengths, the excitation light can be eliminated from the image, leaving only the emitted, fluorescent light. By eliminating the background light, fluorescently stained objects stand out markedly in the image because they are contrasted against a black background. One must be careful to limit the time of exposure of the sample, however, because the intense light beams used to excite fluorescence can generate oxygen radicals causing the fluorescence to be destroyed and the image to fade irreversibly (called photobleaching).
Fluorescence microscopy can allow the precise localization of individual types of molecules within a cell. This is accomplished by linking a fluorophore to a compound that shows specificity for binding to a particular "target" cellular molecule or structure. Thus, the target will appear fluorescent when the fluorophore is appropriately "excited" in the microscope, producing striking images that specifically localize the target within the cell. The process you will use is referred to as "direct immunofluorescence" because it involves the use of fluorescently-labeled antibodies labeled directly with the fluorophore. For example, DAPI is a blue-fluorescent stain for DNA in interphase and mitotic chromosomes. Phalloidin is a fungal toxin that specifically binds filamentous actin. When phalloidin is conjugated to a fluorophore and introduced into a properly treated cell, actin filaments will "glow" in the microscope image, due to the fluorescence emitted by the associated fluorophore.
Another class of very commonly used fluorescently tagged molecules are antibodies. The extreme specificity of antibodies for their particular targets allows localization of their targets at the molecular level. For instance, we know that, during mitosis, chromatids associate with spindle fibers composed of tubulin. In this module, you will use a fluorescently labeled antibody to tubulin to identify spindle fibers and other tubulin-containing structures in the cell.
Teach students how to use the inverted fluorescence microscope:
Students view purchased prepared slides of fluorescently stained cells and identify cellular structures. Ask students to compare the design of a compound light microscope and an inverted fluorescence microscope. Specifically, if using EVOS, the ocular lenses are replaced by a large computer screen. Most importantly, point to the location of the objectives under the stage in an inverted scope. Use of an inverted scope is optimal as the PtK2 cells are attached to the bottom of the slide and reducing the distance between the cells to be imaged and the objectives increases resolution capability.
The PtK2 cell line:
Potorous tridactylus kidney epithelial cells were derived from an Australian rat kangaroo. They were the first marsupial cell line and have a small number of large chromosomes that can easily be visualized. The cells undergo mitosis while adhering to the bottom of a culture flask or glass slide. This website has beautiful photographs of PtK2 cells: https://micro.magnet.fsu.edu/primer/techniques/fluorescence/gallery/cells/ptk2/ptk2cells.html
Discuss the actual experimental design along with a six-week timeline (Table 1):
Each group of students gets a 4-well chamber slide in which PtK2 cells have been cultured. All wells have approximately the same amount of PtK2 cells and were grown under the same conditions in the same culture media. After the cells were grown for a few days, two wells are treated with the same drug (either A = taxol or B = vinblastine, Figure 2).
In lab session 2, students stain the cells for DNA, microtubules, and actin. In lab sessions 3 and 4, each group spends at least one hour at the fluorescence scope to examine and capture images of the stained cells in untreated and drug-treated chambers. In lab session 5, students will collect and analyze data from their cell images. Lab session 6 is set aside for group oral presentations. Each group gives an oral presentation of their data supported by photo-documentation of their observations and includes discussions aimed at identifying their drug and its mechanisms of action.
Table 1. Using Immunocytochemistry: Teaching Timeline
Lab Session 2: Staining Chromosomes, Actin, and Microtubules in Cells.
Quiz and experimental design review:
Information for this section is found in S4. Using Immunocytochemistry – Lab Session 2 Quiz and S5. Using Immunocytochemistry – Lab Sesssion 2 Student protocol. After reviewing microscopy with a short quiz, spend a few minutes reviewing experimental design using the chamber slide (Figure 2). Each group is provided with one 4-well chamber slide in which two wells are treated with the same drug. Slides treated with taxol are labeled A; slides with vinblastine labeled B. Instructors should make a note of which drug each group received to verify and discuss results at the end. Students can use light microscopy to view their cells adhered to the bottom of the chambers. The cells should have attained close to 100% confluency.
Important advice before staining cells:
The staining must be completed in this lab session. Dim the lights and cover the slides with an aluminum foil tent during incubations and washes to prevent quenching of fluorophores. Students should wear lab gloves and proper lab attire. Instruct students to pipette in the same "location" in the well to limit scraping or shearing of cells. Warn students that the cells should be covered in liquid so that the cells do not dry out.
Staining cells:
Note there are two options for DAPI staining. DAPI staining can be done directly after the blocking step as part of the pre-module preparations, or DAPI can be added as part of the mounting media by the students.
Each group will receive a chamber slide of PtK2 cells. Staining for tubulin involves adding 0.4 mL of a 1:400 dilution of dye-labeled anti-tubulin in blocking buffer to each well and incubating for 40 minutes. This is followed by three 5-minute washes with PBS. Next 0.4 mL of 1:40 dye-labeled phalloidin in blocking buffer is added to each well and incubated for 20 minutes. This is followed by three 5-minute washes with PBS. Phalloidin and subsequent wash solutions must be disposed of as hazardous waste (view Safety in S1. Using Immunocytochemistry – Instructor prep slide and reagents).
How are cells prepared for staining:
During the incubation periods, inform students that following drug treatment, the cells were fixed, permeabilized, and blocked. For cell fixation, formaldehyde which crosslinks amino acids and DNA and "freezes" them to preserve them in a life-like state is used. Triton X-100 detergent which solubilizes membrane lipids is used to permeabilize the fixed cells. Permeabilization is necessary as the fluorophore-tagged phalloidin and tubulin antibodies are too large to enter the cells.
Even though the phalloidin and tubulin antibodies are designed to specifically target actin and tubulin within the cells, some non-specific binding can occur and could result in fuzzy images. To avoid this, protein binding sites in the fixed, permeabilized cells must be blocked before staining. Cells are treated with blocking buffer with bovine serum albumin (BSA) that coats these potential non-specific protein binding sites and and will help ensure the fluorescent-tagged molecules with higher binding affinities will bind to their intended target. Phosphate buffered saline, PBS, helps maintain cells at physiological conditions (pH and salt concentration).
Mounting the slide:
Following staining, the slide is coated with purchased mounting media and a coverslip to protect the specimen. This step must be done as quickly as possible to prevent the cells from drying out. You might have to demonstrate for the students. Remove the PBS in the wells. Carefully remove the hard-plastic chamber walls from the glass slide. The walls are attached to the slide by a soft rubber gasket. Using tweezers and scalpel will help with wall removal. Gently tap the side of the slide on a paper towel to remove excess liquid. Place the slide on a paper towel. Add 25 microliters of mounting medium to the center of each of the four areas where the four wells were situated (100 microliters of mounting medium total). The mounting media retains bubbles easily so pipette carefully to avoid bubbles. Burst any bubbles using a clean pipette tip. Carefully place a single, large rectangular coverslip on the entire glass slide. Ensure no bubbles are trapped between the coverslip and the slide. Seal out the air by adhering the coverslip to the slide by painting a thin layer of clear nail polish on all the sides. Cover the slide with foil while the nail polish dries for 15 minutes. Store the slide in a light-impermeable container in the refrigerator until the next lab session.
Lab Sessions 3 & 4: Acquisition of Cell Images
A single fluorescence scope is used by all six lab groups. Each group gets approximately 1 hour at the scope. Three groups acquire images in session 3 and the remaining three in session 4. On the days groups do not have access to the scope, students use the time to work on their presentations and other classwork. Students are asked to bring in their own USB thumb drives to collect and store their images.
Capturing images:
Data collection includes students capturing images of cells in drug-treated and untreated wells. Students must collect images from 5 distinct FOV (Field of View) in untreated wells and 5 FOV in drug-treated wells. First, focus the slide to 10X, look for an area that has many cells, then move to the 40X objective for data collection. For each FOV, capture and save a DAPI image using the DAPI channel (blue). Then for the same FOV, capture an "overlay" image of all three channels, DAPI (blue), phalloidin (red) and tubulin (green). A total of 20 images should be collected for data analysis in lab session 5 (S6. Using Immunocytochemistry – Lab Sessions 3-6 Student protocol, S7. Using Immunocytochemistry – Instructions for EVOS microscope).
Lab Session 5: Data Analysis and Experimental Conclusions
Students use the worksheet S6. Using Immunocytochemistry – Lab Sessions 3-6 Student protocol as a guide for data analysis. Student groups gather their data by counting and comparing the total number of cells, cells in various mitotic stages, and the number of apoptotic cells in drug-treated and untreated cell images. Point to the use of duplicate wells for drug-treated and control to have them better appreciate the degree of natural variation among biological samples. Students will find most cells in interphase, but will also see quite a few apoptotic cells, and cells in prophase, metaphase, anaphase, and telophase (Figure 3).
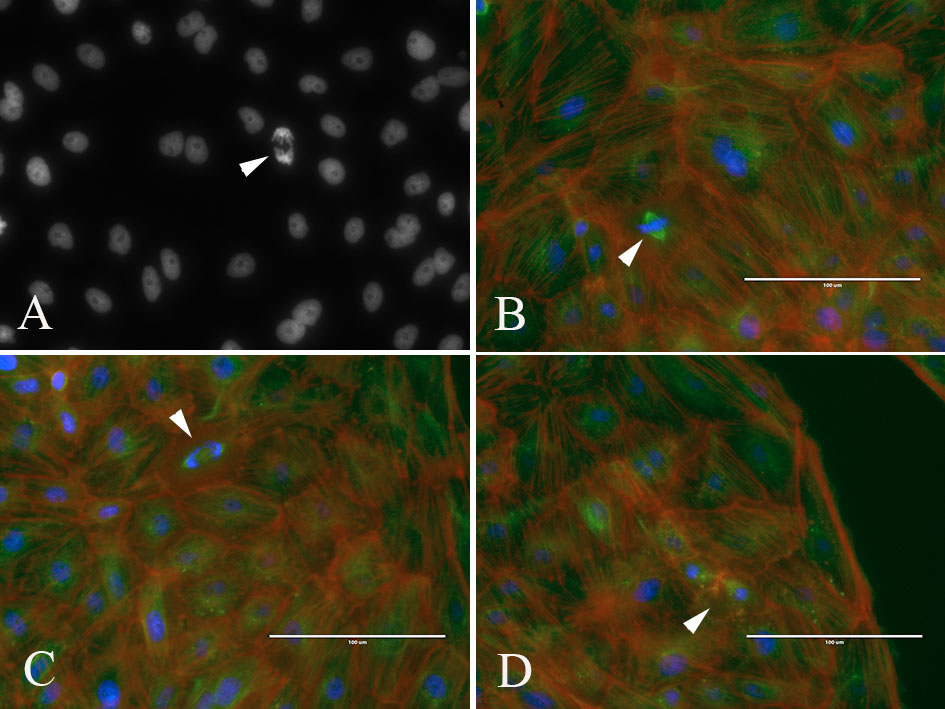
Figure 3: Student images of PtK2 cells in various phases of mitosis. (A) DNA (DAPI) image of interphase nuclei and a cell in anaphase (arrowhead). (B-D) Overlay images of cells stained for DNA (blue), microtubules (green) and actin (red); arrowheads point to metaphase (B), anaphase (C) and cytokinesis (D)
Taxol (also called Paclitaxel) is one of several cytoskeletal drugs that target tubulin. Taxol stabilizes the microtubule polymer and protects it from disassembly into tubulin. Taxol blocks a cell's ability to break down the mitotic spindle during mitosis. As a result, students may visualize cells blocked at metaphase as the cells are unable to proceed into anaphase. This blocks the progression of mitosis and triggers apoptosis. (9; Figure 4). Vinblastine is a vinca alkaloid that binds to tubulin and prevents the polymerization of microtubules, hence hinders assembly of the mitotic spindle (Figure 4). Subsequently, chromosomes at the spindle poles are unable to align at the spindle equator. As a result, students may visualize cells arrested in prophase. The cells may then undergo apoptosis (10).
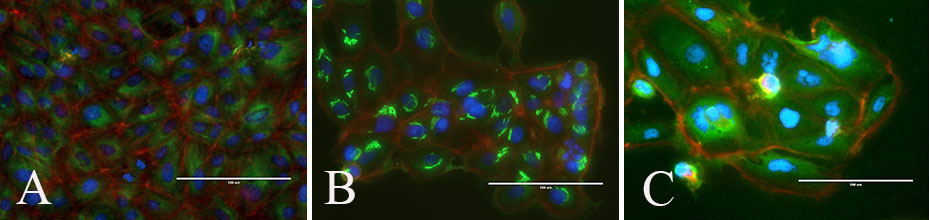
Figure 4: Student images of PtK2 cells (A) untreated, (B) treated with Vinblastine and (C) treated with Taxol. Overlay of DNA (blue), microtubules (green) and actin (red). Cells treated with vinblastine show disorganized microtubules, while cells treated with Taxol show increased apoptosis (nuclei breakdown).
In preparation for their final presentations, students must have quantitative data and selected images to support or refute their initial hypothesis on the mechanism of action of their drug. They must compare images from their untreated versus drug-treated wells to determine if they see differences in the total number of cells, or in the number of mitotic and apoptotic cells. Students can create a bar graph comparing the untreated and drug-treated cells. Students must select a few images (DAPI and overlay) to show chromosomal abnormalities, apoptotic cells, or disorganized microtubule and actin staining.
At this point, the instructor may want to disclose the name of the drugs to the students, but not tell them which drug they actually had. This could also be done after their group presentation (Lab Session 6). Students should be asked to research microtubule assembly and disassembly to help support the mechanisms of action of their drug.
A presentation is prepared by each group that includes supporting microscopic images collected during the lab module, generated tables and graphs, and relevant references.
Lab Session 6: Group Presentations
Student groups present their data and justify why they believe their cells were treated with taxol or vinblastine. Each student in the group is expected to present and participate in the discussion. The student audience is encouraged to participate and ask questions. Student groups with the same drug might discuss their results. (S10. Using Immunocytochemistry – Example student presentation).
TEACHING DISCUSSION
The goal of this lab module is for students to learn fundamental cell biological techniques. One might argue that there is very little inquiry as students are mostly following a protocol. However, the data collection, analysis, and interpretation make this lab module investigative. Students discover differences in the organization of the cytoskeletal elements and chromosomes and apoptosis in drug-treated cells by comparing to untreated cells. Students must then synthesize their quantitative and qualitative data and make a case for the mechanism of drug action. Even though the effects of the drugs used in this module are well known, they are novel to the students, therefore making this lab module an investigative experience for students to learn concepts related to microscopy, build and practice STEM skills (Table 2), and to potentially the gain confidence to do independent research as juniors and seniors (11).
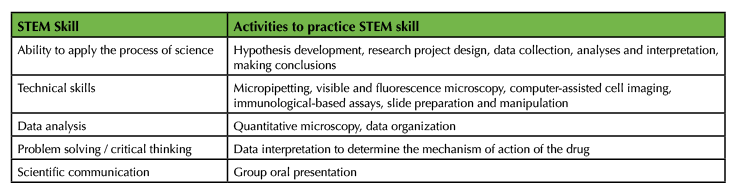
Table 2: STEM skills addressed and practiced in this lab module.
Even though this is a very straightforward lab, we do encounter some areas where students struggle. A common issue is students struggling to identify cells in the different phases of mitosis in their generated slides. Student textbooks and provided websites include drawings and images of mitotic phases that are perfect. However, in a real-world situation with slides generated by students, the cells may be distorted or damaged in the slide preparation. Students get confused when they find cells in stages that are in between the distinct phases of mitosis. For example, students found cells with chromosomes aligned at the equator, but because there was some space between the sister chromatids, students had difficulty deciphering that the cells had just passed metaphase and were in very early stages of anaphase. Mitosis is a fluid process, so understanding that one step flows into the next was a challenge for students.
While analyzing data from drug-treated wells, some students struggled to understand that if cells were arrested in a particular cell cycle stage, it was likely due to the drug preventing the transition to the subsequent stage. For example, if a large number of cells were arrested in prophase by vinblastine, some students assumed that the drug must be acting at the beginning of prophase, rather than the transition from prophase to metaphase. This was clarified by faculty--typically using the "cell cycle washing machine dial" analogy, in which cell cycle control is like the controller arm of a washing machine dial that rotates clockwise and triggers the next essential process when it reaches specific points on the outer dial (12). This analogy can be used to explain that if cells could not form microtubules, the transition to metaphase could not take place, resulting in arrest in prophase.
We also noticed that there was some confusion among students in telling the difference between apoptotic and dead cells (necrotic cells). Cell shrinkage, chromatin condensation and membrane blebbing are indicators of cells undergoing apoptosis, while cell swelling and plasma membrane rupture are characteristics of necrotic cells.
Content and attitudinal assessments were administered at the beginning of the semester and at the end during Fall 2013 and Spring 2013 and 2014 semesters to students enrolled in the Cell Biology with Laboratory course. Of the over 600 students who were enrolled in the course, 98 consented and completed the pre-course content assessment and 92, the post-course assessment. A paired student t-test was used to compare the average percent correct across the participating laboratory sections. The results showed that on questions relating to concepts in light microscopy, post-content assessment scores showed a gain of 11% compared to pre assessment scores. Likewise, the post-content assessment scores were 20% greater than pre on questions relating to fluorescence microscopy (Figure 5).
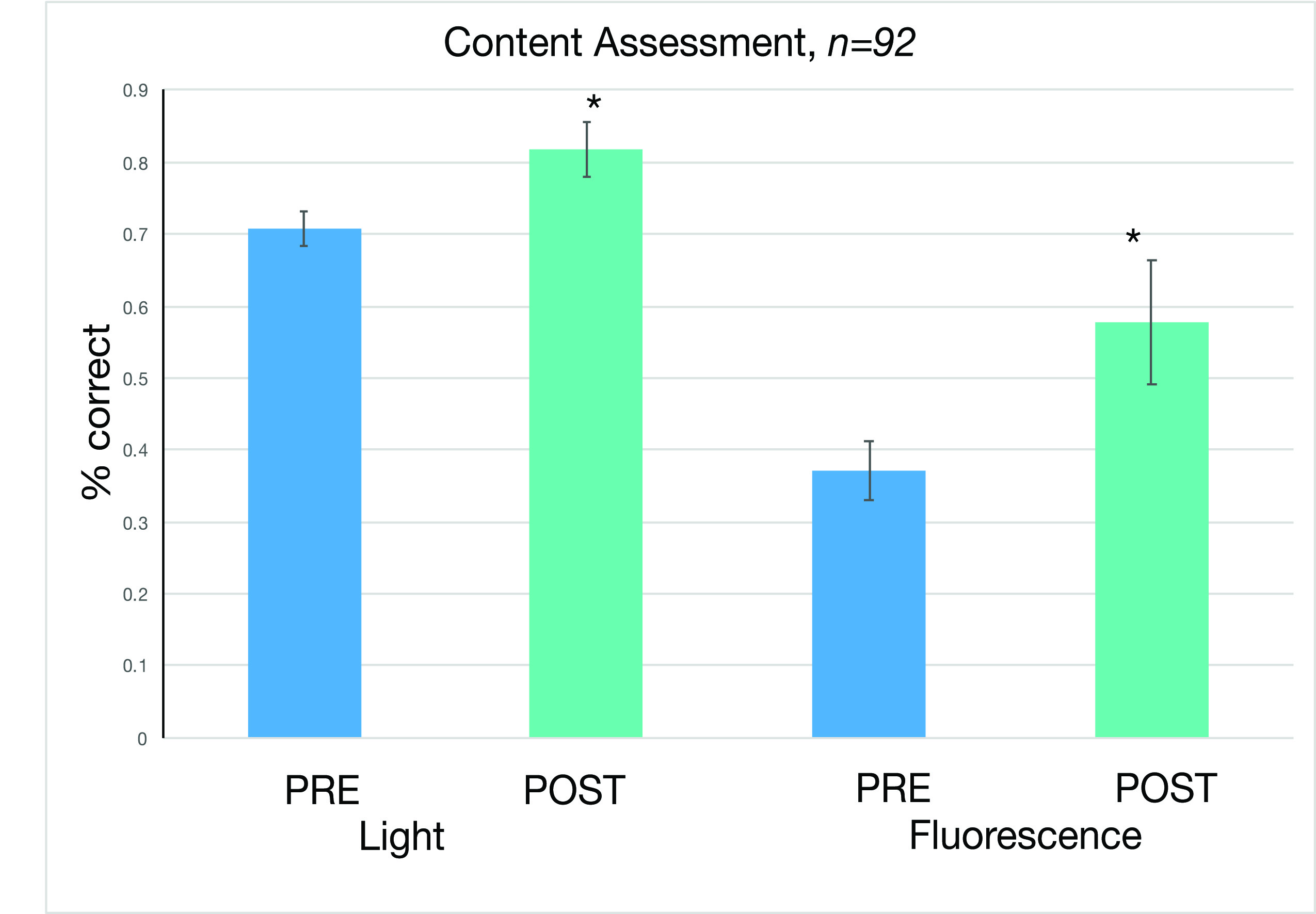
Figure 5: Content assessment on concepts related to light and fluorescence microscopy. The post-assessments when compared to pre-assessments showed a 11% and 20% gain on questions related to light and fluorescence microscopy respectively. The post assessment scores were significantly different from pre assessment scores with a p value of < .05 (paired t test).
We also see an increase in student confidence in the development of various skills gained from this lab module (Figure 6 and Table 2). Greatest gains were seen in students' understanding of the concepts on which fluorescence microscopy is based, and in their ability to design experiments involving fluorescence microscopy. It is interesting to note that the variance in post assessments was much smaller than in pre assessments, likely indicating that students were much more sure of their confidence in these skills at the end of the lab module. Students overwhelmingly valued the research experience as part of the Cell Biology lab. This lab module prepares Biology majors for more advanced courses, and as they progress through matriculation. They will become confident in their ability and proficiency to conduct and understand research in upper-level courses, in graduate school, and/or in biological and biomedical careers.
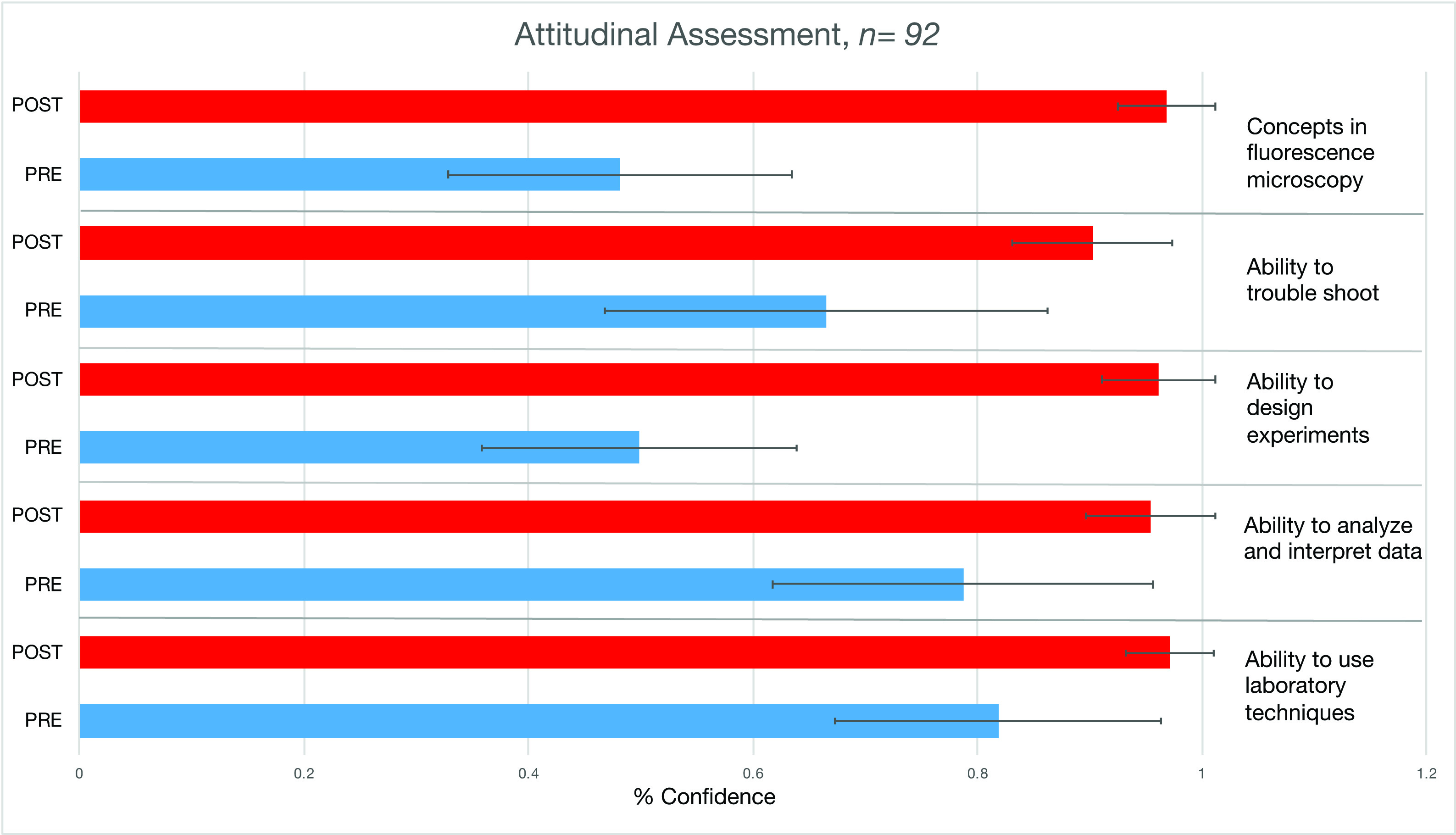
Figure 6: Pre-post attitudinal assessments showed that students gained confidence in their (from top to bottom) (i) understanding of the concepts on which fluorescence microscopy is based; (ii) ability to troubleshoot; (iii) ability to design experiments that take advantage of the unique aspects of fluorescence microscopy; (iv) ability to analyze and interpret experimental data using microscopy; (v) ability to use laboratory techniques involving microscopy. The perceived attitudinal gain in confidence for each of the skills were all statistically different with a p value of < .05 (paired t test).
The lab module is made possible is because of the acquisition of an inverted fluorescence microscope specifically dedicated for the cell biology teaching laboratory. The first inverted microscope was purchased from mini-grant funds and was a refurbished demo model that cost $10,000. Mini-grants and an ASCB fellowship funded additional objectives. Due to the success of these labs and the creation of a cell culture teaching facility, two more new inverted EVOS scopes were purchased with institutional funds. Faculty interested in doing this lab module can use any available fluorescence scope, even one borrowed from a research lab for two weeks. In addition, faculty could ask company reprentatives for availability of refurbished and demo models.
Possible variations
This module involves the use of cells treated with anti-cancer drugs. This strategy could be extended to have students take ownership of their research experiment and ask them to bring in their own experimental house-hold factor to test on the PtK2 cells. Cells can be stained in a similar way to determine if their factor has an effect on mitosis (13,14). Another extension would be to ask students to conduct basic statistics on their data and include error bars on their graphs.
Although none of the students enrolled in the course over the period in which these modules were implemented were blind or visually impaired (BVI), we do have some suggestions for making the student-collected images and other data accessible to those who are BVI. To accommodate students with common visual impairments such a red/green color blindness, one can change the settings on the scope to view the photomicrographs in black and white or blue and yellow. Additionally, we have found that having the students work in small groups and with services of institutionally-provided readers and scribes under an accommodation plan, students with a variety of disabilities are able to engage with the described activities. The group members with the assistance of the readers/scribes can describe the images of the labeled cells and the tabular/graphical data so that all team members are engaged in the data analysis.
SUPPORTING MATERIALS
- S1. Using Immunocytochemistry – Instructor prep slide and reagents
- S2. Using Immunocytochemistry – Lab Session 1 Lecture slides
- S3. Using Immunocytochemistry – Lab Session 1 Fuorescence microscopy introduction
- S4. Using Immunocytochemistry – Lab Session 2 Quiz
- S5. Using Immunocytochemistry – Lab Sesssion 2 Student protocol
- S6. Using Immunocytochemistry – Lab Sessions 3-6 Student protocol
- S7. Using Immunocytochemistry – Instructions for EVOS microscope
- S8. Using Immunocytochemistry – Lab Session 3 Quiz
- S9. Using Immunocytochemistry – Assessment Questions Microscopy and Attitudes
- S10. Using Immunocytochemistry – Example student presentation
- S11. Using Immunocytochemistry – Peer Review
ACKNOWLEDGMENTS
Supported by an internal mini-grant funded by a University System of Georgia STEM Initiative II grant. Supported by a Linkage Fellowship award to D'Costa from the American Society of Cell Biology Minorities Affairs Committee. The majority of this work would not have been possible without support from Dr. Thomas Mundie, Dean of the School of Science and Technology for sharing our vision of training all GGC Biology majors in mammalian cell culture and imaging techniques. With his support and leadership, GGC has a teaching laboratory space with laminar flow hoods, incubators and fluorescence microscopes.
References
- Palombi PSJ, Snell K. 2008. Learning about Cells as Dynamic Entities: An Inquiry-Driven Cell Culture Project. Bioscene: Journal of College Biology Teaching. 33, 27-33.
- Hammamieh R, Anderson M, Carr K, Tran CN, Yourick DL, Jett M. 2005. Students Investigating the Antiproliferative Effects of Synthesized Drugs on Mouse Mammary Tumor Cells. Cell Biology Education Vol 4, 221-234.
- Marion RE, Gardner GE, Parks LD. 2012. Multiweek cell culture project for use in upper-level biology laboratories. Advances in Physiology Education. 36, 154-157.
- McIlrath V, Trye A, Aguanno A. 2015. Using Mouse Mammary Tumor Cells to Teach Core Biology Concepts: A Simple Lab Module. J. Vis. Exp. (100), e52528, doi:10.3791/52528.
- Shelden EA, Offerdahl EG, Johnson GT. 2019. A Virtual Laboratory on Cell Division Using a Publicly-Available Image Database. CourseSource. https://doi.org/10.24918/cs.2019.15
- Howard DR, Miskowski JA. 2005. Using a Module-based Laboratory to Incorporate Inquiry into a Large Cell Biology Course. Cell Biology Education Vol. 4, 249-260, Fall 2005. https://doi.org/10.1187/cbe.04-09-0052
- Tare M, Singh A. 2008. A Cell Biology Laboratory Exercise to Study Sub-Cellular Organelles in Drosophila. https://ecommons.udayton.edu/bio_fac_pub/156.
- Skloot R. 2010. The Immortal Life Of Henrietta Lacks. New York : Crown Publishers, 2010. Print.
- Weaver BA. 2014. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014 Sep 15; 25(18): 2677-2681. doi: 10.1091/mbc.E14-04-0916.
- Jordan MA, Thrower D, Wilson L. 1992. Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles. Implications for the role of microtubule dynamics in mitosis. J Cell Sci. 1992 Jul;102 (Pt 3):401-16.
- Awong-Taylor J, D'Costa A, Giles G, Leader T, Pursell D, Runck C, Mundie T. 2016. Undergraduate Research for All: Addressing the Elephant in the Room. Council on Undergraduate Research CUR Quarterly Fall 2016: Vol 37, No 1.
- Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Components of the Cell-Cycle Control System. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26824/.
- Barrera A, Hurst-Kennedy J. 2019. An Inquiry-Based Laboratory Curriculum Investigating Cell Viability using Mammalian Cell Culture and Fluorescence Microscopy. Tested Studies for Laboratory Teaching: Proceedings of the Association for Biology Laboratory Education, 40, Article 22, electronic publication.
- Hurst-Kennedy J, Saum M, Achat-Mendes C, D'Costa A, Javazon E, Katzman S, Ricks E, Barrera A. 2020. The Impact of a Semester-Long, Cell Culture and Fluorescence Microscopy CURE on Learning and Attitudes in an Underrepresented STEM Student Population. Journal of microbiology & biology education, 21(1), 21.1.25. https://doi.org/10.1128/jmbe.v21i1.2001.
Article Files
Login to access supporting documents
Using Immunocytochemistry and Fluorescence Microscopy Imaging to Explore the Mechanism of Action of Anti-Cancer Drugs on the Cel(PDF | 773 KB)
S1. Using Immunocytochemistry-Instructor prep slide and reagents.docx(DOCX | 31 KB)
S2. Using Immunocytochemistry-Lab Session 1 Lecture slides.pptx(PPTX | 1 MB)
S3. Using Immunocytochemistry-Lab Session 1 Fluorescence Microscopy Introduction.docx(DOCX | 23 KB)
S4. Using Immunocytochemsitry-Lab Session 2 Quiz.docx(DOCX | 20 KB)
S5. Using Immunocytochemistry-Lab 2 Student Protocol.docx(DOCX | 22 KB)
S6. Using Immunocytochemistry-Lab Sessions 3-6 Student Protocol.docx(DOCX | 26 KB)
S7. Using Immunocytochemistry-Instructions for EVOS microscope.docx(DOCX | 17 KB)
S8. Using Immunocytochemistry-Lab Session 3 Quiz.docx(DOCX | 19 KB)
S9. Using Immunocytochemistry-Assessment Questions Microscopy and Attitudes.doc(DOC | 60 KB)
S10. Using Immunocytochemistry-Example Student Presentation.pptx(PPTX | 2 MB)
S11. Using Immunocytochemistry- Peer Review.doc(DOC | 34 KB)
- License terms

Comments
Comments
There are no comments on this resource.