Chilling in the Cold: Using Thermal Acclimation to Demonstrate Phenotypic Plasticity in Animals
Editor: Benjamin Martin
Published online:
Abstract
The lesson plan described here provides students hands-on, scientific experiences that relate to the globally important issue of climate change and its impacts on animals. Students use the scientific method and basic components of experimental design to investigate how animals cope with changing temperatures. In our modifiable 6-week lesson plan, which is appropriate for both science majors and non-majors, students learn how to design, conduct and analyze an experiment addressing how fruit flies can acclimate to changing temperatures and respond appropriately. Following the experiment with flies, students also get first-hand experience with real-time weather data from NOAA databases. In this exercise, students integrate the results from the laboratory experiment to this real-world data set to appreciate how the questions we addressed in the classroom can be applied to a real-world problem of climate change.
Primary Image: Acclimation affects cold tolerance of the fruit fly. This image depicts the difference in time of recovery from a cold shock between flies acclimated to a cold, room temperature or warm environment.
Citation
Potts LJ, Garcia MJ, Teets NM. 2020. Chilling in the cold: Using thermal acclimation to demonstrate phenotypic plasticity in animals. CourseSource. https://doi.org/10.24918/cs.2020.21
Society Learning Goals
Ecology
- Species-Habitat Interactions
- How do species interact with their habitat?
- Impacts of Humans on Ecosystems
- What impacts do humans have on ecosystems?
Lesson Learning Goals
The primary goal of this lesson is to teach students how organisms respond to changes in their physical environment and how we can use this knowledge to predict biological impacts of climate change. We apply the concepts of phenotypic plasticity and thermal biology so that students can appreciate the importance of temperature on all levels of an organism's biology and ecology. Using real-world climate data, students gain an understanding of how changes in our world's temperature regimes can impact biological systems. This lesson aligns with goals contained within the Ecological Society of America Learning Framework, specifically 1) How do species interact with their habitat? and 2) What impacts do humans have on ecosystem?
Lesson Learning Objectives
Students will be able to:
- Describe how the scientific method can be used to answer real-world problems.
- Define the basic components of an experiment.
- Predict how tolerance of extreme temperatures and phenotypic plasticity may influence individual fitness and ultimately shape the evolution of organisms.
- Understand the basic principles of climate change and evaluate how these changes in temperature regimes will impact organisms.
Article Context
Course
Article Type
Course Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
The ability of organisms to cope with changes in their environment is critical for survival. Through years of research, we know that animals and plants can respond to their changing environments through a wide range of mechanisms (1-3). Tadpoles can change their body shape when in the presence of a predator (4,5). Plants can change their floral scent cues based on the scent cues of nearby flowering plants (6-8). Insects can alter their ability to survive cold stress when acclimated to a cold environment (9,10). The ability of an individual to produce multiple phenotypes depending on environmental conditions is termed phenotypic plasticity.
Current rates of global warming and climate change are unprecedented in Earth's history. These rapid changes in temperature and precipitation patterns are having drastic effects on plant and animal life (11-13). In many cases, the rate of climate change exceeds species' ability to adapt to these new environments through evolution, leading to changes in behavior and phenology of organisms, range shifts of their populations, and even extinction (14-17). Thus, phenotypic plasticity is critical for an organism's ability to persist in the face of climate change (18). One of the best studied and understood environmental changes that can elicit phenotypic plasticity is a change in temperature. For insects, the most diverse and abundant land animals on the planet and the focus of this lab activity, temperature has a major influence on their biology, ecology and evolution. Temperature directly impacts insect behavior and activity, which influences how successful they are at producing the next generation (i.e. Fitness; (19)). Climate change is increasing mean temperatures, as well as variability and the frequency of extreme events, and these changes are having major consequences for insects (20,21). Somewhat paradoxically, the winter season is perhaps the most affected by climate change (22); winter is warming faster than other seasons, and these changes in winter conditions can affect insects' ability to survive sudden cold snaps when they do occur (e.g., the polar vortex of 2018-2019).
This lesson incorporates two key concepts, climate change and phenotypic plasticity, to investigate how a common insect can respond to changes in temperature by changing its cold tolerance, and how these biological responses relate to climate change. While our lesson plan does not directly measure fitness, we discuss how thermal acclimation is important for maintaining fitness in a changing climate. While previous studies have used cold tolerance of flies to address the topic of climate change (23,24), our lesson extends these lessons by incorporating phenotypic plasticity to different environments including real weather data to predict responses to climate change.
Intended Audience
This laboratory activity is suitable for both science majors and non-majors and is modifiable for upper and lower level students. Our lesson was given to a small 20-student class of upper-level non-science major students at Transylvania University in Lexington, Kentucky (n=20).
Required Learning Time
This lesson requires time for acquisition of the materials, rearing of the flies, and one week for acclimation treatments. Data collection requires a single 60-minute class period. The lesson is divided into six individual activities, with each activity taking between 60 and 75 minutes (see Table 1).
Prerequisite Student Knowledge
Before this lesson, students were given lectures and materials about the effects of climate change, including major changes in weather patterns such as increased temperatures and increased frequency of extreme events. We also discussed the terms phenotypic plasticity and acclimation in light of climate change. For example, how can an organism cope with an environment that is un-predictable in temperature and weather patterns? We discussed the value of plasticity to survive in unpredictable climate scenarios. Finally, the students were informed about cold-tolerance in flies, including the terms chill-coma and its recovery time, and critical thermal minimum.
Prerequisite Teacher Knowledge
We recommend that teachers familiarize themselves with the basic science of climate change, including the predicted outcomes and causes. Teachers should be familiar with phenotypic plasticity and acclimation in organisms. For in-depth review articles on adaptation and plasticity in response to climate change, see Catullo et al. 2019 and Xue et al., 2019 (23,24). Knowledge on how insects cope with low temperatures will be beneficial (see above). We have included an example powerpoint lecture that teachers can use to familiarize themselves with the lesson topics, and use in their classrooms (Supporting File S1. Chilling in the Cold – Example PowerPoint). Finally, teachers will benefit from a basic knowledge of Google sheets, or another data-analysis software (e.g., SAS, R, STATA) of your choice.
SCIENTIFIC TEACHING THEMES
This lesson incorporates the teaching mode of think-pair-share to facilitate active learning and inclusiveness in the classroom.
Active Learning
Almost all material in this lesson is designed from a student-centered perspective to facilitate active learning, which has been shown to increase student performance in STEM courses (25). Many of the activities are done in a modified form of Think-Pair-Share (26), which is a classroom-based active learning strategy in which students think about problems posed by the instructor, then students work together in pairs (or our case, small groups) to solve the problem and share their ideas with the rest of the class. This model allows students to voice their ideas to a small group of peers, reflect on their own thinking and obtain immediate feedback from peers and the instructor (27,28).
Assessment
Throughout the lesson, there are many opportunities for formative assessments. From the start of the activity, if the instructor chooses to use our included PowerPoint presentation (Supporting File S1. Chilling in the Cold – Example PowerPoint), there are example questions to give the students to assess their knowledge of plasticity, fitness and how climate change may play a role. Once the activity and the experimental procedure are introduced, we have included discussion questions that can be used to gauge student knowledge or generate a pop quiz (Supporting File S3. Chilling in the Cold – Student Handout; discussion questions). We also encourage instructors to use the student handout (Supporting File S3. Chilling in the Cold – Student Handout; procedure) to guide students in constructing the hypotheses for the experiment. We had students draw graphical depictions of their predictions on the board, labeling their independent and dependent variables, as well as brainstorming what variables might function as controls. Prior to the start of the experiment, students were also assessed using formative and summative questions aligned with the learning outcomes: how acclimation impacted flies' ability to withstand cold and how plasticity could enhance survival in a climate-change scenario. During the laboratory exercise where students analyze real-world climate data, we created a worksheet of discussion questions for the student groups to work through (Supporting File S5. Chilling in the Cold – Climate Handout; discussion questions). At the end of the class, we discussed these questions as a group, but could easily be adapted into a more rigid assessment of student knowledge such as a quiz, or short 500-word essay. The summative assessment at the end of the activity is to create a lab report in which students demonstrate knowledge of the scientific method and the specific content related to the lab activity (Supporting File S6. Chilling in the Cold – Lab Report Rubric).
Inclusive Teaching
This lesson seeks to create a collaborative learning environment, open to all students' academic, social and cultural backgrounds. It fosters an inclusive teaching environment by encouraging students to use prior skills and knowledge towards the activity (29). For instance, in our course many students were business or economics majors and had previous experience with Google sheets. Other students were fine arts majors and were able to visualize graphs and experimental outlines with their groups. Additionally, we recommend incorporating a variety of teaching methods to meet the needs of students with diverse learning preferences. For example, we presented ideas and concepts to students via PowerPoint, but also demonstrated point by point on a white board with graphs and drawings. Students were given worksheets and instructions. Finally, oral instructions were given, and students were encouraged to work in groups with peer-led activities.
LESSON PLAN
This lesson plan includes six laboratory exercises during which students conduct and analyze the results of an experiment in a small group setting. Students learn the entire scientific process ranging from experimental design to statistical analysis and presentation of results. For this activity, we recommend using the fruit fly Drosophila melanogaster, a model organism that is inexpensive and easy to maintain (Supporting File S2. Chilling in the Cold – Fruit Fly Information; supplies and fly-handling). Throughout the exercises, students worked in self-selected groups of 2 or 3. Larger group sizes may result in too little work per student, and this lesson would be difficult for a single student working alone. At the beginning of each class, students should be given a brief presentation that provides an overview and purpose of the activity, where students can locate the materials, and opportunities to ask any questions before they begin. Below we outline the lesson plans for each laboratory activity.
Laboratory Exercise #1: Introduction to relevant topics.
The goal of the first lab is to introduce students to climate change, phenotypic plasticity, cold tolerance, and the life cycle of fruit flies.
Lab Overview
This lab introduces students to working with Drosophila while also covering the topic of plasticity and how it relates to cold tolerance. We have provided an example PowerPoint that instructors can use to help introduce students to the concepts of the lesson (Supporting File S1. Chilling in the Cold – Example PowerPoint). Students are provided information on the life cycle of the fly and how it is dependent on temperature at all stages (Supporting File S2. Chilling in the Cold – Fruit Fly Information; fly life cycle).
Next, the basics of insect cold tolerance, thermal acclimation, and phenotypic plasticity are introduced (Supporting File S1. Chilling in the Cold – Example PowerPoint). Because flies are ectotherms, their body temperature is regulated by ambient temperature, such that if the environment cools down, so will the fly. Depending on your class level, you may choose to give a more detailed explanation of cold tolerance, for example by delving into the cellular biology of this trait. Typically, at temperatures between 0-10°C, depending on species, insects enter a reversible state of paralysis known as "chill-coma." In chill coma, all movement ceases, and the insect is unable to walk around, feed or mate. If the chill-coma lasts too long, the insect will die. However, there is a window of time when the insect, if warmed back up, will recover. The time it takes to recover is called "chill-coma recovery time" (CCRT). This trait is an ecologically relevant measure of cold tolerance and assesses the time required to regain ecological functions like finding food and mates. It is also easy to measure, as it does not require any special equipment, and can be directly observed by students. Explain to students that depending on the environment the fly is acclimated to, they may have a shorter or longer chill coma recovery time (30). There is also a genetic component to this trait, such that chill coma recovery time is influenced by both genotype and environment (31,32). Thus, it is a robust way to measure phenotypic plasticity to a temperature cue, and to investigate how climate change may influence fruit flies.
Once students understand the basic biology of fruit flies, cold tolerance metrics and phenotypic plasticity, discuss how climate change relates to this experiment. This concept is effectively conveyed by displaying graphs of temperature regimes during the winter season (22). Not only is winter increasing in average temperature, but the frequency of cold snaps and warm spells is also predicted to increase.
The goal of this lab is for students to think about how ectotherms depend on temperature for their function, and how their entire biology may be impacted by disruption of normal temperature cycles. See the Supporting File S3. Chilling in the Cold – Student Handout; discussion questions, for ways to lead your class, and to assess the extent to which students are grasping the material.
Laboratory Exercise #2: Generating hypotheses and separating flies into acclimation treatments.
The goal for this lab is to separate flies into their acclimation treatments so that their cold tolerance can be measured in the following lab exercise. Students will also be introduced to the scientific method and generate hypotheses and predictions for their experiments.
Lab Prep
Prior to this lab exercise, you will need to have adult flies. You can order adult flies from online vendors such as Carolina or Fisher, or you can rear flies on your own prior to the exercise. The latter option is preferable, because you can control the rearing temperature and the age of the flies, but the first option is easier for those without fly experience. Each student group will need at least 30 adult flies (n=10 per acclimation treatment).
Additionally, this lab requires acclimation treatment areas and materials to handle the flies. A full list of required materials, as well as information for creating cooled and heated areas if you don't have access to temperature-controlled incubators, is provided in Supporting File S2. Chilling in the Cold – Fruit Fly Information; fly handling.
Lab Overview
To begin this lab, introduce students to the tenants of the scientific method, including hypothesis generation, dependent and independent variables, experimental design, analyses, and graphical and written display of results. We found a common source of confusion for students is correctly identifying independent and dependent variables (33). To clarify these terms beyond giving the students definitions and examples of each, draw graphs of example experiments. The independent variable is going to be represented on the x-axis, and the dependent variable on the y-axis. This activity is a good thought exercise for students to visualize the difference between variable types and start thinking about data graphically.
Once the students understand the basics of the scientific method, and are able to define each component, have the student groups generate a set of hypotheses and predictions about the effect of fly acclimation treatment (independent variable) on the flies' CCRT (dependent variable). Depending on your class level, you may find it useful for students draw their predictions in graph form. In other words, have the students predict what their results would look like if the alternate hypothesis is supported. We have provided an example handout to give to your students, with boxes and details for creating the hypotheses and organizing data (Supporting File S3. Chilling in the Cold – Student Handout; procedure).
The next activity is to separate flies into different acclimation treatments. In Supporting File S2. Chilling in the Cold – Fruit Fly Information; fly handling, we give a detailed procedure of the method we used to handle flies. Students will transfer anesthetized flies in groups of ten to new food vials destined for the acclimation treatments. In our classroom, we had access to three incubators, room temperature (22°C), warm (33°C) and cool (16°C). Depending on your space and supplies, you can choose to do only two temperatures, or modify the temperatures (see Supporting File S2. Chilling in the Cold – Fruit Fly Information; acclimation, for additional low-cost ideas for creating temperature controlled environments).
Once each student group has 10 flies (or more) per acclimation group, students will label their vials with markers and tape and place vials in the appropriate temperature chamber. The flies will acclimate to these temperatures for one week. During this time, they should not need any additional food or care.
Laboratory Exercise #3: Cold treatment and data collection.
The goal for this lab is to expose flies to the cold treatment and collect chill coma recovery time data for the experiment.
Lab Prep
For this lab, you will use an ice-water slurry (0°C) to cold-treat the flies. Before the experiment, flies will need to be transferred from the vials with food to clean vials without food (or the flies will drop and stick in the food media). Submerge fly vials in a stryofoam cooler with ice water slurry, using styrofoam tube racks to keep water out. We (the instructors) transferred flies to clean tubes and started the 2 h cold-shock treatment prior to class. Thus, when the students arrived, they only had to collect data on CCRT. For a longer lab period (≥3 h) students may be able to start the cold shock treatment at the beginning of class, although there will be 2 h of downtime afterwards.
Lab Overview
In this exercise, students will perform the cold shock experiment and collect data. Explain the process of measuring CCRT to the class. Remind the students the flies will be in a reversible chill-coma state, and they are to measure the time of recovery. Once the 2 h cold shock treatment is finished, students will dump flies into labeled petri dishes and immediately start their timers. They will record the time it takes for each fly to stand up and begin walking around. Students can divide the tasks so that one group member uses their phone or other device as a stop-watch, another one watches the flies, and a third records the CCRT. Example results from this experiment are provided in Supporting File S4. Chilling in the Cold – Example Data; example student data.
Laboratory Exercise #4: Data analysis.
The goal of this lab is to analyze the results of the experiment. For our group, with limited statistical background, we opted for a simple one-way ANOVA test using an open-access website (https://www.socscistatistics.com/). You may choose to run a more complicated analysis, such as modeling in R, or use other software like JMP or SAS.
Lab Overview
In this lab, students run statistical analyses on their CCRT data. We chose to have students run a one-way ANOVA comparing mean CCRT across the three acclimation treatments. Students can use Google Sheets to organize their data and the website "Social Science Statistics" (https://www.socscistatistics.com/) to run the tests. Be sure to explain to students what this test is comparing (means and variance of CCRT across acclimation treatments) and what its output will be (F statistic and p-value). An example data sheet is provided to illustrate how students can organize their own results (Supporting File S3. Chilling in the Cold – Student Handout; procedure). During this lab, students will also graph their data. We chose to use box and whisker plots and have the students draw them by hand on provided graphing paper. We found this was a useful exercise to not only get students to think about their data graphically, but to use Google Sheets to calculate simple statistics (mean, median, min, max, quartiles) that can be applied outside the classroom. Our students enjoyed the break from using the computer, and many found a creative outlet in drawing the graphs by hand. An example computer-generated box and whisker plot for these data are provided in Supporting Matieral S4. Chilling in the Cold – Example Data; example student data.
Laboratory Exercise #5: Compare results to temperature data.
The goal of this lab is to make a connection between the acclimation experiment and climate change in the real world. Using freely-accessed temperature data, students can see what environmental conditions flies experience, and using the knowledge from the experiment, how flies may survive various temperature conditions. If time allows, use the discussion questions provided in Supporting File S5. Chilling in the Cold – Climate Handout; discussion questions, to guide student engagement, and assessment of real-world data.
Lab Overview
This lab exercise provides students real-world temperature data to make predictions about cold stress and acclimatization for flies in nature. In our class, we provided a 5-year data set on historical temperature data from winters in three distinct areas: Moran, Michigan; Lexington, Kentucky; and Key Largo, Florida. Using a website run by NOAA (www.ncdc.noaa.gov) you can request access to daily summaries which include minimum, maximum and average daily temperatures from a wide range of weather stations. We have included the temperature dataset that we provided to our students (Supporting File S4. Chilling in the Cold – Example Data). Students organized the data by month and calculated monthly averages for all three variables (daily minimum, daily maximum, and daily average) for all three locations. Given your class level, you may opt to have them obtain these data on their own to gain experience using publicly available repositories for climate data.
Using Google Sheets, students can create a table of the temperature data and plot bar graphs of the monthly summaries (see Supporting File S4. Chilling in the Cold – Example Data; example graph of climate data). Remind the students that the minimum daily temperature represents the cold-shock that flies in the wild experience, and the daily average would be equivalent to the acclimation treatment temperature. To assess student understanding of how this activity relates to our experiment, see the discussion questions provided in Supporting File S5. Chilling in the Cold – Climate Handout; discussion questions.
Depending on your class level, it may be useful to include a discussion on the short and long-term effects of climate change on fly populations. In the short-term, insects can cope with changes in climate through phenotypic plasticity. If these environmental changes persist, we would expect to see evolutionary adaptation to the new thermal environment, especially for animals with short generation times like fruit flies (although it is worth noting that the current rate of climate change likely exceeds the capacity for adaptation in most organism). These discussions will enhance student knowledge and understanding of how climate change is expected to cause phenotypic plasticity and adaptation in flies and other organisms. See Sgro et al. 2015 (18).
Laboratory Exercise #6: Present results.
The goal of this lab is to have students explain their findings, present data in a graphical form, and explain the significance of their results in the context of climate change. We opted for a short lab-report and a class discussion between the groups. You may choose to alter this to include poster presentations or oral reports, depending on class level and your preferred method of assessment.
Lab Overview
Students compile their experimental and weather data in a short 2-page lab report (Supporting File S6. Chilling in the Cold – Lab Report Rubric). For our students, we required them to define the components of the scientific method, predict how cold-exposure and phenotypic plasticity may influence individual population structure and ultimately shape the evolution of ectotherms, and evaluate how ectotherms may respond to future temperature regimes predicted by climate change models. As our students were non-majors, we weren't concerned with their mastery of scientific literature, but more so that they could describe how the scientific method could be used to answer a real-world problem. Overall, our goal was for students to understand that climate change is not only an issue for humans but that changes in temperature, including winter conditions, can have significant impacts on living organisms. See our students' average grades in Supporting File S7. Chilling in the Cold – Student Grades.
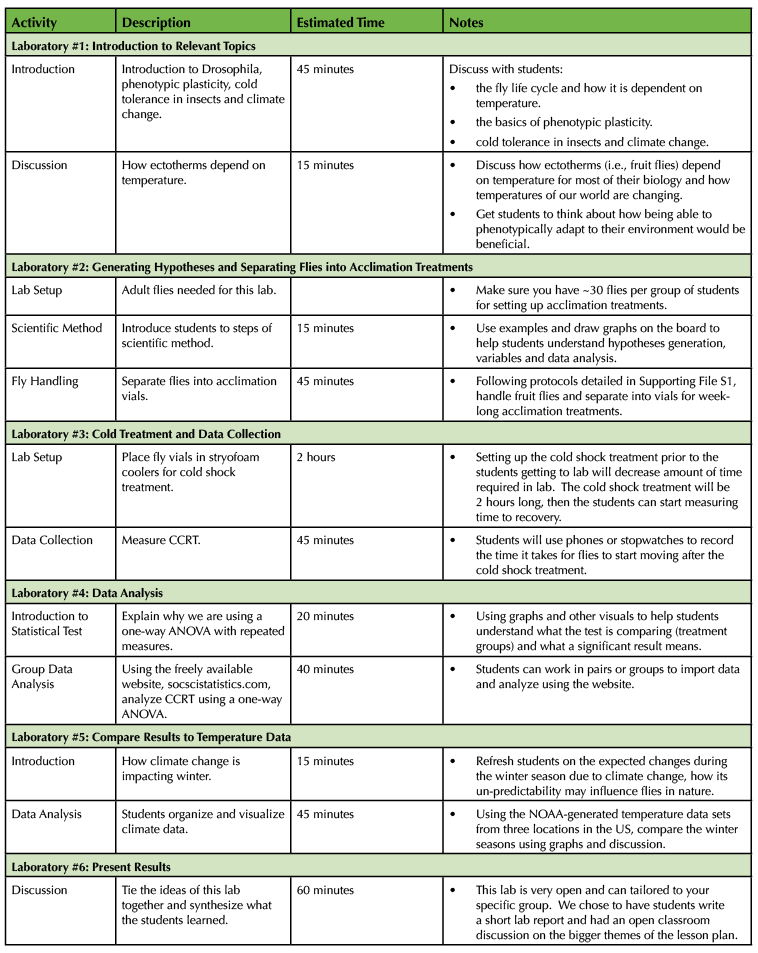
Table 1. Lesson teaching timeline
TEACHING DISCUSSION
The primary goal of this lesson is to understand how organisms' response to changes in their environment and the implications for climate change. We use an experiment on phenotypic plasticity of cold tolerance to demonstrate an animal's ability to acclimate to its environment, and we use the results in conjunction with real climate data to make predications on animal responses to climate change. Within this goal, we had several learning outcomes, discussed below.
Understanding the Scientific Method
Our students were upper level non-majors taking the course as a required science credit, and many hadn't taken a biology course since high school. While they could recite the steps of the scientific method, they initially had difficulty explaining and applying them. For instance, some students had difficulty identifying independent and dependent variables. We incorporated class discussions and drew graphs and charts on the board to help students see what we were about to tackle in the experiment. Once the information was presented to students in multiple formats, they were able to independently come up with hypothesis and draw graphs of their expected data. Student feedback about this outcome was positive, with one student remarking "I appreciated how we went over the scientific method and how it corresponds to real world situations."
Phenotypic Plasticity to Temperature with Climate Change Emphasis
This lesson tests flies' ability to acclimate to different temperature and alter their tolerance to extreme cold. We then used real climatic data to predict insect responses to climate change. For the laboratory portion, students directly observe the connection between cold-exposure and physiological function of the fly, and how tolerance of cold varies depending on what environment the fly lives in. For the activity with climate data, students extrapolate how temperature change may impact insect populations structure and ultimately their evolution.
The activity with real climate data hit home for many students. They were unaware that detailed climate data are freely accessible through the NOAA database, and they appreciated being able to make a real-world connection to their lab-generated data. While organizing the temperature data and creating graphs was initially difficult, by working in pairs and small groups they were able to combine skills to visualize their data. Student feedback about this portion of the activity was again positive, with one student remarking, "I had understood the concepts of phenotypic plasticity and cold tolerance prior to this portion of the lab, but it [i.e., the activity with real climate data] was interesting to look at and was complimentary to what we had already done."
Conclusion
This lesson was designed to reinforce the importance of phenotypic plasticity in dealing with temperature change in an animal system. The activity was presented under the umbrella of climate change and how changes in the winter seasons may impact animals, especially ectotherms. Students were given a problem (how do flies deal with cold exposure) and used the scientific method to discover possible solutions. During this lesson, students are exposed to various skillsets that are useful both in the class and real-life. These skills include using a scientific mindset to solve a problem, how to think of data both graphically and statistically, how to design and interpret graphs, and how to think about complicated problems in a synthesized manner. This lesson also promotes team building skills, oral and visual and written communication and the ability to troubleshoot issues all of which translate both in and outside the classroom.
SUPPORTING MATERIALS
- S1. Chilling in the Cold – Example PowerPoint. Example PowerPoint slides on the topics of phenotypic plasticity and evolution of traits to be used prior to the start of the activity, followed up by example discussion questions
- S2. Chilling in the Cold – Fruit Fly Information. Information on biology of fruit flies, and a list of supplies required for their maintenance and experimental setup.
- S3. Chilling in the Cold – Student Handout. Example questions for the pre-lab discussions and a handout to distribute to students. The handout includes a scenario for the students to refer to for the basic premise of the experiment. Also included are boxes for generating hypotheses, and examples of how students should organize their data.
- S4. Chilling in the Cold – Example Data. Excel document including example data and graphs. Included are example results from the chill coma recovery experiment, climate data from three regions in the US, and example graphs. For climate data each region is included on a separate sheet, and an example graph comparing the regions is provided on a separate sheet.
- S5. Chilling in the Cold – Climate Handout. Example handout for instructors to help explain the NOAA-generated climate data. Included are discussion questions that can be used to guide students and assess their understanding of the data.
- S6. Chilling in the Cold – Lab Report Rubric. The lab report rubric includes information on the format of the report as well as a detailed rubric of student assessment of learning objectives and goals of the lesson plan.
- S7. Chilling in the Cold – Student Grades. The lab report rubric with the average grades from our students, followed up with a discussion of the students' grades.
ACKNOWLEDGMENTS
We would like to acknowledge the students of BIO 1164 at Transylvania University during the Winter term 2019. We would also like to acknowledge the biology faculty at Transylvania, specifically Dr. James Wagner and Dr. Josh Adkins. Dr. Wagner provided mentorship and guidance to the instructor, LJP, and Dr. Adkins helped with the laboratory setup. This work is funded by an Agriculture and Food Research Initiative Education and Workforce Development grant nos. 2018-67011-28054 to LJP and 2018-67012-2800 to MJG from the USDA National Institutes of Food and Agriculture. This work is also supported by the National Science Foundation under Grant No. OIA-1826689 to NMT.
References
- Gomulklewicz R, Kirkpatrick M. 1992. Quantitative Genetics and the Evolution of Reaction Norms; Quantitative Genetics and the Evolution of Reaction Norms. Evolution (N Y). 46(2):390-411. doi:10.1111/j.1558-5646.1992.tb02047.x
- Schlichting CD, Smith H. 2002. Phenotypic plasticity: linking molecular mechanisms with evolutionary outcomes. Evol Ecol. 16(3):189-211. doi:10.1023/A:1019624425971
- Pigliucci M. 2003. Phenotypic Integration: Studying the Ecology and Evolution of Complex Phenotypes. Ecol Lett. 6:265-272.
- Michel MJ. 2012. Phenotypic plasticity in complex environments: Effects of structural complexity on predator- and competitor-induced phenotypes of tadpoles of the wood frog, Rana sylvatica. Biol J Linn Soc. 105(4):853-863. doi:10.1111/j.1095-8312.2011.01831.x
- Relyea RA. 2004. Fine-Tuned Phenotypes: Tadpole Plasticity under 16 Combinations of Predators and Competitors. Ecology. 85(1):172-179.
- Caruso T, Trokhymets V, Bargagli R, Convey P. 2013. Biotic interactions as a structuring force in soil communities: Evidence from the micro-arthropods of an Antarctic moss model system. Oecologia. 172(2):495-503. doi:10.1007/s00442-012-2503-9
- Dicke M. 2016. Plant phenotypic plasticity in the phytobiome: A volatile issue. Curr Opin Plant Biol. 32:17-23. doi:10.1016/j.pbi.2016.05.004
- Valladares F, Gianoli E, Gómez JM. 2007. Ecological limits to plant phenotypic plasticity. New Phytol. 176(4):749-763. doi:10.1111/j.1469-8137.2007.02275.x
- Lee RE, Chen C, Denlinger DL. 1987. A Rapid Cold-Hardening Process in Insects. Science. 238(4832):1415-1417.
- Teets NM, Denlinger DL. 2013. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiol Entomol. 38(2):105-116. doi:10.1111/phen.12019
- Yohe G, Parmesan C. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 421(6918):37-42. doi:10.1038/nature01286
- Menzel A, Bairlein F, Post E, Beebee TJC, Convey P, Fromentin J...Parmesan C. 2002. Ecological Responses to Recent Climate Change. Nature. 2002;416(6879):389-395. doi:10.1038/416389a
- Walther GR. 2010. Community and ecosystem responses to recent climate change. Philos Trans R Soc B Biol Sci. 365(1549):2019-2024. doi:10.1098/rstb.2010.0021
- Descimon H, Thomas JA, Ryrholm N, Tammaru T, Hill JK, Kaila L...Kulberg J. 1999. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 399(6736):579-583. doi:10.1038/21181
- Bradley NL, Leopold AC, Ross J, Huffaker W. 1999. Phenological changes reflect climate change in Wisconsin. Proc Natl Acad Sci. 96(17):9701-9704. doi:10.1073/pnas.96.17.9701
- García-Robledo C, Kuprewicz EK, Staines CL, Erwin TL, Kress WJ. 2016 Limited tolerance by insects to high temperatures across tropical elevational gradients and the implications of global warming for extinction. Proc Natl Acad Sci. 113(3):680-685. doi:10.1073/pnas.1507681113
- Burgess MD, Smith KW, Evans KL, Leech D, Pearce-Higgins JW, Branston CJ,...Phillimore AB. 2018. Tritrophic phenological match-mismatch in space and time. Nat Ecol Evol. 2(6):970-975. doi:10.1038/s41559-018-0543-1
- Sgrò CM, Terblanche JS, Hoffmann AA. 2015. What Can Plasticity Contribute to Insect Responses to Climate Change? Annu Rev Entomol. 61(1):433-451. doi:10.1146/annurev-ento-010715-023859
- Régnière J, Powell J, Bentz B, Nealis V. 2012. Effects of temperature on development, survival and reproduction of insects: Experimental design, data analysis and modeling. J Insect Physiol. 58(5):634-647. doi:10.1016/j.jinsphys.2012.01.010
- Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK,...Whittaker JB. 2002. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Chang Biol. 8(1):1-16. doi:10.1046/j.1365-2486.2002.00451.x
- Block W. 2011. Low Temperature Biology of Insects, edited by David L. Denlinger and Richard E. Lee Jr. Cambridge University Press, Cambridge. ISBN 978-0-521-88635-2 Antarctic science. 23:500-500. doi:10.1017/S0954102011000678
- Williams CM, Henry HAL, Sinclair BJ. 2015. Cold truths: How winter drives responses of terrestrial organisms to climate change. Biol Rev. 90(1):214-235. doi:10.1111/brv.12105
- Catullo RA, Llewelyn J, Phillips BL, Moritz CC. 2019. The Potential for Rapid Evolution under Anthropogenic Climate Change. Curr Biol. 29(19):R996-R1007. doi:10.1016/j.cub.2019.08.028
- XUE Q, Majeed MZ, ZHANG W, MA C. 2019. Adaptation of Drosophila species to climate change — A literature review since 2003. J Integr Agric. 18(4):805-814. doi:10.1016/S2095-3119(18)62042-8
- Freeman S, Eddy SL, McDonough M, Smith MK, Okoroafor N, Jordt H, Wenderoth MP. 2014. Active learning increases student performance in science, engineering, and mathematics. Proc Natl Acad Sci U S A. 111(23):8410-8415. doi:10.1073/pnas.1319030111
- Kaddoura M. 2013. Think Pair Share: A teaching Learning Strategy to Enhance Students' Critical Thinking. Educ Res Q. 36(4):3-24.
- Smith MK, Wood WB, Adams WK, Wieman C, Knight JK, Guild N, Su TT. 2009. Why Peer Discussion Improves Student Performance on In-Class Concept Questions. Science. 323(5910):122-124. doi:10.1126/science.1165919
- Smith MK, Wood WB, Krauter K, Knight JK. 2011. Combining peer discussion with instructor explanation increases student learning from in-class concept questions. CBE Life Sci Educ. 10(1):55-63. doi:10.1187/cbe.10-08-0101
- Armstrong MA. 2011. Small World : Crafting an Inclusive Classroom ( No Matter What You Teach ). Thought and Action. (Fall):51-61.
- Gerken AR, Mackay TFC, Morgan TJ. 2016. Artificial selection on chill-coma recovery time in Drosophila melanogaster: Direct and correlated responses to selection. J Therm Biol. 59:77-85. doi:10.1016/j.jtherbio.2016.04.004
- Hoffmann AA, Anderson A, Hallas R. 2002. Opposing clines for high and low temperature resistance in Drosophila melanogaster. Ecol Lett. 5:614-618. doi:10.1046/j.1461-0248.2002.00367.x
- Kellermann V, Overgaard J, Hoffmann AA, Flojgaard C, Svenning J-C, Loeschcke V. 2012. Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc Natl Acad Sci. 109(40):16228-16233. doi:10.1073/pnas.1207553109
- Picone C, Rhode J, Hyatt L, Parshall T. 2007. Assessing gains in undergraduate students' abilities to analyze graphical data. Teach Issues Exp Ecol. 5(July):1-54. http://www.tiee.ecoed.net/vol/v5/research/picone/pdf/Picone_etal2007.pdf.
Article Files
Login to access supporting documents
Chilling in the Cold: Using Thermal Acclimation to Demonstrate Phenotypic Plasticity in Animals(PDF | 166 KB)
S1. Chilling in the ColdExample PowerPoint.pptx(PPTX | 2 MB)
S2. Chilling in the ColdFruit Fly Information.docx(DOCX | 70 KB)
S3. Chilling in the ColdStudent Handout.docx(DOCX | 22 KB)
S4. Chilling in the ColdExample Data.xlsx(XLSX | 2 MB)
S5. Chilling in the ColdClimate Handout.docx(DOCX | 18 KB)
S6. Chilling in the ColdLab Report Rubric.docx(DOCX | 17 KB)
S7. Chilling in the ColdStudent Grades.docx(DOCX | 16 KB)
- License terms

Comments
Comments
There are no comments on this resource.