Going Remote: An Online Adaptation to Using a Primary Cell Culture Model to Study the Neural Extracellular Matrix
Editor: Scott Gehler
Published online:
Abstract
In light of the COVID-19 pandemic, instructors have modified materials to transition to a remote learning platform. The challenge for science instructors has been to design lab exercises that incorporate both active and experiential learning. In this vein, in this Essay I describe how I modified a previously introduced Lesson using a primary cell culture model to study a specialized structure found within in the neural extracellular matrix, named the perineuronal net. When I taught this exercise originally, the students had completed the majority of the experiment in person, but to adapt to online learning, changes were made to Lab Sessions 3 and 4. Since students were not able to perform the analysis portion of the exercise, I provided images and the students were still tasked with completing a lab report. I made the handouts, information about the lab report, the rubric, and PowerPoints available through the learning management system. Herein, I also reflect on how an instructor could modify the entire four-week experiment to be conducted virtually. In the adapted exercise, students play a leading role in researching protocols, explaining why each step is carried out, establishing hypotheses, and drawing support for these statements from the literature. Therefore, it is possible to have students complete challenging and inquiry-based exercises even if they are not able to carry out the experiment directly. Moving forward, it will be particularly important to readily adapt exercises to fit into a remote learning environment, as described here.
Primary image: Primary cultures were created from the cerebral cortices of wild-type CD-1 mice, cultured, and then stained with anti-aggrecan antibodies and appropriate secondary antibodies to outline a perineuronal net.
The original published lesson: Using a Primary Cell Culture Model to Study the Neural Extracellular Matrix
Citation
Giamanco KA. 2020. Going remote: An online adaptation to using a primary cell culture model to study the neural extracellular matrix. CourseSource. https://doi.org/10.24918/cs.2020.44Society Learning Goals
Cell Biology
- Methods & Tools of Cell Biology
- How do the methods and tools of cell biology enable and limit our understanding of the cell?
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
In the Spring 2020 semester, the COVID-19 pandemic led schools, colleges, and universities, to shutter, thereby canceling in person instruction. This quickly forced students to adapt to learning online and forced instructors to adjust their classes to a remote teaching platform. Due to the nature of the pandemic, both students and teachers alike had little time to prepare for such a transition. This was particularly difficult for large courses, discussion-based courses, and lab classes. In this Essay, I will discuss how the COVID-19 pandemic impacted the delivery of a previous Lesson studying the neural extracellular matrix that was published in CourseSource (1). Additionally, I will detail ways in which the entire exercise can be formatted for virtual instruction. It is my hope that adopting instructors will find this information useful as the pandemic has rendered the immediate future of in-person classes and laboratories uncertain.
In brief, the original Lesson was taught over four weeks with the overall goal of examining the formation and molecular composition of a substructure of the neural extracellular matrix called the perineuronal net. This structure enwraps the cell body and extensions emanating from populations of neurons within the nervous system and has been hypothesized to modulate synaptic plasticity. In terms of composition, perineuronal nets are highly heterogenous and are comprised of sugars, glycoproteins, and proteoglycans (2,3). The exercise described in both the original Lesson and herein focuses on the role of the chondroitin sulfate proteoglycan, aggrecan, in the formation and organization of perineuronal nets.
This exercise is best suited for an upper-level undergraduate course in Cell Biology in which the students are Biology or Biochemistry majors. In Week 1, the instructor dissects the cerebral cortices of embryonic mice and the students use the tissue to isolate and then plate single cells. They then treat the cells with potassium chloride to globally enhance neural activity and with a glial cell inhibitor to eliminate glial cells from the cultures. These treatments are designed to allow students to answer two specific questions: (1) Is aggrecan expression modulated by activity? and (2) Is aggrecan likely made by neurons or glia? The cells are cultured for 14 days after which students fix, block, and stain the cells with primary antibodies aimed at identifying aggrecan and a glial cell specific marker. The instructor finishes the experiment by applying secondary antibodies and cover slipping the cells. The students then image the slides using a fluorescence microscope. During the time in which the cells are cultured, students generate hypotheses and predictions for the aforementioned questions by gathering support from the literature. There are two formative assessments after Lab Sessions 1 and 3, which allow for the instructor to provide meaningful feedback to the students in preparation for the summative assessment, the lab report (1).
LESSON ADAPTATIONS
How the Lesson was Adapted in Spring 2020
When my campus shut down in mid-March, my Cell Biology class had already completed Lab Session 1 of the exercise and the cultures had all been treated and were incubating for the requisite 14 days. All of my students had the opportunity to attend Lab Session 2, where we met in a computer lab to discuss the necessary background information about the experiment. Then students broke out into smaller groups to delve into the literature and state their own hypotheses. The timing of the school closure, allowed for only one of my two lab sections to complete Lab Session 3. This meant that only half of the class was able to fix, block and stain their cultures with primary antibodies. We also discussed the details of the lab report and assembled a list of reagents used in the experiment with associated company information.
To accommodate the switch to remote instruction, I made the pre-lab lectures for Lab Sessions 3 and 4 immediately available for students with notes, and used PowerPoint to record accompanying audio (see Supporting File S7. Studying the Neural Extracellular Matrix – Lab Session 3 Pre-Lab Lecture and Supporting File S9. Studying the Neural Extracellular Matrix – Lab Session 4 Pre-Lab Lecture in the original Lesson (1)). Additionally, I provided all students with a Word document detailing the names of the reagents that they needed to include in their lab report as well as corresponding company information with the intention that the students would find the location of each of the companies to cite in their lab reports (Supporting File S1. Going Remote: Studying the Neural Extracellular Matrix – Reagent Information).
Lab Session 4 also had to be modified. Given that we had invested a significant portion of the semester on this exercise, I still wanted my students to complete a lab report and thus, I opted to provide them with data to analyze. More specifically, I provided them with images of cultures stained with anti-aggrecan antibodies and anti-glial fibrillary acidic protein markers as well as Hoechst dye. To provide some context, I included written information as to what the students should be paying attention to when analyzing these images (Supporting File S2. Going Remote: Studying the Neural Extracellular Matrix – Lab Session 4 Modified Handout). Even though the students were not able to use the fluorescence microscopes firsthand, the lab handout still included instructions on how to operate the microscopes, and students took an online pre-lab quiz on this topic. The structure of the lab report remained the same even though the students could not analyze their own work. Instead, they used the provided data and then determined whether they could support their initial hypotheses. As explained in the original Lesson, the goal was not to have students generate a "correct" hypothesis, but rather to find support for their hypotheses and then discuss their own results in the context of a greater body of work (1).
Since I was not able to meet with the students face-to-face about the lab report, I continued to offer office hours remotely through Cisco WebEx. I also highly encouraged my students to email me with questions if they could not attend office hours as well as submit either complete or incomplete drafts of their lab report. Roughly 40% of my students submitted drafts of the lab report and those that took advantage of this opportunity reported being grateful for the feedback.
How the Entire Lesson Could be Adapted
In the following section, I will discuss how instructors wishing to adopt this lesson could modify the entire exercise to be delivered remotely. I also include information about how each session is carried out in a face-to-face versus a remote setting. Additionally, I provide tables comparing and contrasting these two ways of organizing the exercise.
Lab Session 1
In the face-to-face Lab Session 1, as detailed in the previously published Lesson, students were provided with dissected cerebral cortices from mice and they then carried out a series of steps to isolate and plate single cells from this material (1). Given the amount of time needed for the exercise, I typically do not give a pre-lab lecture, but I do administer a lab quiz to ensure that the students have read the lab handout (see Supporting File S4. Studying the Neural Extracellular Matrix – Lab Session 1 Handout in the original Lesson (1)). The questions on this lab quiz are confined to procedural questions instead of questions on the background material. After the students finish, they have to write the materials and methods in their own words and create a table that details their total cell counts, the calculated density, as well as how their cells were plated (i.e., how the cells were diluted to achieve the desired density). All of this information helps the students prepare their lab reports (Table 1).
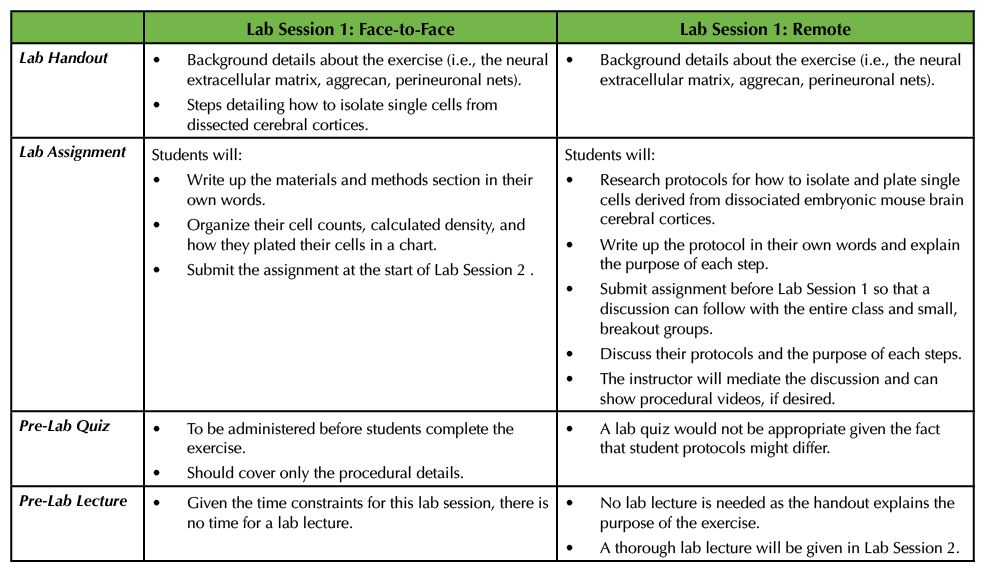
Table 1. This table outlines what material is covered in the lab handout, the particulars of the corresponding lab assignment as well as information about the lab quiz and lab lecture for Lab Session 1. In addition, this table compares and contrasts Lab Session 1 in a remote versus a face-to-face setting.
In the remote Lab Session 1, the instructor could start by stating that the objective of the exercise is to study aggrecan, a molecular constituent of the perineuronal net found in the neural extracellular matrix. They could provide the part of the original lab handout that explains the background information, but not any procedural details (see Supporting File S4. Studying the Neural Extracellular Matrix – Lab Session 1 Handout in the original Lesson (1)). The students could be tasked with delving into the literature to find protocols that allow investigators to isolate single cells from cerebral cortices derived from embryonic mice. More specifically, the students could write up the protocol using proper citations since they are not able to complete the procedure in person (see Supporting File S1. Studying the Neural Extracellular Matrix – Lab Session 1 Assignment in the original Lesson (1)). In addition, the students could explain the purpose of each of the steps in the protocol (Table 1). There are a number of different resources that students can consult, some of which are included here (4-8). Some articles even have accompanying videos for students to view part or all of a procedure (5).
This open-ended assignment would lend itself well to a discussion, as students could meet in groups to outline the procedures they found. The instructor could hold these discussions through their preferred platform, using Breakout rooms to allow for smaller discussions. In the smaller groups, the students could share the details of the protocols and explain to their peers the purpose of each step. For example, one can use trypsin or papain along with manual trituration to isolate single cells from the dissected cortices. Students should be able to explain why such chemicals are used in addition to how trypsin or papain are inhibited prior to plating. Additionally, students should also determine the composition of the media that the cells should be incubated in, and this can provide another discussion point. To wrap up the session, the entire class should convene so that students can ask follow-up questions, or the instructor can pose questions to the students about the procedural details. Given that in this exercise students will likely have varying steps in writing their own protocols, I would not recommend having a pre-lab quiz (Table 1).
Culture Treatments and care of cultures (in between Lab Sessions 1 and 3)
After the cultures are generated in the original Lesson, the cells are be broken up into experimental and control groups to manipulate overall neuronal activity with potassium chloride (KCl) and block glial cell growth with cytosine-β-D-arabinofuranoside (AraC). Students are responsible for treating the cultures on 1 day in vitro (DIV). Then the instructor removes these chemical treatments on 3 DIV by performing a full media exchange. Thereafter, the cultures are monitored, and half media exchanges are performed on 6, 9, and 12 DIV (1) (Table 2).
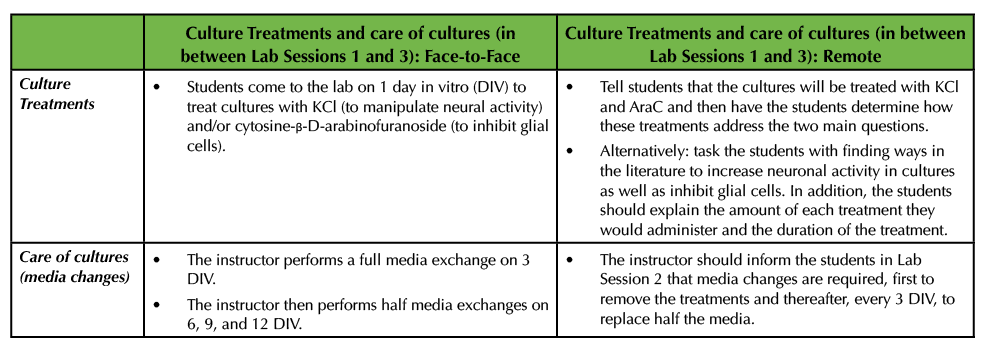
Table 2. In between Lab Session 1 and 3, cultures are treated with chemicals to increase bioactivity as well as inhibit glial cells. This table details how the cultures are treated and cared for in both a virtual, remote environment as well as in a face-to-face environment.
In the remote environment, the instructor has a few options. The instructor may wish to tell the students that the cultures will be treated with KCl and AraC. Then the students can come to Lab Session 2 having done some research into what these chemicals will do to the cells and how these treatments will help address the two main questions (Table 2). For example, there is an extensive body of literature detailing how the application of KCl increases bioactivity in neurons and this work delves into the underlying molecular mechanisms (9-16). The application of AraC has been widely used to limit glial cell proliferation (17-18) and students can be asked to provide support from the literature as to how both KCl and AraC have been used.
Alternatively, the instructor may wish to have the students propose ways to inhibit glial cell growth and increase neural activity by surveying relevant literature (Table 2). For example, investigators have reported that calcium permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptors and L-type calcium-voltage gated channels mediate the activity-dependent formation of perineuronal nets (4,19). In addition to AraC being used as an anti-mitotic agent, others have employed the application of fluorouracil (20). If the instructor chooses to have the students research ways to inhibit glial cell growth and increase the levels of neural activity, the students should also be asked to explain the concentration and the duration at which they would use the various chemicals (Table 2).
Lab Session 2
Lab Session 2 will be very much the same in a remote setting as it would be in a face-to-face environment. Both sessions are focused on students stating their own hypotheses and predictions based on the two questions provided by the instructor. The students survey the literature to make such assertions. They can work in small groups as well as with the instructor during this process (Table 3).
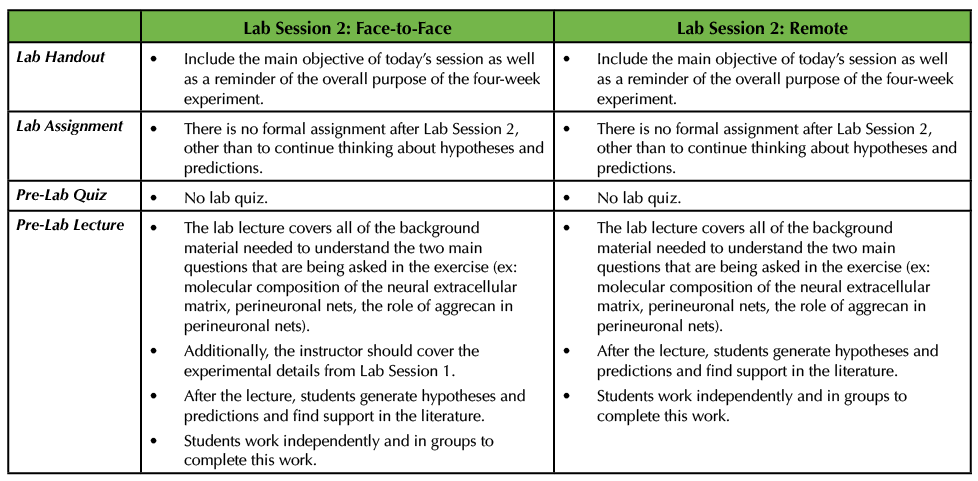
Table 3. In Lab Session 2, in both the remote and face-to-face circumstances, the instructor delivers a pre-lab lecture on all of the pertinent background information on the project. The students then work in groups to pose hypotheses and predictions to address the two main questions. This table explains how this would work remotely versus an in-person scenario.
The pre-lab lecture on the background material about the exercise, including how the neural extracellular matrix is structured, what perineuronal nets are, where they are found within the nervous system, and the proposed function of perineuronal nets will be the same in both settings, with a few minor modifications (consult Supporting Files S5 and S6. Studying the Neural Extracellular Matrix – Lab Session 2 Pre-Lab Lecture and Lab Session 2 handout, respectively in the original Lesson (1)). In the remote setting, the pre-lab lecture does not need to cover the experimental details for how to create primary cultures, as students have already researched and written up their protocols. Secondly, in the remote situation, the instructor should inform the students that full media exchanges must occur to remove the treatments and then after that, every 3 DIV half of the media is exchanged for each culture well (Table 3).
If the adopting instructor wishes to perform this lab session remotely, then they should hold a live meeting so that students can meet in small groups to discuss their hypotheses and predictions and use the time to find relevant literature. The instructor should design time in the session to move between these small groups and then holder a larger discussion at the end. In a face-to-face setting, I would recommend that the students meet in a computer lab to facilitate discussions and group work (Table 3).
Since the pre-lab lecture is focused on the background information about the experiment, I do not recommend a lab quiz for this session, in either format. In my opinion, it is best to allow the students time to synthesize the information about the neural extracellular matrix and perineuronal nets before being tested (Table 3).
Lab Session 3
In the original Lesson, the instructor provided the students with a lab handout detailing how the cells would be fixed, blocked, stained, and coverslipped (see Supporting File S8. Studying the Neural Extracellular Matrix – Lab Session 3 handout (1)). Students also took a pre-lab quiz based on the information in the lab handout and then the instructor delivered a brief pre-lab lecture explaining why cells are fixed, the importance of blocking, and the details of indirect immunofluorescence (see Supporting File S7. Studying the Neural Extracellular Matrix – Lab Session 3 Pre-Lab Lecture (1)). The students carried out these procedures, and the instructor finished the exercise the next day by adding secondary antibodies, staining the cells with a DNA dye, and mounting the coverslips onto glass slides (see Supporting File S8. Studying the Neural Extracellular Matrix – Lab Session 3 handout (1)). In the corresponding assignment, the students were told to write the protocols employed in Lab Session 3 in their own words. In addition, they were asked to state their hypotheses and predictions for the two main questions that were being asked in this experiment and include support from the literature. Lastly, this assignment also tasked the students with gathering background information on what perineuronal nets are, where they are found in the nervous system, and which molecules comprise perineuronal nets (see Supporting File S2. Studying the Neural Extracellular Matrix – Lab Session 3 Assignment (1) (Table 4).
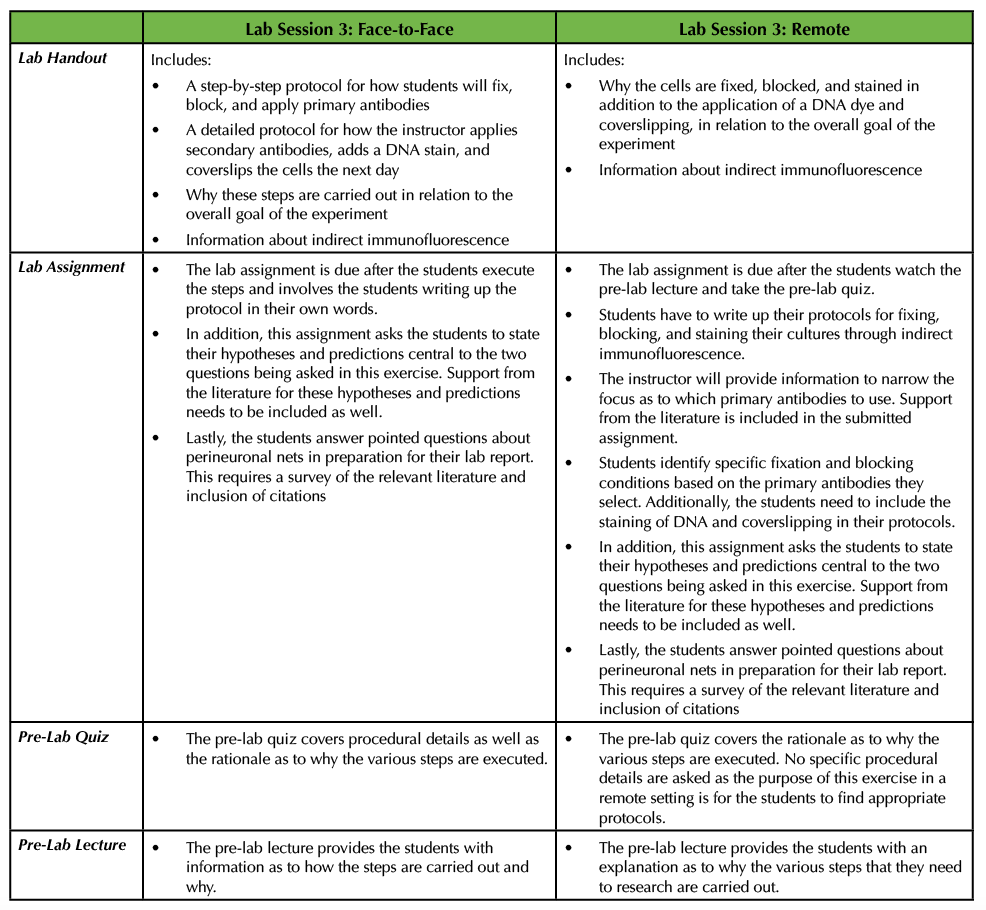
Table 4. In the third lab session, students perform portions of an indirect immunofluorescence protocol by fixing, blocking, and applying primary antibodies to the cultures. This table discusses how this can be adapted from a face-to-face exercise to a remote exercise.
Since Lab Session 3 requires a number of steps and the protocols for all of these techniques can vary widely, it is a wonderful opportunity to have students do research and write up their own step-by-step procedures with appropriate citations. This can be easily executed in the remote environment. In advance of the exercise, the instructor can provide a pre-lab lecture and a lab handout similar to the ones explained above for the face-to-face experiment with slight modification. More specifically, the instructor should not provide experimental details, but instead allow for the students to write their own protocols. The instructor should let the students know that they need to stain their cultures using anti-aggrecan and anti-glial fibrillary acidic protein (GFAP) primary antibodies and that the main goal is to analyze the results using indirect immunofluorescence (meaning that a secondary antibody is conjugated to a fluorophore). First, the students should determine which primary antibodies to use as there are different aggrecan and GFAP antibodies that are commercially available. To do this, the students will survey the literature to specifically find antibodies that have been used in primary cultures derived from embryonic mouse cortices rather than just searching more generally for anti-aggrecan and anti-GFAP antibodies. Next, the students need to identify appropriate secondary antibodies to use, as well as which dye to apply to stain DNA, and which cover slipping reagent to use. As part of their assignment for Lab Session 3, students should attach the articles that they found in which investigators used those primary antibodies. In doing so, the students will identify which fixative solution and blocking solutions to use. It is important for the students to understand that often times investigators try several different fixatives and blocking solution combinations to determine the best conditions. There are many resources for such staining procedures, both in the literature (21) and on the websites of companies that produce antibodies. The students should submit their complete protocols as part of their Lab Session 3 assignment in addition formalizing their hypotheses and predictions as well as answering specific questions about perineuronal nets as described above. In a remote setting, students would take a pre-lab quiz as to why the various steps enumerated above are performed (Table 4).
Lab Session 4
In the original Lesson in Lab Session 4, the students analyzed their results using a fluorescence microscope after taking a pre-lab quiz that covers the various components of the specific instrument that is being used in the experiment. The professor also delivered a pre-lab lecture (see Supporting File S9. Studying the Neural Extracellular Matrix – Lab Session 4 Pre-Lab Lecture (1)), which instructs students on how to use the microscopes, also detailed in the lab handout (see Supporting File S10. Studying the Neural Extracellular Matrix – Lab Session 4 Handout (1)). Each group worked together to interpret their results and then joined a larger discussion with the entire class so the instructor could determine how the students were answering the two central questions. The summative assessment is due two weeks after this exercise to give students time to prepare their formal lab reports (see Supporting File S12. Studying the Neural Extracellular Matrix – Lab Report and Rubric Information (1)) (Table 5).
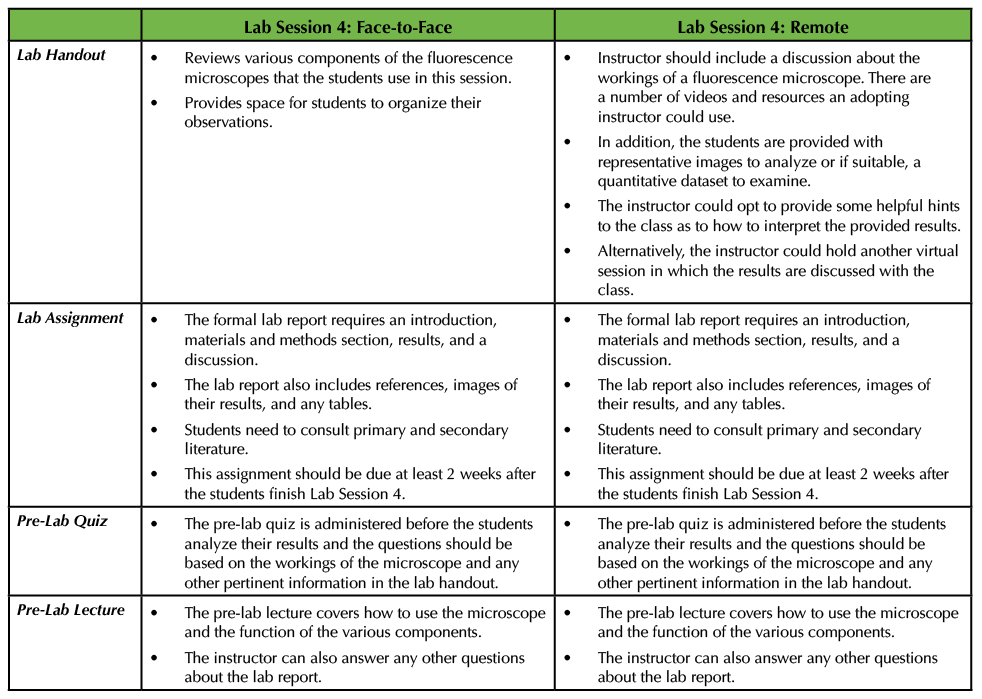
Table 5. In the last lab session, students analyze their results using a fluorescence microscope and gather observations to complete their formal lab report. In a remote situation, the instructor can provide a dataset for the students to analyze instead. This table summarizes how this lab session would run in these two situations.
In a remote setting the instructor will have to provide data for the class to examine since they cannot perform the microscopy work themselves. This can be accomplished by finding images from the literature and telling the students that these images are representative of what they would see under the microscope. Moreover, if the instructor wishes, the analysis could be more quantitative in nature, where the students are given a dataset to work with in addition to microscope panels. The students would then be tasked with analyzing the results in the context of the two questions that were posed (Supporting File S2. Going Remote: Studying the Neural Extracellular Matrix – Lab Session 4 Modified Handout). The instructor should also provide a brief pre-lab lecture that the students can access just as they would in a face-to-face setting. I would recommend that the instructor still includes information about the fluorescence microscopes as it would be beneficial for the students to understand how the equipment works. If the instructor opts to do this, then a pre-lab quiz could be administered about the components of a fluorescence microscope (Table 5). Many companies that manufacture and sell microscopes have videos that detail how a fluorescence microscope works (ex: a video from Leica: https://bitesizebio.com/webinar/fundamentals-of-fluorescence-microscopy/). The adopting instructor might wish to hold an additional virtual session in which the students can talk in small groups or with the instructor about the provided images or dataset.
SCIENTIFIC TEACHING THEMES
Active Learning
Since the students will not be able to execute this experiment in a hands-on manner, it is particularly imperative for the instructor to engage with the class throughout the four-week exercise. For example, the instructor will provide feedback on the two formative assessments and can choose to hold one-on-one or small group meetings with students either through formal virtual office hours or by appointment. Lab Session 2 is an important exercise in that the students will work with their classmates as well as the instructor to establish hypotheses and find supportive evidence for those statements. For the analysis portion of the experiment, the instructor can provide notes or information on how to interpret the data in the lab handout, or again, could choose to meet with students individually or in small groups. Given the remote setting, the instructor should make themselves available for students to ask questions and should create breakout discussion groups to promote peer discussion. In the online adaptation of the exercise, the students have more opportunities to explore the literature, research protocols, and understand at a deeper level why these steps are carried out.
Assessments
I recommend two formative assessments after Lab Session 1 and 3 and the major lab report as the final assignment. This format could be modified if the instructor wishes to have students prepare posters or create oral presentations. The weekly quizzes allow for the instructor to monitor if the students have been reading the handout and preparing for the exercises.
Inclusive Teaching
Since this experiment will be carried out remotely, the instructor should make sure that all students have access to computers, the internet, and any other software or technology that is needed. Some campuses are able to provide such equipment to students and instructors alike given the need for virtual learning and teaching. As it possible that not everyone has a dedicated and private workspace, the instructor should reassure the class that they do not need to use video, but instead can rely or audio or even just typing in a chat box.
I would recommend that students form their own discussion groups, just as they would if the experiment was conducted in person. This will provide a sense of familiarity and comfort, which is likely to be appreciated given the fact that the experiment is being carried out virtually.
In addition, when designing videos for the pre-lab lectures, the instructor should keep in mind that they should provide notes, audio, as well as video to accommodate students with disabilities. I also recommend following up individually with students to ensure that the material has been presented suitably.
Lastly, the instructor needs to make sure that the individualized feedback that the students receive on both of the formative assessments is thoughtful, constructive, and well-explained. Because the students do not have traditional face-to-face time, they will rely on this feedback to prepare their lab reports. In addition, the instructor should be open to suggestions or modifications from the students given the unconventional way of completing this experiment.
SUMMARY
This Essay describes a remote adaptation of a previously published Lesson using a primary cell culture model to study the neural extracellular matrix to a remote or virtual learning environment. Although the students do not carry out the actual experiments, they can still accomplish the main objective of answering two questions about how the perineuronal net of the neural extracellular matrix forms. More specifically, the students are responsible for finding appropriate protocols, setting forth hypotheses, finding support for these hypotheses and completing both formative and summative assessments. Given the unique and unusual situation of the current pandemic, this adaptation can still provide students with a thought-provoking, challenging, and exciting Lesson.
SUPPORTING MATERIALS
- Supporting File S1. Going Remote: Studying the Neural Extracellular Matrix – Reagent Information. This Word document contains a list of reagents and equipment that were used in Lab Sessions 1 and 3. Some of the reagents and equipment are considered to be common use or standard items in which the students do not need to include the company and the location of the company. For the other items, the instructor has included the name of the company that makes the product and for the lab report, the students should add that information in along with the location of the company.
- Supporting File S2. Going Remote: Studying the Neural Extracellular Matrix – Lab Session 4 Modified Handout. This handout has been modified from the original Lesson to reflect the fact that the students were not able to analyze the slides they created. Instead, the instructor provided microscope images for the students to examine and analyze, which served as the basis of the lab report.
- Additional supporting files used in the original version of the lesson and referenced in this article are available at https://doi.org/10.24918/cs.2020.9.
ACKNOWLEDGMENTS
I would like to thank the Cell Biology students enrolled in my class in Spring 2020. Thank you for being patient, accommodating, and persevering through this difficult semester. I appreciate all your hard work. Additionally, thank you to Kelly Nealon and Emma Baxley for taking care of the mice that were used during this past semester. I also want to thank my colleagues in the Department of Biological and Environmental Sciences for thoughtful discussions on how to adjust to remote teaching and Western Connecticut State University for supporting faculty, staff, and students during this challenging semester.
References
- Giamanco KA. 2020. Using a primary cell culture model to study the neural extracellular matrix. CourseSource. doi: 10.24918/cs.2020.9.
- Bandtlow CE, Zimmermann DR. 2000. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol. Rev. 80:1267-1290. doi: 10.1152/physrev.2000.80.4.1267.
- Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. 1998. Perineuronal nets: past and present. Trends Neurosci. 21:510-515.
- Dityatev A, Brückner G, Dityateva G, Grosche J, Kleene R, Schachner M. 2007. Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev. Neurobiol. 67:570-588. doi: 10.1002/dneu.20361.
- Hilgenberg LGW, Smith MA. 2007. Preparation of Dissociated Mouse Cortical Neuron Cultures. J. Vis. Exp. 10:562. doi: 10.3791/562.
- Giamanco KA, Morawski M, Matthews RT. 2010. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience. 170:1314-1327. doi: 10.1016/j.neuroscience.2010.08.032.
- Giamanco KA, Matthews RT. 2012. Deconstructing the perineuronal net: cellular contributions and molecular composition of the neuronal extracellular matrix. Neuroscience. 218:367-384. doi: 10.1016/j.neuroscience.2012.05.055.
- Sahu MP, Nikkilä O, Lågas S, Kolehmainen Su, Castrén E. 2019. Culturing primary neurons from rat hippocampus and cortex. Neuronal Signal. 3: NS20180207. doi: 10.1042/NS20180207.
- Franklin JL, Fickbohm DJ, Willard AL. 1992. Long-term regulation of neuronal calcium currents by prolonged changes of membrane potential. J. Neurosci. 12:1726-1735. doi: 10.1523/JNEUROSCI.12-05-01726.1992.
- Vallano ML, Lambolez B, Audinat E, Rossier J. 1996. Neuronal activity differentially regulates NMDA receptor subunit expression in cerebellar granule cells. J. Neurosci. 16:631-639. doi: 10.1523/JNEUROSCI.16-02-00631.1996.
- Oberdorf J, Vallano ML, Wojcikiewicz RJ. 1997. Expression and regulation of types I and II inositol 1,4,5-trisphophate receptors in rat cerebellar granule cell preparations. J. Neurochem. 69:1897-1903. doi: 10.1046/j.1471-4159.1997.69051897.x.
- Mellor JR, Merlo D, Jones A, Wisden W, Randall AD. 1998. Mouse cerebellar granule cell differentiation: electrical activity regulates the GABAA receptor alpha 6 subunit gene. J. Neurosci. 18:2822-2833. doi: 10.1523/JNEUROSCI.18-08-02822.1998.
- Fickbohm DJ, Willard AL. 1999. Upregulation of calcium homeostatic mechanisms in chronically depolarized rat myenteric neurons. J. Neurophysiol. 81:2683-2695. doi: 10.1152/jn.1999.81.6.2683.
- Tremper-Wells B, Mathur A, Beaman-Hall CM, Vallano ML. 2002. Trophic agents that prevent neuronal apoptosis activate calpain and down-regulate CaMKIV. J. Neurochem. 81:314-324. doi: 10.1046/j.1471-4159.2002.00829.x.
- Bui CJ, Beaman-Hall CM, Vallano ML. 2003. Ca(2+) and CaM kinase regulate neurofilament expression. Neuroreport. 14:2073-2077. doi: 10.1097/00001756-200311140-00013.
- Moulder KL, Cormier RJ, Shute AA, Zorumski CF, Mennerick S. 2003. Homeostatic effects of depolarization on Ca(2+) influx, synaptic signaling, and survival. J. Neurosci. 23:1825-1831. doi: 10.1523/JNEUROSCI.23-05-01825.2003.
- Negishi T, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. 2003. Primary culture of cortical neurons, type-1 astrocytes, and microglial cells from cynomolgus monkey (Macaca fascicularis) fetuses. J. Neurosci. Methods. 131:133-140. doi: 10.1016/j.jneumeth.2003.08.006.
- Rhodes KE, Moon LDF, Fawcett JW. 2003. Inhibiting cell proliferation during formation of the glial scar: effects on axon regeneration in the CNS. Neuroscience. 120:41-56. doi: 10.1016/S0306-4522(03)00285-9.
- Brückner G, Grosche J. 2001. Perineuronal nets show intrinsic patterns of extracellular matrix differentiation in organotypic slice cultures. Exp. Brain Res. 137:83-93.
- Pinedo HM, Peters GF. 1988. Fluorouracil: biochemistry and pharmacology. J.Clin. Oncol. 6:1653-1664. doi: 10.1200/JCO.1988.6.10.1653.
- Marchenko S, Flanagan L. 2007. Immunocytochemistry: Human Neural Stem Cells. J. Vis. Exp. 7:267. doi: 10.3791/267.
Article Files
Login to access supporting documents
Going Remote: An Online Adaptation to Using a Primary Cell Culture Model to Study the Neural Extracellular Matrix(PDF | 197 KB)
S1. Going Remote Studying the Neural Extracellular Matrix - Reagent Information.docx(DOCX | 14 KB)
S2. Going Remote Studying the Neural Extracellular Matrix - Modified Lab Session 4 Handout.docx(DOCX | 824 KB)
- License terms

Comments
Comments
There are no comments on this resource.