Nanoparticles and Shrimp: An Interdisciplinary Lab Series in Chemistry and Biology for Undergraduate Engineering Students
Editor: Valerie Haywood
Published online:
Abstract
The parallels between engineering and science are vast, as both disciplines apply theory and inquiry to solve complex problems. In order to prepare students for interdisciplinary careers in STEM, we have developed a lab project in which engineering students in an Engineering Chemistry course pair with engineering students taking Cellular and Molecular Biology to investigate the biological relevance of superparamagnetic iron oxide nanoparticles (SPIONs). SPIONs are at the forefront of investigation across several different fields, which makes them an exceptional interdisciplinary educational tool. The collaboration takes place over the course of three (3), 2-hour lab periods, with additional checkpoints during the biology component. Two of these periods are designated for synthesis and characterization of SPIONs by the chemistry students, and one lab period is designated for induction of toxicity assays and cytotoxicity analysis by the biology students. Rather than a traditional written lab report, we have challenged our students to infuse their analysis with creativity by filming a video lab report. Scores are generated based on presentation style and creativity, scientific accuracy, ability to develop and explain their hypothesis, data analysis, and interpretation of findings on a larger scale.
Primary image: SPIONs Lab Project Outline: Outline of lab sequence for a collaborative project between biology and chemistry students.
Citation
Grove LE, Rogers RP, Alibeik S. 2020. Nanoparticles and shrimp: An interdisciplinary lab series in chemistry and biology for undergraduate engineering students. CourseSource. https://doi.org/10.24918/cs.2020.52
Society Learning Goals
Ecology
- Impacts of Humans on Ecosystems
- What impacts do humans have on ecosystems?
Science Process Skills
- Process of Science
- Interpret, evaluate, and draw conclusions from data
- Construct explanations and make evidence-based arguments about the natural world
- Modeling/ Developing and Using Models
- Recognize the important roles that scientific models, of many different types (conceptual, mathematical, physical, etc.), play in predicting and communicating biological phenomena
- Make inferences and solve problems using models and simulations
- Build and evaluate models of biological systems
- Communication and Collaboration
- Share ideas, data, and findings with others clearly and accurately
Lesson Learning Goals
From the Ecology Learning Framework:
- What impacts do humans have on ecosystems? Students will explore the properties of different types of SPIONs and investigate their toxicity effects on an organism.
From the Science Process Skills Learning Framework:
- Predicting Outcomes. Students will generate a hypothesis on the effect of different types of SPIONs on brine shrimp.
- Interpreting results/data. Students will analyze data from several different experiments to assess toxicity.
- Communicating results. Students from different courses will work together as part of a larger group to contribute to the project; and students will work together to develop a video lab report that communicates the experimental details and results.
Lesson Learning Objectives
Students will be able to:
- design a synthesis for SPIONs given the basic formulation.
- use spectroscopy to measure the quantity of a solute in solution.
- conduct an NMR experiment to measure T1 relaxation.
- develop a hypothesis related to toxicity of SPIONs based on functionalization.
- use trypan blue for preparing biological samples to study mortality rates.
- set up a population growth experiment.
- analyze data from multiple experiments to draw a conclusion about the effects of a pollutant on an organism.
- collaborate with individuals from different backgrounds to produce an interdisciplinary final product.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
The first year for a typical engineering major at any school tends to be intensive in science and math courses, with very little actual coursework in engineering. Thus, with minimal expertise in their engineering field of interest, it can be challenging for an engineering major to recognize how science course content is relevant to their discipline (1). Additionally, with nanotechnology becoming an increasing presence in science and technology, there is a growing need to educate future engineers on the properties and applications of this technology (2,3). With these issues in mind, we developed a collaborative project investigating the synthesis, properties, and toxicity of superparamagnetic iron oxide nanoparticles (SPIONs) for introductory chemistry and biology courses at Wentworth Institute of Technology (Figure 1). In addition to enhancing technical components in chemistry and biology labs, the primary learning outcomes of this lab series focus on understanding the interaction between metallic compounds and cells through collaboration.
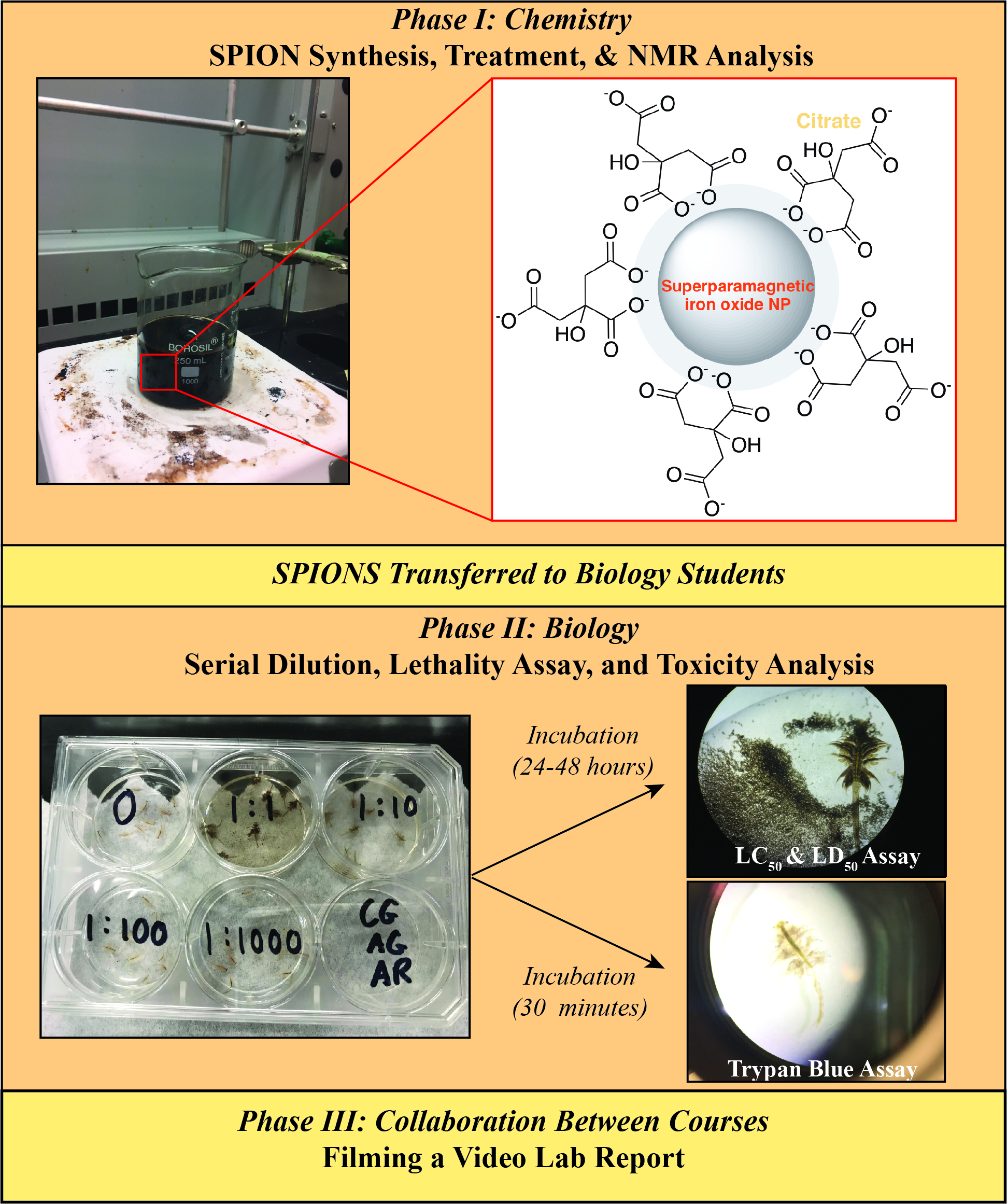
Figure 1. Flow chart of combined chemistry and biology labs and dissemination.
Besides the growing importance of nanotechnology, nanoparticles were selected due to the ease of synthesis (4,5) and the variety of properties that can be investigated (6,7). A number of lab experiments on the synthesis of nanoparticles have been reported (2,3,8-12); however, this lab series brings together engineering students from two different science curricula, exposing them to the interconnections of science and technology and how the development of a technology can be multidisciplinary (13-15). Specifically, this project was framed as an investigation into the use of SPIONs as contrast agents in MRI (16), which directly relates to future coursework for our biomedical and biological engineering majors. Since numerous synthetic variations are available, students can develop and test a hypothesis relating to either the magnetic properties or toxicity of the nanoparticles. Furthermore, background related to the construction of the NMR and MRI instruments, including the superconducting coil, provides interest for our electrical and mechanical engineering students.
To begin, chemistry students synthesize the SPIONs and determine their effect on the T1 relaxation of water hydrogens using NMR spectroscopy (17). SPIONs are readily prepared by mixing ferrous and ferric solutions in a 1:2 ratio (Eqation 1) under basic conditions (4,18). However, synthesis must be performed under inert atmosphere to ensure formation of the superparamagnetic magnetite Fe3O4 form; otherwise, the sample may oxidize to the maghemite Fe2O3 form. A synthesis under normal atmospheric conditions has been reported and is more suitable for introductory lab experiments (19). A ratio of Fe(II) to Fe(III) of 2:3 and total Fe concentration of 200 mmol were found to produce nanoparticles with magnetization properties similar to those produced under inert atmosphere (20). After SPION formation, students determine the iron content by dissolving the SPIONs in acid and generating the red ferrous o-phenanthroline compound, which is detected using US/Vis spectroscopy (21,22). Afterwards, students assess the effect of their SPIONs on the T1 NMR relaxation of water. A discussion of UV/Vis and NMR spectroscopy are used to support chemistry class content in electronic structure theory (2,8,9,23).
Equation 1. Fe2+ + 2 Fe3+ + 8 OH- → Fe3O4 + 4 H2O
A number of common functionalization routes have been reported, including the use of tethered gadolinium complexes to improve T1-T2 contrast (24) and groups such as Tween 80, polyvinyl alcohol, or polyethylene glycol to improve biocompatibility (25-29). For this lab, some student groups functionalized their nanoparticles with citrate and some did not, the latter serving as a control. Chemistry students performed their own research into the chemistry of citrate and developed a hypothesis on how citrate will influence the T1 relaxation of the water protons. Students on the biology team are familiar with the role of citrate in cellular respiration and contributed to discussion surrounding impact on uptake and toxicity. Citrate-functionalization of SPIONs has been shown to improve cellular uptake, minimize nanoparticle aggregation, and have minimal effect on the T1 relaxation time (30).
Upon completion of chemical analysis, the biology students assessed toxicity of the nanoparticles by investigating lethality and cytotoxicity using brine shrimp (Artemia salina) as a model. While nanoparticles have a diverse range of promising applications, there is concern regarding the impact on living organisms and ecosystems (8). Making connections between the biological impact of nanoparticles and their toxicology is very relevant to the engineers who may use them (31-34). Systems previously used in the undergraduate setting to assess toxicity include paramecia and E. coli (7,8). However, brine shrimp can withstand extreme conditions and environmental changes; thus, exposure to agents that result in substantial demise serves as an adequate reflection of the overarching impact of a given event. In this specific experiment, citrate is used because it is naturally utilized by cells to facilitate cellular respiration. However, a synthetic variant could become toxic past a certain concentration and should be assessed. Since Artemia are multicellular eukaryotic organisms that participate in such respiratory activities, any death following exposure to the student-made SPIONS would reflect toxicity and thus assist in identifying the ideal concentration for uptake without lethality. Based on these factors, it can be suggested that Artemia salina is an ideal organism for this particular experiment (10,35).
In an undergraduate setting, it is challenging to employ common assays that are used to assess viability and lethality because of cost and biosafety restrictions (36). We have circumvented this issue by employing the trypan blue stain, which is a safer alternative that can be used to assess cell survival following exposure to chemicals, nanoparticles, and experimental drugs. The ratio of live:dead cells can be used as a preliminary assessment of cytotoxicity and translated to tissue viability. In addition to having a low biosafety risk, increased convenience, and a low cost, brine shrimp-based assays allow students to investigate the immediate impact of SPION samples in a time frame conducive with undergraduate coursework (35).
Upon completion of this lab series, students completing the chemistry portion of the lab should be able to follow simple synthetic chemistry techniques, demonstrate knowledge of the origin of magnetism, and perform a T1 relaxation NMR experiment. Students completing the biology lab should be able to explain the relationship between LD50 and LC50 as it applies to cytotoxicity and cell death in a model organism. Students from both courses should be able to collaborate to synthesize complimentary information during data analysis to develop and communicate a conclusion.
Intended Audience
This lab series was designed for first-year college students in introductory biology and chemistry courses. It could be modified to do at the high school level. In this case, the T1 NMR measurement could be dropped, as most high schools do not have access to NMRs. Students could focus simply on synthesis of the SPIONs and assessment of toxicity. Alternatively, high school classrooms could collaborate with their local university to access NMR instrumentation.
Required Learning Time
The following procedure is split into three (3), 2-hour labs: (1) Synthesis, (2) Chemical and NMR Analysis, and (3) Toxicity Studies. However, the chemical synthesis and NMR analysis could be fit into a 4-hour lab, followed by a 2-hour biology lab.
Prerequisite Student Knowledge
Students in biology should be familiar with microscopy and cellular respiration. Students in chemistry should be familiar with solution chemistry, atomic structure, and oxidation-reduction chemistry.
Prerequisite Teacher Knowledge
Teachers should know microscopy for the biology component. For the chemistry component, teachers should have an understanding of nanotechnology. They should also know how to run simple UV/Vis and NMR experiments. For both, they should know how to use Excel for simple data analysis. For UV/Vis and NMR spectroscopies, we used equipment from Vernier and Nanalysis, respectively. Both company’s websites have comprehensive information for educators on how to setup instrumentation and run experiments.
SCIENTIFIC TEACHING THEMES
Active Learning
This lab series highlights the natural relationship between chemistry and biology. In order to highlight the symbiotic nature of these fields, we specifically chose to have students work separately on components and then come together to promote collaboration. This is a common occurrence in the biomedical field; thus, it recapitulates the working field model. Throughout the lab sequence, students in chemistry and biology sections are required to meet and discuss their data to prepare for a final video lab report. In completing this project, students must be capable of explaining their data and analysis to peers in the opposite class and work together to assess their hypotheses, thus reinforcing the learning experience. Students are given very little restrictions and are asked to creatively, but clearly, describe the background theory, their hypothesis, results, and analysis of hypothesis.
In addition to containing an interdisciplinary educational component, the labs are also excellent tools to support classroom instruction. For the biology component, this lab is conducted in parallel with a module on cellular respiration, and the role of citrate is highlighted as an integral component of the Kreb’s cycle. In chemistry, the lab experiments overlap with lecture material in solid state systems and molecular orbitals, thus linking nanoparticles and their unique size-dependent properties to theory. Additionally, the measurement of the SPIONs’ paramagnetic behavior using NMR spectroscopy directly relates to conversations on molecular orbital and band theory on how electronic structure dictates magnetic properties. This lab activity helps students to understand the utility of such abstract concepts. The biology and chemistry components can be strengthened by allowing students to do some data analysis in class, ending with a discussion of results. The students are provided with limited background on SPIONs prior to the lab in effort to promote independent learning via consultation of primary publications.
Assessment
There are several different checkpoints to provide learning support and encourage communication across the group. Both chemistry and biology students individually turn in their written lab analyses to their respective instructor for feedback before the final lab report. The instructors promptly return these analyses with feedback so that the students can make appropriate changes and thus learn from their errors. Students must also submit evidence (in the form of a photo) of their joint meeting to work on the project. In addition to the photo, students complete a partner evaluation for each member of the combined group (Figure S4). This provides insight on the dynamics of the group as a whole and contributes to the overall faculty assessment of individual students. The final assessment is a video lab report, completed by the group of chemistry and biology students. The video lab report should address: an introduction to nanotechnology, their hypotheses, results from the chemistry and biology labs, and an overall assessment of their hypotheses based on the gathered data. Faculty from both the chemistry and biology components grade the video lab reports and then average their results to obtain the final grade.
Inclusive Teaching
Our university has incorporated elements of inclusivity throughout all curricula. In the lab, specifically, we follow the inclusive-by-design approach as opposed to being inclusive-on-request (37). There are four (4) lab stations in the biology and chemistry labs that are specifically designed to accommodate those with physical disabilities as well as processes for chemical, equipment, and waste handling that allow students to participate in all aspects of the experiment.
With respect to fostering an environment that promotes productivity and elevates the learning experience for all students, we have diversified the materials and models used to deliver protocols and assessments of student work. Specifically, we provide all lab protocols in advance so that students can interpret and paraphrase the methods in a way that is most accessible for their learning. In each discipline, students work in self-formed groups. However, during the formation of the larger combined groups, faculty are intentional, making certain that each group is representative of the school’s population as a whole. In doing so, the final groups have a mix of students from different majors, years, demographic backgrounds, and sexes.
With regards to assessment, we have students produce both written and oral presentations, which allows students to demonstrate their learning in more than one format. Support for the production of the final video is available in the form of lab access, faculty access, sample work, and detailed guidelines. At all points during the lab series, students are encouraged to interact with one another and with faculty to ensure their educational needs are being met.
LESSON PLAN
In the procedure described below, chemistry student groups vary the amount of citrate used to functionalize their nanoparticles during the synthesis step, with some groups acting as the control and adding no citrate (Supporting File S1. Toxicity of SPIONs – Lab Handout). Biology students receive the nanoparticles following NMR analysis and perform toxicity assays with SPIONs diluted in seawater. While it rarely occurred that a student was enrolled in both biology and chemistry courses during the same semester, we did make sure to choose student groups such that there would only be one such case per biology/chemistry student group. Details on the Lesson Plan can be found in Table 1.
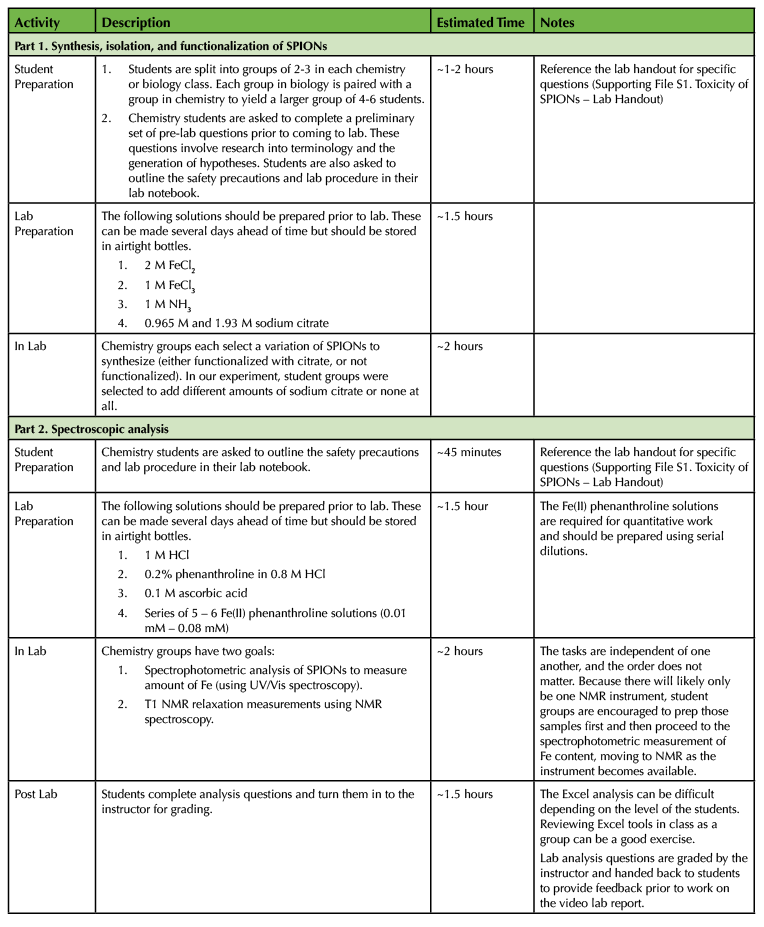
Table 1. Toxicity of SPIONs lab – Lesson Plan Timeline.

Table 1. Toxicity of SPIONs lab – Lesson Plan Timeline.
Materials and Instrumentation
For the chemistry labs, this experiment uses standard laboratory glassware, standard 5 mm NMR tubes, coaxial insert tubes for 5 mm NMR tubes (Norell), 2-by-1/16 inch Neodymium rare earth disc magnets (CMS Magnetics), 2 M iron (II) chloride, 1 M iron (III) chloride, 1 M ammonia, 1.93 M sodium citrate, hydrochloric acid, ascorbic acid, 1,10-phenanthroline monohydrate, and deuterium oxide (Aldrich). The Beer's Law analysis was completed using the Vernier SpectroVis Plus and LabQuest 2. NMR data were collected using the Nanalysis NMReady-60 60 MHz benchtop NMR. NMR data analysis was completed using the ACD/Spectrus Processor software.
For the biology lab, brine shrimp (Carolina, Supporting File S2. Toxicity of SPIONs – Maintenance of brine shrimp), Instant Ocean Sea Salt 33g/L (sodium chloride, magnesium chloride, sodium sulfate, calcium chloride, and potassium chloride; Carolina), four (4) 15 ml conical tubes, one (1) 6-well plate, three (3) petri dishes, trypan blue, phosphate buffered saline (PBS), paintbrush for collecting brine shrimp, mechanical tissue disruptor tool, plastic pestles that fit into the 1.5 ml microfuge tubes, 1.5 ml microfuge tubes, micropipettes and micropipette tips, glass slides, a dissecting microscope, and a compound light microscope were used.
Laboratory Exercise
Synthesis, Isolation, and Functionalization of SPIONs
The aerobic synthesis of iron oxide nanoparticles is dependent on the ratio of Fe2+ to Fe3+ in solution (20,30). The procedure given to students uses a 2:5 ratio of ferrous to ferric iron. 1.5 mL of 2 M FeCl2 was mixed with 7.5 mL of 1 M FeCl3, in which concentrated HCl has been added until FeCl3 is fully dissolved. Fifty (50) mL of 1 M NH3 was added as the solution stirred to bring the pH of the solution above 9. At this point, students can either allow the nanoparticles to continue stirring for 30 minutes or add an assigned quantity of 1.93 M sodium citrate solution and stir for 30 minutes. For the sample data provided within, 3 different samples were prepared: (1) a control, in which students added 50 mL of DI water, (2) a sample made using 50 mL of 0.965 M sodium citrate solution (final sodium citrate concentration of 0.483 M), and (3) a sample made using 50 mL of 1.93 M sodium citrate solution (final sodium citrate concentration of 0.965 M).
To isolate the SPIONs, 2-by-1/16 inch Neodymium rare earth disc magnets were used to magnetically decant and rinse the nanoparticles. The disc size was chosen to cover the base of the beaker. Students can then easily decant most of the solution with minimal loss of the nanoparticles. Four rinses with DI water were typically performed. The SPIONs can be dried or re-dispersed in 100 mL of water. If brine shrimp are used for toxicity studies, then the SPIONs were re-suspended in saline solution.
For the NMR and toxicity studies, the Fe content of the SPIONs was determined using spectrophotometric analysis. A 5 mL SPION sample was diluted to 100 mL using DI water. The SPIONs were then re-dispersed, and a 1 mL aliquot was transferred to a 100 mL beaker. One (1) mL of 50 - 60°C 1 M HCl was added while the solution stirred to dissolve the nanoparticles. Twenty (20) mL of 0.1 M ascorbic acid was added to reduce all iron to Fe2+, followed by the addition of 3 mL of a 0.2% solution of phenanthroline in 0.08 M HCl. The solution was stirred for 10 minutes. A Beer's Law relationship at a wavelength of 512 nm was determined using a series of Fe(II)-phenanthroline solutions with concentrations of 0.01 mM to 0.08 mM (21,22).
T1 NMR Relaxation Measurements
NMR samples of the SPIONs were created using standard 5 mm NMR tubes with coaxial inserts. The target total Fe concentration was 0.1 - 1.5 mM. To prepare the NMR sample, 700 µL of D2O was added to an NMR tube. To a coaxial NMR tube insert, 80 µL of the SPION sample was added. This sample was then sonicated for approximately one minute to ensure suspension of nanoparticles. Nanoparticles will remain suspended for approximately 20 - 30 minutes, during which time the insert tube was added to the NMR tube and NMR data collected (17,38). The Nanalysis NMReady 60 uses an inversion recovery pulse sequence, which is a 180-degree pulse, followed by a tau delay and then a 90-degree pulse. The scan parameters were: ten (10) points with a tau stop of 20000 ms. The spectral width was set to 10 ppm with a resolution of 4K. Depending on the T1 experiment parameters, data acquisition typically takes 5 to 10 minutes.
Toxicity Studies
Biology students received SPION samples from the chemistry students. Serial dilutions of 1:1, 1:10, 1:100, and 1:1000 using seawater were created for brine shrimp assays. Lethality assays were conducted in a 6-well plate filled with corresponding dilutions in addition to a well filled with pure seawater as a control. Ten (10) brine shrimp were transferred into each well using a paintbrush, observed using a stereoscope, and incubated for 2 - 5 days. After 2 - 5 days, students returned to quantify live brine shrimp and calculate the % dead brine shrimp in each sample using Equation 2.
Equation 2. % dead= (Number of dead brine shrimp/total number of brine shrimp)*100%
Cellular toxicity was assessed by transferring two (2) brine shrimp into a petri dishes containing 2 ml of either pure seawater, 1:10 SPION dilution, or 1:1000 SPION dilution. After incubation for 25 - 35 minutes, one specimen from each petri dish was placed on a glass slide and washed in 50 µL of PBS. PBS was removed via wicking before 60 µL of trypan blue was added directly to the brine shrimp on the slide. Brine shrimp were incubated in the trypan blue for approximately one minute at room temperature before excess liquid was wicked away. Specimens were once again washed with PBS to remove residual stain before blue:clear cell quantifications were made using a compound light microscope.
Following live observations, the specimen was transferred to a 1.5 ml microfuge tube with 50 µL of PBS. The mechanical tissue disruption tool was employed to grind the sample into a cellular suspension for two minutes. After the sample was completely homogenized, 40 µL of the cell suspension was used to make a wet mount of the sample to be observed on high power. Cytotoxicity was qualitatively assessed using the scale in Table 2.
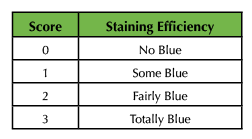
Table 2. Qualitative scale to assess cytotoxicity using trypan blue.
Potential modifications
There are a number of alternative syntheses that can stem from this basic synthesis, including the use of other functional groups or other metal solutions in place of Fe (II) chloride. These variations can impact both biological function and magnetic properties (4,5). For example, gadolinium complexes can be tethered to the outer shell of SPIONs to produce T1-T2 dual modal contrast agents (24). Biocompatibility and a reduction in toxicity has been achieved using Tween 80 (25), polyvinyl alcohol derivatives (26,27), and polyethylene glycol (28,29). Besides surface functionalization, varying the metal content can yield SPIONs with improved magnetic behavior (39). Substitution with Mn2+ has been shown to produce SPIONs with improved MRI relaxivity (r2) values (40). Surprisingly, substitution with Zn2+ also yields improved magnetic saturation behavior due to Zn2+ disrupting the antiferromagnetic coupling between Fe ions (41).
Hazards
Good lab hygiene should be practiced at all times, including wearing gloves, lab coats, and safety goggles. All materials should be handled with extreme caution to prevent accidental exposure. Iron (III) chloride and 1,10-phenathroline monohydrate are very toxic if ingested. 1,10-phenanthroline monohydrate, ascorbic acid, sodium citrate, iron (III) chloride, and iron (III) chloride are hazardous in case of contact with skin or eyes. Ammonia and hydrochloric acid are contact and inhalation hazards and should be handled in the fume hood. Nanoparticles created in this experiment are toxic and must be disposed of according to local and state regulations. The rare earth magnets are strong magnets; they can pinch fingers and should be segregated from another and other objects that they may be attracted to. Trypan blue can pose health hazards if ingested and may cause respiratory sensitivity if directly inhaled. To prevent direct exposure, students should handle the stain under a snorkel or in a biosafety cabinet.
Expected Results
SPION Synthesis and Characterization
The synthesis of SPIONs and resulting functionalization with citrate yielded jet black nanoparticles suspended in basic media (Figure 1, inset). Both the control and citrate-functionalized SPIONs were attracted to the rare earth magnets. After a 1:20 dilution in DI water, the students dissolved their SPIONs by acidifying their SPION solution and reduced all Fe3+ to Fe2+ via the addition of 0.1 M ascorbic acid solution. The addition of a 0.2% phenanthroline solution yielded the ferrous phenanthroline coordination compound, which is red in color. After this process, there were no visible remaining SPIONs. The students were given ferrous phenanthroline solutions with concentrations ranging from 0.01 to 0.08 mM to determine the Beer's Law relationship. The extinction coefficient of the ferrous phenanthroline solution was found to be 10.5 mM-1 cm-1 at 512 nm. Resulting concentrations of the diluted SPIONs ranged from 0.012 to 0.061 mM.
NMR samples for the T1 calculations had concentrations ranging from 0.1 to 1 mM. Samples that were higher than 1 mM tended to give poor results. Peak intensity at 4.7 ppm was measured for each time point, and the data were fit to the linearized equation for T1 saturation (Equation 3). Averaged T1 values for the SPION samples are presented in Table 3. As shown, the T1 relaxation of water is decreased substantially in the presence of SPIONs, as expected. The addition of citrate had no observable effect on the T1 behavior of the SPION-water samples.
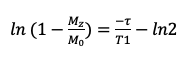
Equation 3.
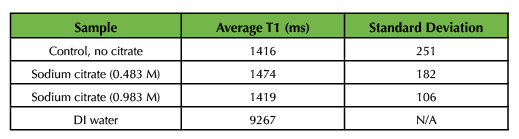
Table 3. Average T1 values and standard deviations for SPIONs synthesized with and without citrate functionalization.
Assessment of Toxicity
The brine shrimp toxicity analysis was completed for the three different sets of SPIONs. Following serial dilution of samples, brine shrimp were added to designated environments for incubation (Figure 2A). Samples incubated with control SPIONS showed little uptake and rapid mortality over the course of 24 hours, suggesting heightened toxicity. Samples incubated with 0.483 M and 0.983 M citrate-treated SPIONs showed immediate uptake (Figure 2B and 2C), yet variable cytotoxicity and lethality across groups. Compiled data suggest that a 1:1000 dilution of 0.983 M citrate-treated SPIONs results in lowest toxicity at both cellular and organismal levels between 24 - 48 hours. After 72 hours, all samples, including those incubated in pure seawater, demonstrated a rapid decline in survival (Table 4 and Table 5).
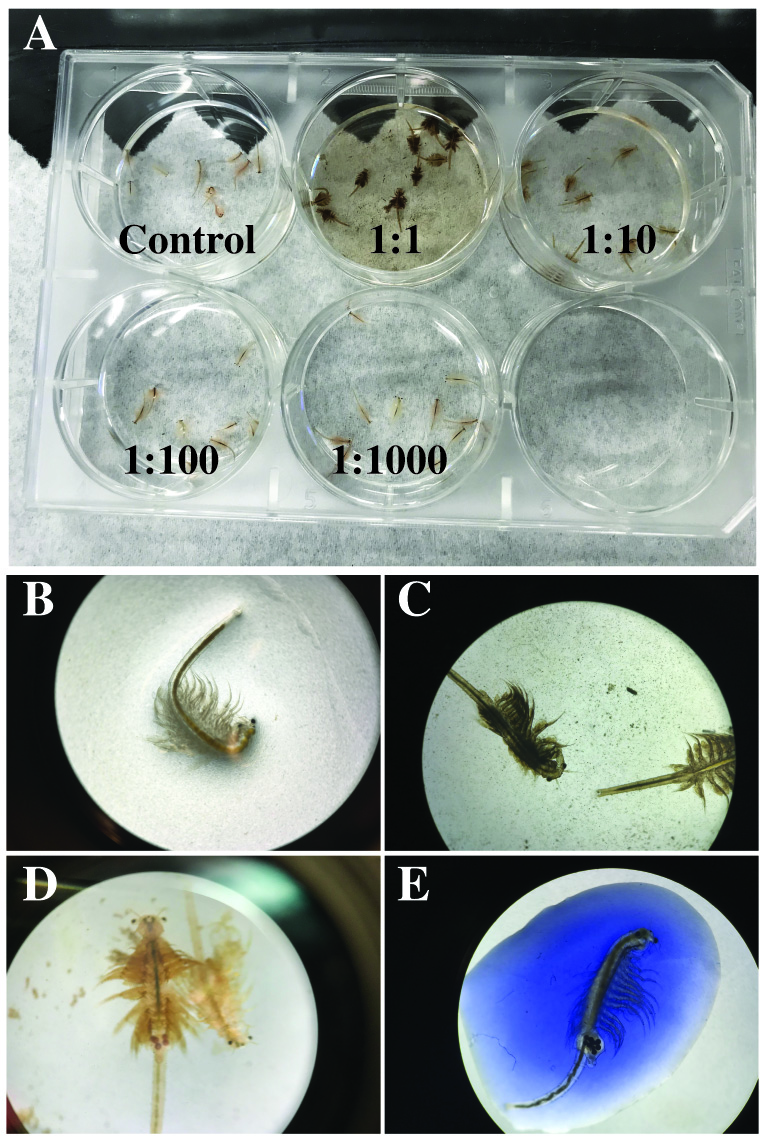
Figure 2. Biological analysis of brine shrimp during lethality and toxicity assays. (A) Brine shrimp were incubated in a 6-well plate containing 2 ml of SPIONs that were resuspended in seawater at concentrations of 0, 1:1, 1:10, 1:100, and 1:1000. Each well contained ten (10) brine shrimp that were incubated for 24 - 48 hours. (B) High-power magnification of a single brine shrimp in the control well. (C) High-power magnification of a single brine shrimp in a well containing 1:1 dilution of untreated SPIONs. (D) High-power magnification of brine shrimp carrying cysts incubated in a 1:1 dilution of 0.983 M citrate-treated SPIONs. (E) Trypan blue staining of brine shrimp following a 30-minute incubation with 0.983 M citrate treated SPIONs.
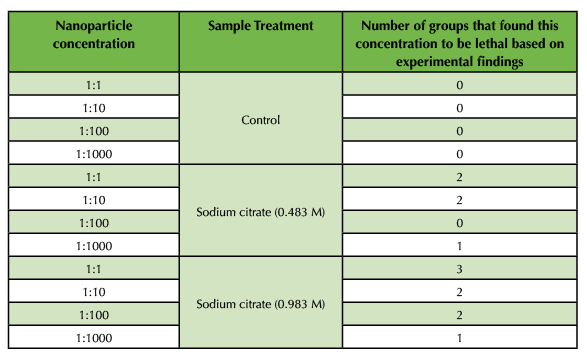
Table 4. Summary of Lethality Findings.
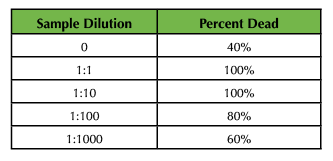
Table 5. Sample lethality data for brine shrimp incubation with 0.965M sodium citrate-coated nanoparticles.
Cytotoxicity assays revealed a direct relationship between concentration of SPIONs and cytotoxicity. In that regard, specimens incubated in the 1:1 dilution of SPIONs demonstrated the largest intake of trypan blue and were consistently assigned a score of '3' on the stain scale for all treatments (Figure 2E and Table 6). These data were consistent for live shrimp and cell suspensions. Interestingly, brine shrimp carrying eggs showed the highest degree of SPION uptake and trypan blue staining, which suggests eggs have a high affinity for citrate but that inclusion of SPIONS is extremely toxic for developing brine shrimp (Figure 2D).
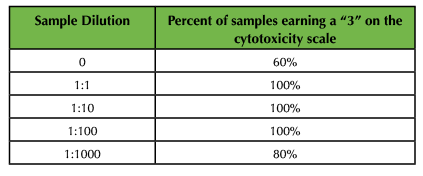
Table 6. Sample cytotoxicity data using the scale from Table 2.
TEACHING DISCUSSION
The interdisciplinary nanoparticle project completed by students in introductory chemistry and biology courses is an opportunity for students to work in teams and learn from one another. They apply the background knowledge provided by their colleagues to answer questions that are relevant to biology, chemistry, and human health as a whole. Students are challenged to think critically by applying foundational concepts and themes in both disciplines as they develop an interdisciplinary application that is both helpful and potentially harmful if not controlled. Moreover, students are actively employing the scientific method in both chemistry and biology to synthesize and test SPIONs for efficacy in the context of magnetism, toxicity, and lethality.
The chemistry and biology students are further challenged to work in interdisciplinary groups to create a video instead of the traditional lab report (Supporting File S3. Toxicity of SPIONs – Rubric). Groups are made taking into consideration the educational background of students (major, year, etc.) as well as the general demographics of each group in an effort to have each larger group best reflect the general population. If there is a student in both classes at the same time, they will be in the same group for each course to avoid duplication and doubling the level of expectation for one particular individual.
The final assessment is that of a video with predefined criteria that emphasize communication of key scientific findings to promote expansion and application of knowledge outside of what is taught in the classroom. Whether or not students actually learned the material and satisfied the desired learning outcomes is assessed using a rubric (Supporting File S3. Toxicity of SPIONs – Rubric). The rubric has course-specific content that is assessed only by the corresponding faculty; however, both faculty members assess execution of the scientific method, creativity, and communication. We found that this approach increases student creativity and also presents opportunities for further interdisciplinary applications in computer science, graphic design, and humanities. Furthermore, students are held accountable for their contributions, as they each participate in peer assessment by completing an in-depth partner evaluation (Supporting File S4. Toxicity of SPIONs – Peer Evaluation). Since students are required to evaluate each other, the efficacy of communication is considered by the instructor, as well as by student colleagues. The peer evaluation also gives students an opportunity to reflect on their contributions to the project and how the interdisciplinary nature of the topic contributed to their understanding of the chemical and biological principles.
The overall efficacy of this experiment with regard to meeting the specified learning outcomes is reflected by the student's ability to articulate their findings in both written and oral formats. The rubric provides a framework for students to use during development of the video; thus, the ability of students to accurately present the information and provide a detailed analysis of the data, in addition to infusing the content with creativity, is a reflection of understanding. Had the students simply answered a question on a worksheet, they would not have had the opportunity to critically evaluate the results to discern the most effective way of delivering the conclusions. Creation of a video challenges students to find meaningful ways to communicate findings that would be easily understood by an interdisciplinary, yet novice, audience without sacrificing technical depth. While some groups produced entertaining videos, the content was lacking, which was suggestive of poor understanding. Other videos were overly technical, which suggested too much reliance on the supporting materials as opposed to a unique analysis. Students that had the best video not only met the specified technical criteria, but also took the time to learn about the content to best support presentation of data in the oral video format. Since many careers in STEM require collaboration between different, yet related, fields, these labs provide an excellent opportunity for the students to gain experience on how to work in multidisciplinary teams to build a cohesive and complete product.
SUPPORTING MATERIALS
- S1. Toxicity of SPIONs – Lab Handout
- S2. Toxicity of SPIONs – Maintenance of brine shrimp
- S3. Toxicity of SPIONs – Rubric
- S4. Toxicity of SPIONs – Peer Evaluation
ACKNOWLEDGMENTS
The authors acknowledge Wentworth Institute of Technology for supporting the development of this activity. They also wish to thank Kimberly Foster, the biology laboratory technician, for all of her assistance in developing and preparing laboratory materials.
References
- Basu-Dutt S, Slappey C, Bartley JK. 2010. Making chemistry relevant to the engineering major. J Chem Educ 87:1206-1212.
- Jenkins SV, Gohman TD, Miller EK, Chen J. 2015. Synthesis of hollow gold-silver alloyed nanoparticles: A galvanic replacement experiment for chemistry and engineering students. J Chem Edu 92:1056-1060.
- Paluri SLA, Edwards ML, Lam NH, Williams EM, Meyerhoefer A, Pavel Sizemore IE. 2015. Introducing green and nongreen aspects of noble metal nanoparticle synthesis: An inquiry-based laboratory experiment for chemistry and engineering students. J Chem Edu 92:350-354.
- Wu W, He Q, Jiang C. 2008. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res Lett 3:397-415.
- Yoo D, Lee JH, Shin TH, Cheon J. 2011. Theranostic magnetic nanoparticles. Acc Chem Res 44:863-874.
- VanDorn D, Ravalli MT, Small MM, Hillery B, Andreescu S. 2011. Adsorption of arsenic by iron oxide nanoparticles: A versatile, inquiry-based laboratory for a high school or college science course. J Chem Edu 88:1119-1122.
- Tarkington LM, Bryan WW, Kolhatkar T, Markle NJ, Raska EA, Cubacub MM, Rittikulsittichai S, Li CH, Chen YT, Jamison AC, Lee TR. 2017. Magnetic microorganisms: Using chemically functionalized magnetic nanoparticles to observe and control paramecia. J Chem Edu 94:85-90.
- Amaris ZN, Freitas DN, Mac K, Gerner KT, Nameth C, Wheeler KE. 2017. Nanoparticle synthesis, characterization, and ecotoxicity: A research-based set of laboratory experiments for a general chemistry course. J Chem Edu 94:1939-1945.
- Brittle SW, Baker JD, Dorney KM, Dagher JM, Ebrahimian T, Higgins SR, Pavel Sizemore IE. 2015. Measuring the silver composition of nanocolloids by inductively coupled plasma-optical emission spectroscopy: A laboratory experiment for chemistry and engineering students. J Chem Edu 92:1061- 1065.
- Maurer-Jones MA, Love SA, Meierhofer S, Marquis BJ, Liu Z, Haynes CL. 2013. Toxicity of nanoparticles to brine shrimp: An introduction to nanotoxicity and interdisciplinary science. J Chem Edu 90:475-478.
- Metz KM, Sanders SE, Miller AK, French KR. 2014. Uptake and impact of silver nanoparticles on brassica rapa: An environmental nanoscience laboratory sequence for a nonmajors course. J Chem Edu 91:264-268.
- Winkelmann K, Bernas L, Swiger B, Brown S. 2017. Measurement of chlorophyll loss due to phytoremediation of Ag nanoparticles in the first-year laboratory. J Chem Edu 94:751-757.
- Ramsey LL, Radford DL, Deese WC. 1997. Experimenting with interdisciplinary science. J Chem Edu 74:946.
- Wolfson AJ, Hall ML, Allen MM. 1998. Introductory chemistry and biology taught as an interdisciplinary mini-cluster. J Chem Edu 75:737.
- Hooker PD, Deutschman WA, Avery BJ. 2014. The biology and chemistry of brewing: An interdisciplinary course. J Chem Edu 91:336-339.
- Jarockyte G, Daugelaite E, Stasys M, Statkute U, Poderys V, Tseng TC, Hsu SH, Karabanovas V, Rotomskis R. 2016. Accumulation and toxicity of superparamagnetic iron oxide nanoparticles in cells and experimental animals. Int J Mol Sci 17:1193.
- Dawsey AC, Hathaway KL, Kim S, Williams TJ. 2013. Introductory chemistry: A molar relaxivity experiment in the high school classroom. J Chem Educ 90:922-925.
- Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN. 2008. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108:2064-2110.
- Tsung F. Accessed Dec 12, 2017. Synthesis of Iron Oxide Nanoparticle. https://www2.bc.edu/frank- tsung/PDF/Workshop.
- Karaagac O, Kockar H, Beyaz S, Tanrisever T. 2010. A simple way to synthesize superparamagnetic iron oxide nanoparticles in air atmosphere: Iron ion concentration effect. IEEE T Magn 46:3978-3983.
- Kolthoff IM, Leussing DL, Lee TS. 1950. Reaction of ferrous and ferric iron with 1,10-phenanthroline. iii. The ferrous monophenanthroline complex and the colorimetric determination of phenanthroline. J Am Chem Soc 72:2173-2177.
- Szpak A, Kania G, Skórka T, Tokarz W, Zapotoczny S, Nowakowska M. 2013. Stable aqueous dispersion of superparamagnetic iron oxide nanoparticles protected by charged chitosan derivatives. J Nanopart Res 15:1372.
- Baer C, Cornely K. 1999. Spectroscopy of simple molecules. J Chem Edu 76:89.
- Szpak A, Fiejdasz S, Prendota W, Straçzek T, Kapusta C, Szmyd J, Nowakowska M, Zapotoczny S. 2014. T1-T2 dual-modal MRI contrast agents based on superparamagnetic iron oxide nanoparticles with surface attached gadolinium complexes. J Nanopart Res 16:2678.
- Naqvi S, Samim N, Abdin MZ, Ahmed FJ, Maitra AA, Prashant CK, Dinda AK. 2010. Concentration- dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int J Nanomed 5:983-989.
- Schulze F, Dienelt A, Geissler S, Zaslansky P, Schoon J, Henzler K, Guttmann P, Gramoun A, Crowe LA, Maurizi L, Válle JP, Hofmann H, Duda GN, Ode A. 2014. Amino-polyvinyl alcohol coated superparamagnetic iron oxide nanoparticles are suitable for monitoring of human mesenchymal stromal cells in vivo. Small 10:4340 - 4351.
- Strehl C, Gaber T, Maurizi L, Hahne M, Rauch R, Hoff P, Häupl T, Homann-Amtenbrin M, R P, Hofmann H, Buttgereit F. 2015. Effects of PVA coated nanoparticles on human immune cells. Int J Nanomed 10:3429-3445.
- Hadjipanayis CG, Bonder MJ, Balakrishnan S, Wang X, Mao H, Hadjipanayis GC. 2008. Metallic iron nanoparticles for MRI contrast enhancement and local hyperthermia. Small 4:1925-1929.
- Wahajuddin M, Arora S. 2012. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int J Nanomed 7:3445-3471.
- Mary APR, Narayanan TN, Sunny V, Sakthikumar D, Yoshida Y, Joy PA, Anatharaman MR. 2010. Synthesis of bio-compatible SPION-based aqueous ferrofluids and evaluation of radiofrequency power loss for magnetic hyperthermia. Nanoscale Res Lett 5:1706-1711.
- Oberdörster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H. 2005. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part Fibre Toxicol 2.
- Nel A, Xia T, Mädler L, Li N. 2006. Toxic potential of materials at the nanolevel. Science 311:622-627.
- Rushton EK, Jiang J, Leonard SS, Eberly S, Castranova V, Biswas P, Elder A, Han X, Gelein R, Finkelstein J, Oberdörster G. 2010. Concept of assessing nanoparticle hazards considering nanoparticle dosimetric and chemical/biological response-metrics. J Toxicol Env Health A 75.
- Kurwadkar S, Pugh K, Gupta A, Ingole S. 2015. Nanoparticles in the environment: Occurrence, distribution, and risks. J Hazard Toxic Radiant Waste 19:04014039.
- Lieberman M. 1999. A brine shrimp bioassay for measuring toxicity and remediation of chemicals. J Chem Edu 76:1689.
- Rajabi S, Ramazani A, Hamidi M, Naji T. 2015. Artemia salina as a model organism in toxicity assessment of nanoparticles. DARU 23:20.
- Hackl E, Ermolina, I. 2019. Inclusion by design: Embedding inclusive teaching practice into design and preparation of laboratory classes. Curr Pharm Teach Learn 11:1323.
- Miguel OB, Gossuin Y, Morales M, Gillis P, Muller R, Veintemillas-Verdaguer S. 2007. Comparative analysis of the 1H NMR relaxation enhancement produced by iron oxide and core-shell iron-iron oxide nanoparticles. Magn Reson Imaging 25:1437-1441.
- Yang H, Zhang C, Shi X, Hu H, Du X, Fang Y, Ma Y, Wu H, Yang S. 2010. Water-soluble superparamagnetic manganese ferrite nanoparticles for magnetic resonance imaging. Biomaterials 31:3667.
- Beeran AE, Shaiju SAN, Fernandez FB, Muvvala KS, Wunderlich W, Anil S, Vellappaly S, Ramachandra Rao MS, John A, Jayasree RS, Harikrishna Varma PR. 2015. An aqueous method for the controlled manganese (Mn(2+)) substitution in superparamagnetic iron oxide nanoparticles for contrast enhancement in MRI. Phys Chem Chem Phys 17:4609.
- Jang J, Nah H, Lee JH, Moon SH, Kim MG, Cheon J. 2009. Critical enhancements of MRI contrast and hyperthermic effects by dopant-controlled magnetic nanoparticles. Angew Chem Int Ed 48:1234.
Article Files
Login to access supporting documents
Nanoparticles and Shrimp: An Interdisciplinary Lab Series in Chemistry and Biology for Undergraduate Engineering Students(PDF | 1 MB)
S1 Toxicity of SPIONs-Lab Handout.docx(DOCX | 494 KB)
S2 Toxicity of SPIONs-Maintenance of brine shrimp.docx(DOCX | 13 KB)
S3 Toxicity of SPIONs-Rubric.docx(DOCX | 23 KB)
S4 Toxicity of SPIONs-Peer Evaluation.docx(DOCX | 15 KB)
- License terms

Comments
Comments
There are no comments on this resource.