Converting a Face-to-Face Lab to Online: An Example of Process and Outcomes for a CRISPR-Based Molecular Biology Lab
Editor: Srebrenka Robic
Published online:
Abstract
The COVID-19 pandemic created a need to convert in-person laboratory courses into an online format in a short amount of time. For this reason, we converted our face-to-face molecular biology lab course to an online version in Spring 2020. The lab course focuses on using CRISPR-Cas9 editing to introduce mutations into the ADE2 gene in Saccharomyces cerevisiae. When planning the conversion to online, we identified a number of challenges to addressing the course goals, such as including technology, accessing real data for analysis, and engaging students in collaboration. After brainstorming ways to address these and other challenges, we developed an online lab course for which the “in lab” portion was largely focused on working collaboratively on data analysis, troubleshooting, and drawing conclusions, as well as experimental design and examining primary literature. Surveys suggest students perceived significant gains in key scientific thinking and process skills including forming scientific arguments, data analysis, and working collaboratively. Students also reported multiple different ways in which the online courses made them feel like scientists. Here we describe key parts of the course structure and our experience in teaching the course to over 200 students. We also describe the process of converting from face-to-face to online, as the process could be applied to any existing hands-on lab. The process enabled us to quickly adapt important information, course goals, and experimental contexts from existing labs, reducing the workload involved in creating a new lab while still providing the experience of focusing on scientific thinking skills to students.
Primary image: A CRISPR-focused lab course moved online, where students analyze data, write about results, and discuss experiments in a virtual environment. Part of this image was created using non copyrighted images from Open Clipart (openclipart.org), Creative Commons Zero 1.0 labelled for unrestricted reuse.
Citation
McDonnell LM, Reuther K, Cooper A, Day C, Gustafson-Brown C. 2021. Converting a face-to-face lab to online: an example of process and outcomes for a CRISPR-based molecular biology lab. CourseSource. https://doi.org/10.24918/cs.2021.22Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
The goals of a lab course are often varied, and may include developing practical, hands-on skills, developing critical thinking and analytical skills, and learning biological concepts. Given the typical nature of biology lab courses, involving hands-on, wet-lab work, it can be challenging and daunting to convert such a course to an online mode. When our university shut down face-to-face operations in mid-March, 2020, we had about three weeks to convert our face-to-face, molecular-biology-focused lab course to an online version. Due to the quick turn-around time required, we did not attempt to have students do at home, hands-on, kit-based experiments (such as The Home Scientist Illustrated Guide to Home Biology Experiments, Udemy Hands-On Biology Lab Skills at Home: Real world project). Instead, we focused on using existing elements from the course to engage students in tasks that centered around experimental design, results/data analysis, troubleshooting, drawing conclusions, and learning about the protocols associated with the experimental context. Below we describe the typical face-to-face course, and elaborate on the process we used to decide what elements of the face-to-face course we could use in the online version. This process is useful for creating new courses, regardless of the format.
Face-to-face course: structure, themes, and goals
Our face-to-face lab course is geared towards upper-level biology students and is 10 weeks long (quarter system). The lab meets twice per week at scheduled times for which students register. Each time block is three hours and 50 minutes; however, some labs use only two hours, while others take the full time, depending on the tasks being completed each day. Each lab section has 24 students and a teaching assistant. The instructor oversees and attends all lab sections. Over the quarter, there are 12-16 sections total. There is also a lecture portion associated with the course, which meets for three x 50-minute sessions per week, taught by five or six different instructors. All classes do the same activities in the lab, following the same weekly schedule, but each instructor tailors their own lectures. Table 1 is included as a PDF document along with supporting materials and shows a detailed weekly schedule for both in-person and online. Figure 1 has an example of activities from one week of the course. Students in all classes engage in keeping a lab notebook, complete scientific writing assignments, data analysis assignments, regular quizzes (or multiple "midterms") and a written final exam (not a practical final exam). All of the assignments and tests are constructed by each individual instructor, although there is considerable overlap between multiple instructors who collaborate on developing and sharing course material.
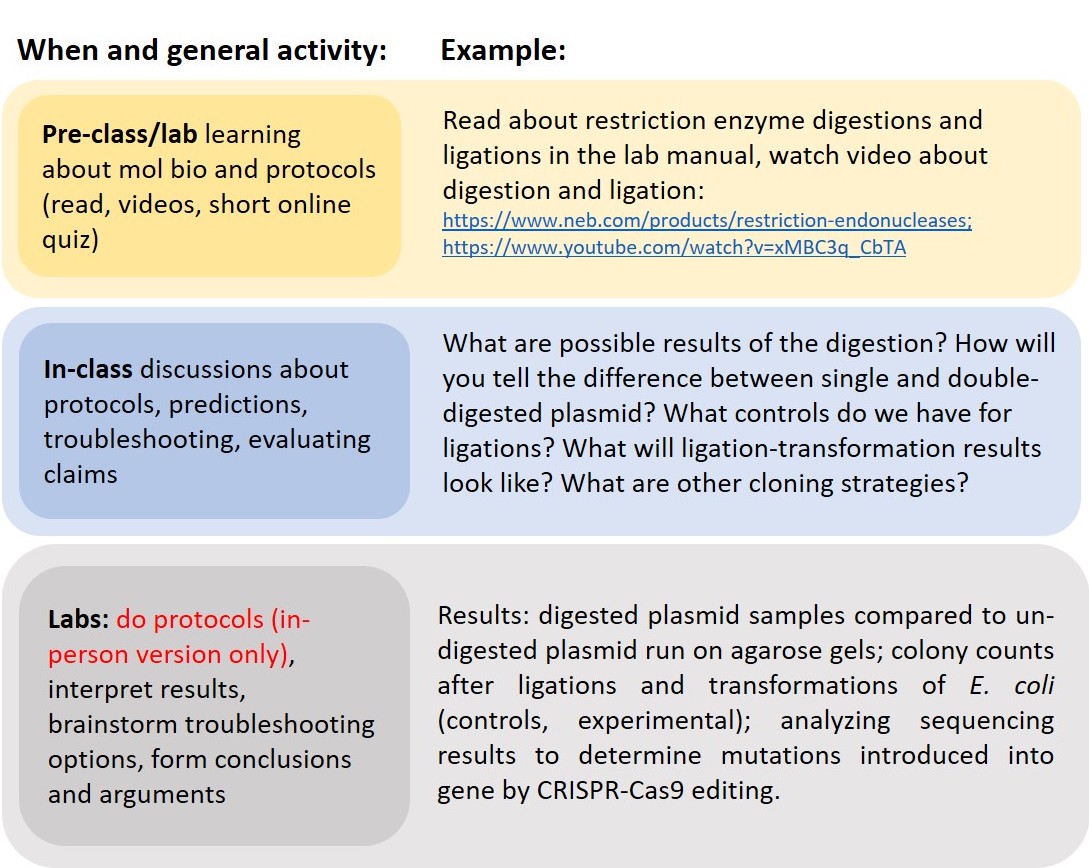
Figure 1. Example weekly structure of the lab course where students engage in pre-class/lab learning, deepen understanding from lecture discussions, and during lab time they do the lab work and interpret results (face-to-face) or do interpreting and discuss conclusions drawn (online). Pre-class/lab video links included: New England BioLabs Restriction Endonucleases and New England BioLabs DNA Ligation YouTube video.
The broad goals of this particular lab course are for students to:
- Apply knowledge of molecular biology concepts and molecular laboratory techniques (e.g., CRISPR Cas9-editing) to design experiments, predict outcomes, explain and troubleshoot results.
- Use various molecular biology techniques to create recombinant DNA molecules, introduce mutations, and examine effects of mutations using phenotype and genotype data.
- Engage in collaboration to learn and analyze data.
- Draw conclusions based on evidence and reasoning and clearly communicate their findings.
- Find, read, and evaluate primary literature.
To achieve these goals, students engage in an eight-week experiment to use CRISPR-Cas9 to edit a specific locus in the genome of a haploid strain of Saccharomyces cerevisiae. Specifically, we target the ADE2 gene, which is involved in de novo purine biosynthesis. Mutations that render the gene-product non-functional result in build-up of a precursor in the purine biosynthesis pathway, which turns the yeast cells red in color. In this way, we can easily screen for loss-of-function mutations after editing. We also use sequencing of the gene to determine precisely what mutations are introduced after the CRISPR-Cas9 editing experiment. The inspiration for this lab came from Seghal et al. (1), with several modifications that make it similar to one described by Ulbricht, 2019 (2). For background reading on how the CRISPR editing process works and other examples of its use in a lab course, please see (2-5). Students engage in the entire experimental process: designing guide RNA (gRNA) and homology-directed repair (HDR) sequences, cloning the gRNA sequence into the transformation plasmid, making PCR copies of the HDR template, transforming yeast, evaluating phenotype, generating and sending a PCR product of the ADE2 gene for sequencing, and analyzing the results (see Table 1 for the week-by-week lab activities). Aside from the CRISPR experiment, the students also do additional mini-experiments to learn other techniques and concepts (such as RNAi, and SNP genotyping). Throughout the course, students learn about the various lab techniques and analyze the results generated to evaluate how well each part of the experiment is working. As results do not always match expectations, students are also engaging in frequent troubleshooting.
Each section tests multiple gRNA/HDR combinations, targeting different parts of the gene and adding different mutations. For example, students can identify predicted transcription factor binding sites using the Cold Spring Harbor Promoter Database for Saccharomyces cerevisiae. Based on this, we recently tested a gRNA that targeted the promoter region of ADE2 and an HDR that would mutate the two predicted transcription factor binding sites. We also used a gRNA and HDR combination that targeted the ADE2 locus just after the start codon, introducing multiple nonsense mutations, respectively (similar to that presented in Seghal et al. (1)). Students collect yeast phenotype data (red versus white colonies) and sequencing results (which mutations were introduced) and pool their data into a shared database (Google Sheets). In this way, the students have a larger data set from which to evaluate the effects of various gRNA/HDR combinations on yeast survival, gene function, and specific genetic edits, as well as multiple biological replicates of each gRNA/HDR experiment. As we are using new gRNA/HDR combinations each quarter, we do not always know what the effects of the mutations will be on gene function, or the frequency that all mutations designed in the HDR will be incorporated, and as our data set grows, we are learning more and more about varying efficiencies of gRNAs and HDR repair.
At the end of the CRISPR experiment, students are tasked with using the pooled class phenotype and sequencing data sets to answer the following questions:
- What is the frequency of loss-of-function mutations in ADE2 when specific (HDR) and non-specific (NHEJ) mutations are introduced after Cas9-induced cutting?
- Is there an impact on colony survival depending on the gRNA?
- How efficiently is each HDR mutation incorporated?
- What types of NHEJ mutations caused LOF of ADE2?
Converting the face-to-face lab to an online version
We had approximately three weeks to plan how to deliver the above course in a fully online mode. We started by making big-picture decisions about goals and outcomes followed by decisions on how to implement, akin to a backward design approach. To engage in the conversion process, we discussed and addressed these questions:
- What goals do we want students to achieve with this online lab?
- What will students do during "lab time" to achieve these goals?
- What challenges might we face in trying to achieve these goals online, and how we will address these challenges?
With the exception of using the wet-lab techniques, we wanted students to achieve all the broad goals of the face-to-face course (listed above). With these goals in mind, we identified the major learning and teaching components of the face-to-face course, what challenges we would face to achieve them online, and how to address these challenges (summarized in Table 2). In general, we wanted to put a greater emphasis on goals that would encourage scientific thinking: analyzing and interpreting results, drawing conclusions, troubleshooting, experimental design, and evaluating primary literature, because these could still be achieved during the online lab sessions. We decided to continue using the existing CRISPR-Cas9 experimental context, as this would reduce the amount of new material that would have to be developed on short notice. Given that many students and instructors were feeling stressed about the online transition, we also decided to cut out some parts of the course, freeing time to get acquainted with the online format, and allowing more time spent on our desired goals.
Cutting some material out of the course was also an excellent way to ensure the course was largely focused on one main experimental context (CRISPR-Cas9 editing). We decided to deliver the lecture portion of this course either synchronously (with recordings made available to students) or asynchronously. Our synchronous online teaching took place using Zoom web conferencing. In this paper we will not be focused on the process of transitioning face-to-face lectures to online. Please see the following for some ideas on converting face-to- face lectures to online (6,7, and Boston University's Quick Guide to Converting your Face-to-Face Pedagogical Approaches to the Online Environment).
CHALLENGES OF CONVERTING TO ONLINE
Below we elaborate on some of the challenges posed by converting the face-to-face wet-lab course to online, and how we addressed these challenges (Table 2).
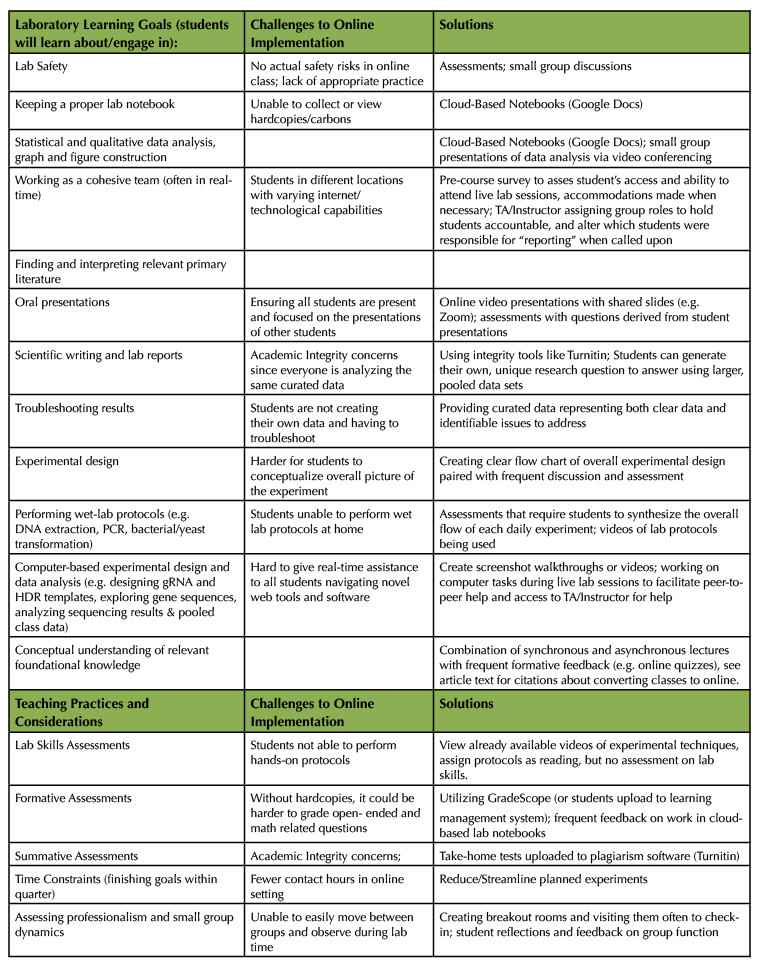
Table 2. Challenges and solutions to achieving broad course and teaching goals when converting our face-to-face lab to an online version.
Challenge 1. Students are in different locations with varying access to internet and a private place to engage in live online meetings
Typically, students are working in pairs or small groups in the lab to conduct experiments and interpret/troubleshoot results. These peer-peer interactions as well as frequent student-teaching assistant and student-instructor interactions are extremely important for clarifying thinking and receiving feedback. Our goal was to make the lab time synchronous, such that students would connect via Zoom and assemble into breakout groups of four to five people to work together on the daily tasks. To determine if this was feasible, we ran a survey at the start of the course to identify student's access to a reliable internet connection and ability to connect synchronously. A very small fraction of students (1-2%) reported having limited access that would negatively impact their ability to succeed in the course. For this reason, we proceeded with synchronous lab time and made case-by-case adjustments such as completing tasks offline and submitting later for those students who faced significant barriers to access. If a larger portion of the class were unable to connect synchronously, one approach could be to have students work independently on tasks, but implement a structured peer-review system using various tools such as monitored online discussion boards, shared Google Docs, or peer-review features in the campus learning management system.
We also used freely available programs for our computer-based lab exercises, such as: Yeast Genome Browser, ApE (a plasmid editor), and Google Sheets/Docs for data analysis and documenting work.
In person, the lab time is scheduled for 3 hours and 50 minutes. During this time students are doing bench work, analyzing and interpreting results, making notes in their lab notebooks, and occasionally working on other assignments such as write-ups of their work, additional data analysis or bioinformatics exercises. Lab time is sometimes also used for quizzes/midterms, or doing peer-review of writing. As an instructional team we felt that video-conferencing (via Zoom) for nearly four hours would be too draining for both students and the teaching team. For this reason, we scheduled labs for two hour blocks, using the additional time for office hours and individual work time on assignments or take-home quizzes. Since the first iteration of the online lab, we have extended the online lab time to be about three hours, because we found there were often students who needed more than two hours to finish the work and get sufficient feedback. Others may need to consider Instructor scheduling when deciding on the length of a virtual lab. For example, when the lab was 2 hours long, a single instructor could be teaching two sections that ran back-to-back. When there were three-hour labs, an instructor had two sections that had slight overlap, and would bounce between these classes, using Slack (or other communication tools) to allow for quick questions to be asked and to flag the instructor to rejoin a particular section.
We also had to cut material from the course to allow for instructors, teaching assistants, and students to get acquainted with the new online format. The first week had structured activities to practice joining the live lab session, engaging with peers in breakout groups, and uploading work to the learning management system and GradeScope (which we were using to score tests). Reducing course content also allowed us to use more time for reviewing journal articles and engaging students in designing and delivering presentations, compared to the face-to-face version. Even now that instructors and students are more familiar with online teaching and learning, we have still found it beneficial to reduce content, as it allows for more time to dive deeply into a topic. We plan to keep this reduced content once we return face-to-face.
Challenge 2. Performing wet-lab protocols (e.g., DNA extraction, PCR, bacterial/yeast transformation) and access to real data for students to analyze
To avoid the need to develop new experiments or contexts, we decided to have the online version walk through the same experimental goals and methodologies as the face-to-face version. We knew the students were not going to be assessed on proficiency of using various techniques, but should understand how they work and what kinds of results are generated from using these procedures. For example, several labs in the face-to-face version normally engage students in setting up a restriction enzyme digestion of the plasmid, checking the digestion using agarose gel electrophoresis, and then setting up a ligation of the gRNA fragment into the digested plasmid. In the online version, students learned about the protocols from readings, lectures, the lab manual, and sometimes videos (e.g., from the Journal of Visualized Experiments). Then, during the online lab time, students briefly discussed some of the protocol steps and spent the bulk of the time analyzing results. For example, they started out by discussing how they would have set-up restriction enzyme digestions (e.g., how many microliters of DNA to add to the digestion reaction given the initial and desired final concentration of DNA). In a face-to-face version, students would have run digestions on a gel to analyze the results and assess if they had a successful digestion. So, in the online version students spent a good deal of time interpreting gel electrophoresis results we provided to them, from several different digestions.
This meant that we needed access to real results from prior experiments. Thankfully, when we had run the face-to-face version of the lab course in the previous quarter, many instructors had copies of student's gel images (as well as many other results and data from the experiment, see * note in Table 1). This meant we had access to a spectrum of "successful" through "unsuccessful" results. Providing students with multiple results encouraged them to discuss and decide which were successful and which were not, and brainstorm troubleshooting approaches for unsuccessful results. An example of some results they analyzed, as well as instructions for analysis in lab that day, can be found in Supporting File S1. Converting face-to-face lab to online – Example Lab Results for Analysis. Engaging in troubleshooting provided opportunities for students to apply their understanding of the procedures and experimental goals.
The need for curated data to use in the online version prompted us to create a database of results for the course, now shared with all course instructors. We have found this resource of expected and unexpected results incredibly valuable for teaching and analysis. Unexpected results, such as a different banding pattern from a restriction enzyme digestion, or only some of the designed HDR mutations incorporated into the genome, has increased the complexity of the discussions we have with students as they try to reason through the results. Moving forward, as we collect data again in person, we plan to continue to collect and curate results to expand this database. An important caveat is to be sure there is an easy way for students to submit results (e.g., upload to a google form via a link on the LMS).
Challenge 3. Keeping a proper lab notebook
Normally, students document their lab work, results, and thinking in a paper lab notebook. Many instructors collect notebooks on a regular basis, or do random spot-checks, to provide feedback to students on their thinking and lab notebook recording practices. We chose to use cloud- based methods for student lab notebooks. Each student was assigned their own Google Doc which they, the instructor, and the TA could access and edit. Students were often tasked with reviewing specific sections of the lab manual that covered background information on the relevant techniques, and providing specific details about how they would set-up various reactions (e.g., volumes of reagents, etc.) as well as predicting results. Students were also asked to do pre-lab drawings of the protocols to help them visualize and think about what happens during various experimental steps.
To see an example portion of the Lab Manual, a student Lab Notebook Entry, and the Lab Notebook Rubric, see the following Supporting Files:
- Supporting File S2. Converting face-to-face lab to online – Example Lab Manual Section
- Supporting File S3. Converting face-to-face lab to online – Student Lab Notebook Entry and Rubric
If students felt that using a text editor such as Google Docs was not an efficient way for them to draw protocols, we allowed them to paste in pictures of hand-drawn protocols or anything made using different software. For more information about the utility of drawing protocols, see (8). Students were responsible for individually summarizing their analysis and interpretations in their lab notebooks during and after each lab session. The benefit of using Google Docs for lab notebooks is that the teaching assistant and instructors could access them after each lab and give feedback on work and writing. It is important to note that a Google Docs lab notebook can be set up to remain anonymous, as the student does not have to add any personal identifying information to the file in order for the instructor to grade their notebooks. This may be relevant if you are teaching at an institution that does not support the use of Google (for example, many Canadian schools do not allow storing student information on Google platforms because the servers are not managed in Canada). We managed this by labelling the Google docs A through Z, and used a separate sheet to indicate which student was assigned which Google doc.
Challenge 4. Students working as part of a cohesive team
One of our goals was for students to engage in the same kind of collaborative work they would have done during the face-to-face lab. For this reason, the live, video-conferencing lab sessions were largely spent with the students working in small breakout groups (four to six students) to discuss experimental plans, analyze and interpret results, and draw conclusions. This size group was chosen because larger groups might make it easier for some students to minimally participate while smaller groups (two to three students) make it harder for the instructor and teaching assistants to adequately spend time with each group individually. Breakout groups were hosted through a video-conferencing tool that allowed the teaching assistant and instructor to circulate through the groups, answering and asking questions. In breakout groups, we often assigned students to be either a reporter or a manager (rotating who held these responsibilities) to ensure more equal participation. Reporters were asked to share analysis or ideas with the class, and managers were responsible for keeping the group on task and ensuring everyone had a chance to contribute ideas or questions. Some instructors had students work in the same group all quarter, while others randomized the groups in each session to maximize the number of different peer interactions.
One of the labs early in the course is a journal article discussion that both reinforces CRISPR-Cas9 concepts as well as important considerations for experimental design. We provide this as an example of how a structured small-group and whole-class discussion can occur, be effective at engaging students in group-based learning, and engage students with challenging material. We structured the journal article discussion in the following manner: 1) Students were introduced to the core concepts necessary to understand the paper and some methods in lecture prior to the lab discussion and as part of the pre-lab preparation assignment; 2) During lab time, students worked in their breakout groups to answer questions about the methods and what could be concluded from a specific figure from the article; 3) After sufficient time in groups, the whole class was pulled back together and reporters from a subset of groups were randomly selected to summarize their group's thinking. In this way, students had time to receive feedback from peers and instructors during group time, and be exposed to various ways of thinking about the journal article by hearing other groups' ideas. The materials used for pre-lab and in-lab discussions can be found in Supporting File S4. Converting face-to-face lab to online – Journal Article Discussion Materials. The discussion questions used for this activity were based on an in-class worksheet activity from the face-to-face course in which students worked in small groups. In this way, we took an activity from what would have been lecture time and added it to the lab time to ensure students would be able to engage in discussion, rather than work individually. The group work is especially helpful for this activity because of the challenges many students face when deconstructing primary literature. In general, and especially in an online teaching and learning environment, highly structured discussions and analysis activities are helpful to keep groups on track, hold groups and individuals accountable, and to achieve the desired goals.
Challenge 5. Assessments
The main challenge with assessments was 1) Ensuring they were done fairly and with integrity, and 2) creating varied opportunities for students to demonstrate their understanding and scientific thinking. For this reason, there were varied ways that students were assessed. It was common for instructors to have students complete a short quiz online (via our learning management system) to check their understanding of key concepts and protocol information from the lectures and lab manual, prior to the lab session. Students engaged in frequent writing, especially by providing comprehensive explanations for results of experiments in the lab notebooks. Quizzes and exams were converted to a take-home format, in which students had at least 24 hours to complete the assessments. Access to GradeScope and Turnitin streamlined the grading and plagiarism checks, respectively. Quizzes/exams focused on high-level questions requiring data analysis, interpretation, forming conclusions, and critiquing claims.
Avoiding lower level recall questions eliminated the chance that students could look up answers online. An example of some take-home exam questions can be found in Supporting File S5. Converting face-to-face lab to online – Take Home Assessment Examples. Students also produced very short presentations at the end of the course, which they delivered during the last couple of live lab sessions (or recorded and delivered to the Instructor if they could not attend the live session).
The goal for the presentations was to provide students with an opportunity to learn more about a laboratory/research method of their choosing, and practice collaborating with a peer to learn the material and create a presentation. Students were assigned to work in randomly-generated pairs (or individually, depending on instructor preference) and given a list of topics to choose from, although they could propose a method not on the list. For example, a group could choose RNAi, present the mechanism, show results from a paper that used RNAi , and explain what conclusions could be drawn. By having either pairs or individuals, present, all students were held accountable for contributing. Engaging in collaboration to create and deliver a presentation was no different online compared to face-to-face, aside from the fact that they had to work in an online environment (Zoom). On presentation days, students shared their screen (slides) and presented while the rest of the class listened and provided feedback using an online form. We specifically dedicated one or two lab sessions as group-work time so students did not have to schedule meeting with a partner outside of class. For an example presentation, rubric, and the comment form, see Supporting File S6. Converting face-to-face lab to online – Presentation Documentation. Additional information and comments on assessments are provided below.
OUTCOMES
Gains in scientific thinking skills
Students were asked to complete a survey after the course which asked students to evaluate if their skills in a variety of areas had changed as a result of the course, if there were any parts of the course that made them feel like a scientist, and what improvements they recommend for future online iterations. Questions about the impact of the course on their experience with various course elements and skills were based on the "course elements" questions from the CURE survey, developed by Lopatto.
Results of students reporting the impact of the course on their experience with various course elements and skills are summarized in Table 3. In general, students felt that the course helped them improve either "a lot" or "a little" in all skill areas. Of the skills surveyed, over 50% of students reported that they "improved a lot" in the following areas:
- generating biological/molecular explanations for results obtained (62.5%)
- working collaboratively in small groups (61.4%)
- analyzing research data (60.8%)
- presenting research results in written papers (55.3%)
- maintaining a laboratory or research notebook (54.2%)
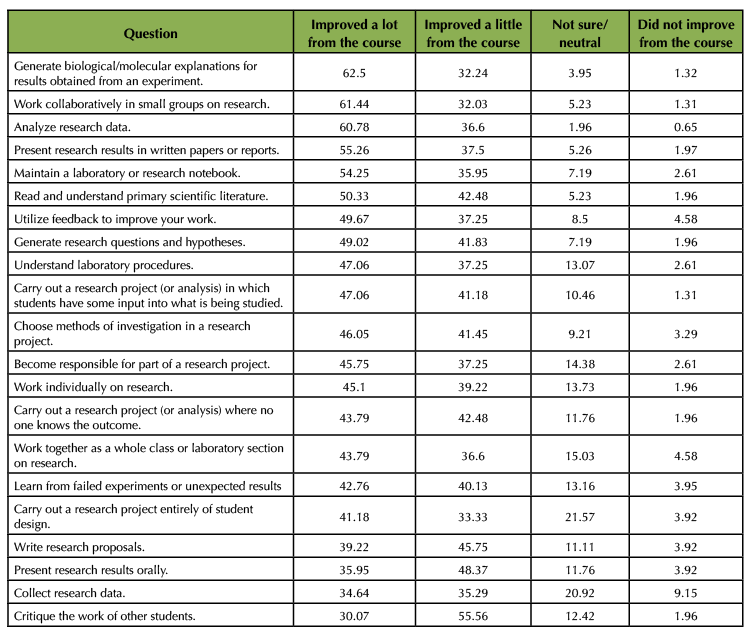
Table 3. Student perceptions on how the online lab course contributed to their skill development. On the post-course survey, students were asked, “Indicate if you improved upon the skills below from the experiences you had in BIMM 101 (the course code),” and were presented with four options: “improved a lot,” “improved a little,” “not sure/neutral,” and “did not improve.” Values in the table are the percentage of students who selected each option. Each question had either 152 or 153 students responding (some students skipped a question). Questions are organized in the table based on the percentage of “improved a lot from the course” (highest to lowest).
Combining the "improved a lot" and "improved a little" responses, over 80% of students reported some improvement in understanding laboratory procedures and in learning from failed experiments of unexpected results. These student perspectives reflect some of the key goals of the course: collaborating to analyze data, applying understanding of molecular biology concepts and techniques to interpret results, and forming conclusions backed up with reasoning. Perceived gains in these areas have been reported by others studying the impacts of course-based research experiences (9,10), and are articulated in Biological Experimentation Competencies Map (11). Our survey results suggest that an online lab course can have positive impacts on student attitudes, despite the lack of hands-on and authentic research experience.
Students reported the lowest gains in critiquing the work of others, collecting research data, presenting results orally, and writing research proposals. Although students in some lab sections had the chance to engage in peer-reviewing, and all did give oral presentations, these experiences would have only occurred once during the quarter. This may not have been enough exposure for students to feel they had made significant gains in those areas. It was not surprising that students did not report gains in collecting research data, as all the results were provided to them. Significant gains in writing research proposals were also not expected to be high because, although students spent significant time thinking and writing about experimental design, they did not write a research proposal. Combined, these results suggest that, from the student perspective, the online course was effective at developing important scientific thinking skills despite the lack of hands-on work. Students still gained a sense of understanding of a variety of procedures and how to engage in troubleshooting.
Students also answered an open-ended question that asked "were there any parts of the course that made you feel like a scientist?" Several frequent themes emerged from coding student responses (Table 4), including:
- writing and reviewing primary literature (39% of responses)
- data analysis (35%)
- experimental design and forming conclusions (31%)
- learning about techniques or concepts (22%)
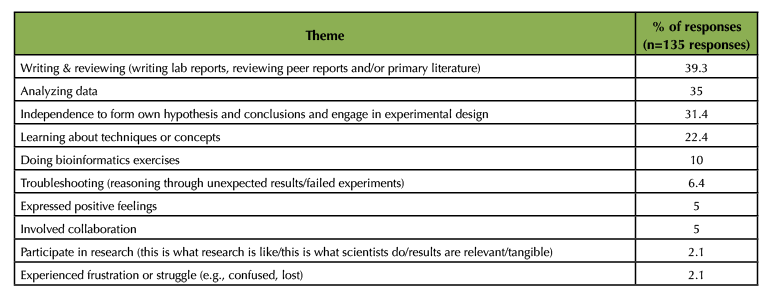
Table 4. Frequency of themes from student responses to the question “Did any parts of this course make you feel like a scientist? If so, what parts and why?” Responses from 140 students were coded, and of those five responded “no.” Responses below are from the 135 “yes” responses (students can list more than one theme, so the percentages add up to more than 100% of responses).
These were also frequent themes that emerged when students were asked this same question in the face-to-face version of the course (not reported here), and some were also identified from responses when students took a course-based undergraduate research experience (10). So, although lacking the face-to-face interactions and bench experiences, students still report gaining scientist-like experiences from the online lab.
RECOMMENDATIONS
The main recommendations we have for converting a face-to-face lab to online, are:
1. Identify your goals – what do you want students to achieve from the online lab experience?
It is our experience, and opinion, that online labs are an excellent opportunity to focus on developing scientific thinking and process skills such as experimental design, data analysis, communicating findings, and forming and evaluating scientific arguments and primary literature. Based on our experience, we plan to modify the face-to-face version to include more of these experiences, as they were so successful in the online version of the course.
2. Trim course material to focus on goals.
We also found that a lab course in an online environment is a lot of work, and culling course material to streamline and really focus student and instructor energy on the chosen goals is very important to ensure everyone has the cognitive energy to fully engage. We recommend starting the course slowly, perhaps with technology training/orientation for instructors, teaching assistants and students, ice breakers to foster an active environment, and low-value assessments to ease into the course material and new mode of online assessment.
3. Identify existing labs that help achieve these goals or have an interesting/relevant scientific context for your course.
Students can walk through experimental protocols to learn about techniques, and focus on analyzing results and data from said hypothetical experiments. Students engage in troubleshooting and learn that experimental results are not always as expected.
4. Implement a system to foster collaboration in groups.
For example, rotating roles within small breakout groups (e.g., acting as manager, note-taker, reporter) and holding students accountable to share, discuss, present and document their work in a lab notebook.
Student responses to the survey question asking for suggestions to improve the online lab course were coded to identify themes among their answers. Table 5 summarizes the themes that emerged from coding 100 responses. The most common recommendation was to include more instructional videos on the protocols discussed in this lab course (34% of responses). Students suggested this would help them better understand the techniques and how they work, and visualize what would have happened in the lab. Although we occasionally assigned protocol videos from the online source JoVE, Journal of Visualized Experiments, the step-by-step protocols in our lab manual did not always align with how the methods were visualized in the videos. In response to the students' suggestion, we have two recommendations: 1) if possible, record videos of someone doing the physical lab work that aligns with how the procedures are described in the manual; 2) assign pre-made videos (e.g., through JoVE or similar) but make time to explicitly discuss how the protocol students read about may vary from what was depicted in the video, and why this is relevant (e.g., when we move from lab to lab in research, there are multiple ways to do a similar protocol). If possible, once university courses are back to face-to-face, we are considering offering a laboratory bootcamp to past students, allowing them the opportunity to experience some of the essential tasks in lab (e.g., micropipetting, vortexing, centrifugations, bacterial transformations, cell culturing, aseptic technique, PCR, gel electrophoresis).
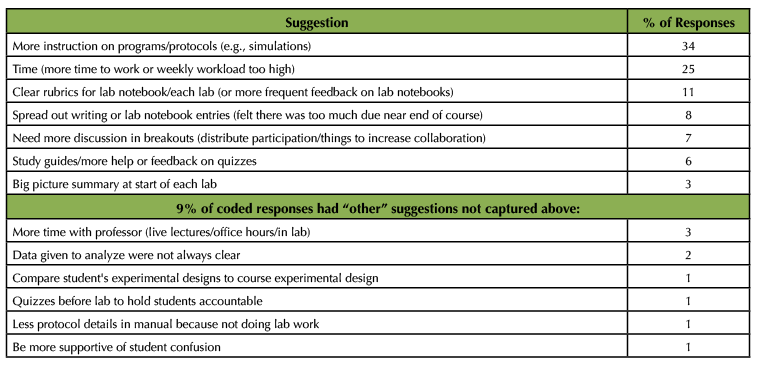
Table 5. Student suggestions to improve the online course. Students were asked to provide recommendations to improve their learning in the online lab course. Suggestions that emerged were labeled and counted out of 100 student responses, presented here as % of responses that contained the suggestion (students could make more than one suggestion, so percentages add up to more than 100).
Another frequently made suggestion by students was to reduce the workload, and provide more time for large writing assignments. Part of this common response is likely the result of the stress associated with quickly shifting to online learning during a pandemic. In fact, the workload (contact hours, writing assignments, and quizzes) was lower in the online than in the typical face-to-face version. However, we recognize that the self-directed and self-paced learning in an online environment may pose an additional real or perceived sense of workload. For this reason, moving forward, we will provide detailed and clear schedules, describe how the online course workload relates to the face-to-face version, provide tips and tools to manage learning in an online course, and work towards spreading out some of the larger writing assignments with more opportunities for feedback and revision.
SCIENTIFIC TEACHING THEMES
Active Learning
The general format of the online lab was to engage in some pre-lab learning, discuss, analyze and problem solve in groups, and then have time to consolidate experiences in the form of individually-produced lab notebook entries and take-home quiz responses. In this way, students were actively involved in both individual and group-based learning. The instructor and teaching assistant being able to circulate through the student groups to ask and answer questions also provided lots of opportunity for real-time feedback, another major benefit of active learning.
Assessment
Instructors measured learning in a variety of ways, all of which were common modes of assessment from the face-to-face version of the course, including
- Evaluating students' predictions, analysis, and conclusions drawn (scientific arguments) in their lab notebooks, typically on a weekly basis.
- Multiple take-home tests, with questions that tested for ability to apply understanding of experiments and techniques to troubleshoot, draw conclusions from results, plan experiments, and critique claims based on evidence given.
- Scientific writing in the form of a short journal article-style report, summarizing their findings of the CRISPR-Cas9 editing of the ADE2 gene in yeast.
Many instructors also awarded points for participation in pre-lab learning and during the lab (e.g., arriving on time, engaging in group discussion).
Students had a chance to assess or reflect on their understanding and progress throughout the course by receiving regular feedback in their lab notebooks (which could be used to improve future lab notebook entries). For the final CRISPR-Cas9 write-up, multiple instructors gave students a chance to submit a draft, engage in peer-review, and then submit a revised version. For informal assessment of progress and to solicit student input, there were a couple of opportunities to share their perspectives on the course and their learning (through a mid-course and end-of-course survey). We would like to make a special comment about the take-home testing. In order to test at higher cognitive levels (analysis, synthesis, evaluation) we wanted to have open-ended responses. We also wanted students to have time to think and work on their tests, to avoid issues with timed online testing (especially for students who had competing interests such as taking care of family members, jobs, and unstable internet connections). For this reason, we chose take-home testing. Students uploaded their responses to our learning management system, and we often also had them upload responses to a plagiarism detection software (Turnitin).
The modes and specific examples of assessment are not the focus of this article, rather the process of converting a face-to-face lab to online is the focus, and for this reason we have not included test questions nor examples of other assignments. However, we are willing to share additional materials (please email the corresponding author).
Inclusive Teaching
Highly structured courses have the potential to be more inclusive (12). This online lab course is highly-structured because it contains pre-lab learning with opportunities for feedback, frequent feedback on thinking and writing in the form of laboratory notebooks, peer-instruction during lab sessions, multiple summative assessments, and opportunities to demonstrate mastery in multiple modes, including writing, take-home tests, as well as oral presentations. Providing students with the opportunity to frequently work at their own pace ensures that those who need differing amounts of time with material have this access (see comments about take-home tests in "Assessment"). Also, accommodating students who have unavoidable conflicts with live video conferencing lab sessions is important in creating an inclusive environment. We emphasized flexibility in this course, encouraging students to participate in the live lab sessions as often as they could but accommodating when they could not (over 90% of students were in the live sessions, all quarter long). We allowed for missed sessions and time to make-up missed lab notebook entries when inevitable conflicts arose such as sick family members or students, work conflicts (some students picked up extra shifts to supplement their family's income), anxiety and depression exacerbated as a result of the pandemic and civil uprising. Many of us took a mastery approach to grading, e.g., weighting the lowest scoring take-home quiz lower than the highest scoring quiz, and using a score of 85% or higher on lower stakes items as a cutoff for full points.
When creating lab discussion breakout groups, some instructors created groups to ensure that there were no gender imbalances or, for example, one student from a traditionally under-represented background being the only under-represented student in their group. Some instructors randomly assigned groups each week, providing opportunities for students to meet more peers and be exposed to more diverse ways of thinking and working. Further, assigning roles to different group members each week ensured that all students had opportunities to speak, report, and be heard by their peers.
Lastly, providing opportunities to engage in a lab course online may increase accessibility for students who normally are unable to participate because of disability, geography, or other constraints. For this reason, it may be worth considering offering online versions of a lab course even when instruction returns face-to-face.
SUPPORTING MATERIALS
- S1. Converting face-to-face lab to online – Example Lab Results for Analysis
- S2. Converting face-to-face lab to online – Example Lab Manual Section
- S3. Converting face-to-face lab to online – Student Lab Notebook Entry and Rubric
- S4. Converting face-to-face lab to online – Journal Article Discussion Materials
- S5. Converting face-to-face lab to online – Take Home Assessment Examples
- S6. Converting face-to-face lab to online – Presentation Documentation
ACKNOWLEDGMENTS
We would like to thank all of the teaching assistants who helped implement the online lab under very stressful times! We are also very thankful to the students, who engaged in fruitful and interesting discussions and provided invaluable feedback on their experiences. We thank Dr. William McGinnis for helping to develop and pilot the CRISPR-Cas9 experiments in the face-to-face version of the course. Use of anonymized student survey results was approved by our Human Research Protections Program at UC San Diego (protocol 170886).
References
- Sehgal N, Sylves ME, Walker SE, Cullen PJ, Berry JO. 2018. Gene Editing in Yeast: An Experimental Protocol for an Upper-Division Undergraduate Laboratory Course. Biochem Mol Biol Educ, 46: 592-601. doi.org/10.1002/bmb.21175
- Ulbricht RJ. 2019. CRISPR/Cas9 in yeast: a multi-week laboratory exercise for undergraduate students. CourseSource. doi.org/10.24918/cs.2019.19
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. 2013. Genome engineering using the CRISPR-Cas9 system. Nat Protoc, 8: 2281-2308. doi.org/10.1038/nprot.2013.143
- Kee HL, Kushner JK, Deuchler CP, Becker AK, Clarke, DG, Pieczynski JN. 2019. Using CRISPR-Cas9 to teach the fundamentals of molecular biology and experimental design.CourseSource. doi.org/10.24918/cs.2019.21
- Samsa LA, Anderson L, Groth A, Goller C. 2020. A CRISPR/Cas guide RNA design in silico activity. CourseSource. https://doi.org/10.24918/cs.2020.46
- Secret M, Jennings Ward C, Newmark A. 2019. Converting a Face-To-Face Introductory Research Methods Course to an Online Format: Pedagogical Issues and Technological Tools, J Teach Sco Work, 39:, 455-476. DOI: 10.1080/08841233.2019.1635558
- Godlewska A, Beyer W, Whetstone S, Schaefli L, Rose J, Talan B, Kamin-Patterson S, Lamb C, Forcione M. 2019. Converting a large lecture class to an active blended learning class: why, how, and what we learned, J Geogr Higher Educ, 43:1, 96-115, DOI:10.1080/03098265.2019.1570090
- Burnette KAS. 2020. Drawing flowcharts of lab protocols helps students prepare for biology labs. CourseSource. doi.org/10.24918/cs.2020.2
- Brownell SE, Koser MJ, Fukami T, Shavelson R. 2012. Undergraduate biology lab courses: Comparing the impact of traditionally based "cookbook" and authentic research-based courses on student lab experiences. J Coll Sci Teach, 41: 36-45.
- Brownell SE, Hekmat-Scafe DS, Singla V, Chandler Seawell P, Conklin Imam JF, Eddy SL, Stearns T, Cyert MS. 2015. A high-enrollment course-based undergraduate research experience improves student conceptions of scientific thinking and ability to interpret data. CBE Life Sci Educ, 14:ar21. doi.org/10.1187/cbe.14-05-0092
- Pelaez N, Andrerson T, Gardner SM, Yin Y, Abraham JK, Bartlett E, Gormally C, Hill JP, Hoover M, Hurney C, Long, T, Newman DL, Karen S, Stevens M. 2017. The Basic Competencies of Biological Experimentation: Concept-Skill Statements. PIBERG Instructional Innovation Materials. Paper 4. http://docs.lib.purdue.edu/pibergiim/4
- Eddy SL, Hogan, KA. 2014. Getting Under the Hood: How and for Whom Does Increasing Course Structure Work? CBE Life Sci Educ, 13: 453-468. doi.org/10.1187/cbe.14-03-0050 606
Article Files
Login to access supporting documents
Converting a Face-to-Face Lab to Online: An Example of Process and Outcomes for a CRISPR-Based Molecular Biology Lab(PDF | 494 KB)
Table 1 - in-person and online lab schedule_0.pdf(PDF | 214 KB)
S1. Converting face-to-face lab to online - Example Lab Results for Analysis_0.pptx(PPTX | 3 MB)
S2. Convering face-to-face lab to online - Example Lab Manual Section_0.docx(DOCX | 116 KB)
S3. Converting face-to-face lab to online - Student Lab Notebook Entry and Rubric.docx(DOCX | 3 MB)
S4. Converting face-to-face lab to online - Journal Article Discussion Materials.docx(DOCX | 25 KB)
S5. Converting face-to-face lab to online - Take Home Assessment Examples.docx(DOCX | 778 KB)
S6. Converting face-to-face lab to online -Presentation Documentation.docx(DOCX | 186 KB)
- License terms

Comments
Comments
There are no comments on this resource.