Fly Exercise: A Simple Experiment to Test the Physiological Effects of Exercise on a Model Organism
Editor: Neena Grover
Published online:
Abstract
Model organisms are commonly used to study and experimentally explore biological processes in ways that are technically or ethically not possible in humans. For this reason, model organisms are an essential component of scientific research and exposing students to practical applications of data collection using model organisms is an essential component of a thorough scientific education. In this paper, we use the fly model species, Drosophila melanogaster, and a fly treadmill to perform an exercise physiology experiment. The goal of the experiment is to expose students to the process, value, and importance of using model organisms in scientific research. The instructor will assist students in collecting, exercising, and sacrificing the flies from various lines and sexes. The students will quantify a biologically meaningful suite of enzyme activities and metabolite concentrations, assemble the results from the assays in a spreadsheet document, plot the data from the results and perform statistical analyses to quantify and, finally, graphically present physiological changes associated with the different exercise regimes. The experiment can be conducted in two three-hour sessions; the first to collect, exercise, and homogenize the flies and then perform enzymatic kinetic assays, the second to quantify metabolite concentrations. After the second lab session, the students will have the complete experimental data set and will be able to perform data analysis on their results. The experiment is designed to suit second to fourth year undergraduate students, depending on their past laboratory experience and skillset.
Citation
Hick JPS, Eng MG, Parrotta MD, Merritt TJS. 2019. Fly Exercise: A Simple Experiment to Test the Physiological Effects of Exercise on a Model Organism. CourseSource. https://doi.org/10.24918/cs.2019.46
Lesson Learning Goals
Students will:
- learn the fundaments of experimental design setup.
- learn how to apply fundamental laboratory techniques in a research environment.
- understand the importance and utility of model organisms in science.
- learn post-experimental analysis with raw data to gain insight into the effect exercise has on a model organism's physiology.
- demonstrate the ability to interpret and present raw data in a graphical form and perform statistical analyses.
Lesson Learning Objectives
Students will:
- demonstrate understanding of the concept and details of experimental design.
- perform an organic lipid extraction to determine total lipid content.
- quantify enzyme activity, as well as triglyceride, glucose, and glycogen concentrations.
- organize their collected data into spreadsheets for statistical analyses.
- interpret the results to gain insight on the varying effects exercise has on an organism's physiology.
- graphically present their results so that trends can be easily identified.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
INTRODUCTION
At its heart, science is asking--and sometimes answering--questions. In biology, selecting a study organism appropriate for your question is central to effectively answering questions. The importance of this selection was clearly articulated by the physiologist August Krogh in a classic address to the American Physiological Society (1); this address is widely credited as the first clear statement of the fundamental value of proper model organisms (2). In this exercise, we combine Drosophila melanogaster (the fruit fly), one of the simplest model organisms to work with and to exercise--an intrinsically interesting biological phenomenon--to explore the biology of physical activity.
Exercise can have an enormous positive impact on health and wellbeing. As the frequency of sedentary lifestyles is increasing, and in the face of the associated global obesity epidemic, healthcare workers, researchers, students, and the general public are becoming more interested in the broad effect of physical exercise on physiology (3). The connection between health and physical exercise is of general interest to researchers and the public, including students and teachers, and other lessons have used similar diet-related topics as a subject to interest students (e.g., 4). One striking feature of exercise, and in fact many biologically interesting phenomena, is that the activities affect various people differently (5). Many of the fundamental physiological effects of exercise are, however, poorly understood, let alone how these effects vary across individuals (5). One reason for this gap in our understanding is the factors, such as genetic background and environment, that are difficult or impossible to control in human-based exercise research (6), but that can more easily be controlled when working with model organisms. In fact, controlling for these factors is one of the primary advantages of model system-based research (7,8). Our experiment allows students to perform a laboratory-based research experiment to observe, quantify, and explore the effects of physical exercise on a living organism, and builds on previous similar lessons to incorporate individual diversity (4).
Model organisms and fruit flies
The term "model organism" is commonly used in modern biology but rarely defined (9). Essentially, model organisms are species that are studied when it is impractical to study the organism of primary interest, and studied with the intent to understand a concept not just in that species or individual, but in a broader variety of organisms (7). Importantly, not all experimental organisms are model organisms (7), and the term model organism is generally reserved for species that are studied across many different laboratories under the principle that many researchers working on the same system can come to a deeper understanding of fundamental concepts than those same laboratories would if they worked with different species (10,11).
Working with model species allows us to ask questions that are fundamentally about human biology, but that we would never ask in humans. For example, it is relatively straightforward to create a four-winged fruit fly (flies are Diptera and typically have only two wings). These experiments have told us a great deal about the genetics and evolution of wings and limbs in general (e.g., 12) and were central to some of the research that led to the 1995 Nobel Prize in Physiology or Medicine (Why Fly; https://droso4schools.wordpress.com/why-fly/). In this research, we are ultimately interested in how human limbs develop, but we would never perform such experiments in humans. On the other hand, recent advances in genetic engineering may make such experiments possible now or in the near future (13), and student feelings about such experiments would make for an interesting classroom discussion.
Different model species have distinct strengths and weaknesses, and biologists need to match their species to the questions they are asking (10,11). The most frequently used animal species in areas such as drug research, testing of vaccines, and biological and cancer research are mice and rats (14). These organisms are, however, expensive to work with, and research using vertebrates still involves sensitive ethical issues and tight regulations. Using animal models in general comes with discomfort at the animal's expense, for example minor discomfort (e.g. single blood sampling), moderate discomfort (e.g. recovery from anesthesia), and severe discomfort (e.g. toxicity tests; 14). Working with an invertebrate model eliminates many of the ethical issues associated with manipulation of humans or even other vertebrates, while providing researchers with the ability to control factors like genetic backgrounds and the environment of the organism. An interesting concept to discuss with a class is the ethical or moral considerations of working with animals. Why is it that we will perform experiments on rats that we wouldn't on humans? Why are we typically even more comfortable with experimenting on and sacrificing invertebrates like flies than rats or mice, let alone humans? A typical project in our laboratory, for example, involves hundreds or even tens of thousands of flies - all of which are sacrificed during the experiments.
With the use of any animal model, a scientist is responsible for making sure the model does not undergo any unnecessary suffering and must be treated with respect at all times. The quality of human life depends on these advances, and the development of new medicines and treatments are all made possible by animal research (15). Working with model organisms is generally conducted with a view of the three R's: Replacement, Reduction, and Refinement (16, 17; The Canadian Council on Animal Care; https://www.ccac.ca/en/training/modules/core-stream/three-rs-of-humane-animal-experimentation.html), with the aim of respecting the organisms that we are working with while improving and advancing our science. Replacement can mean replacing study of a more sentiment animal, for example humans, with a less sentient, e.g. fruit flies. It can also mean replacing animal models with cell-line models or a computer model. Here, reduction means using the fewest number of organisms necessary to answer the question of interest. This point can lead to an interesting conversation of balancing statistical power from larger samples sizes with the ethical concerns of sacrificing more animals. In our context, refinement means refining handling of the study animals to eliminate unnecessary pain or discomfort and can include appropriate animal housing and the methods used to sacrifice organisms. All three terms are open to interpretation and may mean different things in different contexts of biological research (2). These points are, themselves, possible topics of discussion with students.
The common fruit fly, Drosophila melanogaster, is one of the most widely and successfully used model organisms in biological research (8); the 2017 Nobel Prize in Physiology or Medicine was the sixth Nobel awarded based on research using this species (Why Fly; https://droso4schools.wordpress.com/why-fly/). However, when talking to students and the general public about science based on fruit flies, we are frequently asked why we use fruit flies in our research, and how research on flies could apply to humans. The answer is simply that the common origin of life on this planet means that, fundamentally, all known life is related, and asking questions in one species can provide answers for all species. Further, in fruit flies, we have a research tool that provides researchers with a model organism whose small size, short life cycle, and ease of genetic manipulation make them far easier to work with than a human model. In addition to their ease of use as a model organism, flies share with humans approximately 75% of known disease-causing genes and the majority of metabolic pathways and conserved genetic mechanisms (18). These biochemical and genetic similarities make the fruit fly an excellent candidate to study how biological mechanisms are affected by different stressors like physical exercise (19).
Fly exercise
Perhaps not surprisingly, the question we are most frequently asked is also the most basic question: "how does one exercise a fly?" Basically, we simply need to promote increased physical activity in flies. A few methods have been developed to induce physical activity in fruit flies, all of which take advantage of an innate reaction flies have called negative geotaxis, the fly's innate reaction of climbing up against gravity (20). This reaction means that when vials containing flies are tapped on a hard surface or gently rotated, the flies climb up or walk against the direction of rotation. Exercise can be induced simply by repeatedly tapping vials to knock the flies down in the vials as they will then climb back up. In addition, simple machines to tap down or rotate vials can effectively induce exercise simultaneously in large numbers (hundreds or even thousands) of flies and several research groups have been using such apparatuses to induce physical activity to explore exercise physiology using flies (21,22,23). We have developed our own fly exercising machine, called the Flygometer, in which vials containing flies are placed on their side and slowly rotated around their long axis on a conveyer belt to induce flies to walk away from the direction of movement (picture a corner store hotdog cooker, without the heat or hotdogs). The Flygometer, or similar equipment, is an effective tool for promoting physical activity in large numbers of flies for short periods of time. Alternatively, fly vials can simply be "tapped down" to promote increased activity in the absence of any dedicated equipment. Undergraduate students can be taught either approach simply and quickly. After exercise, experimental and control flies can be collected, sacrificed (by quick freezing), and analyzed for enzyme activities or metabolite concentrations. The duration of exercise, the exercise regime (e.g., long/endurance or short/intense), and the time between exercising and freezing can be changed (allowing a focus on immediate or more long-term effects). In addition, the specific biochemical assays performed can be varied (e.g., different enzyme activities and metabolites can be quantified), allowing the complexity of the experiment and analysis to be scaled to the experience level of the students. The enzyme assays that were selected represent an enzyme network responsible for the reduction of NADP+ to NADPH (24). This enzyme network responds to environmental conditions, and a reduction in one enzyme's activity has been shown to actually increase another enzyme's activity (24); the network likely works together to maintain a cellular pool of NADPH to help neutralize radical oxygen species (ROS) (25,26,27). The metabolites chosen in this experiment are commonly measured metabolites in fruit flies to measure responses in environmental or dietary conditions (21,28,29).
Experimental setup and variation
We present here a fly exercise experiment designed around a simple physical activity regime incorporating an interesting and biologically relevant set of metabolites. The complexity of the experimental design, protocol, and equipment, is scalable.
We use flies from our laboratory stocks, but educators can order Drosophila melanogaster, and Drosophila husbandry supplies (e.g. vials and pre-made food powder) from various commercial suppliers (e.g. Carolina Biological or even Amazon). Educators can also order flies directly from the same international Drosophila stock center that we use for our research, The Bloomington Drosophila Stock Center (https://bdsc.indiana.edu/index.html; keep in mind, though, that this is a scientific resource center not a retailer, and they require at least a few weeks to process orders). Drosophila can also be collected from the wild in the summer and fall. We recommend using the standard wild type (i.e., not genetically engineered), control lines Canton-S (Bloomington stock # 64349) and Oregon-R (#5), although we used a small set of lines from the Drosophila Genetics Reference Panel (DGRP lines, a set of wild type fly lines established for genomics research) (30). Instructors can find information on DGRP fly lines as well as transgenic fly lines on FlyBase (http://flybase.org, a database of Drosophila genes and genomes). The DGRP lines are essentially homozygous, so there is no genetic variation within a line, but comparing lines allows researchers to study genetic variation within the population from which the lines were derived. The specific fly lines chosen in this experiment, DGRP 852, DGRP 315, DGRP 380, and DGRP 307, have different levels of motivation related to mitochondrial efficiencies that are shown to play a role in physiological response when exercised (21,31). DGRP 852 and DGRP 315 are high motivation lines while DGRP 380 and DGRP 307 are low motivation lines (21,31). This genetic diversity of the fly lines gives a range of physiological responses. In addition, males and females have different metabolic requirements (32), and their performance will likely vary in this experiment.
We used the Flygometer (Figure 1), but the exercise routine can be conducted using only a flat surface to tap down, and a timer (Tap Down Assay; http://www.flyfacility.manchester.ac.uk). The different exercise treatment options, and the multitude of possible biochemical assays, creates a flexible experimental design that can vary in length and complexity, depending on the level of students, and can be modified to fit student needs and the resources available. For example, a student may choose one sex and one fly line to exercise in ten-minute intervals, with five minutes of rest, for one hour. The student can compare triglyceride content between exercised flies and non-exercised flies. Endpoint biochemical assays that quantify concentrations are generally simpler than kinetic assays measuring, for example, enzyme activities. Having students discuss and choose different lines, sexes, exercise regimes and times between exercise and sacrifice can introduce students to the learning goal of basic experimental design. The ability to easily compare and contrast, and predict, the different effects of long- and short-term exercise regimes is one of the strengths of this experiment. Similarly, instructors can scale statistical analysis and interpretation of data to the level of the students. The experiment also allows students to apply skills learned in lower-year undergraduate labs (e.g., pipetting, practicing sterile techniques, and solution preparation), in the context of a research lab. Working with a commonly-used model organism in scientific research can introduce students to the utility and potential of that model organism, and model organisms in general. In addition, having a simple and quick experiment studying a highly talked-about topic will spike interest, enthusiasm, and intrigue into the course material.

Figure 1. The Flygometer 2.0.
Intended Audience
The intended demographic for this experiment is second- to fourth-year undergraduate level students. In our example, the experiment was performed as part of a third-year Experimental Biochemistry class by two separate cohorts of students. In general, the experiment is best suited for students who have prior experience with pipette usage, standard curve construction, and solution making, although instruction in these skills could be incorporated into the experiment.
Required Learning Time
The required in-class learning time varies, depending on the proportion of the exercise that the instructor elects to perform themselves, versus the proportion the instructor allocates to the students. If the instructor collects and sorts the flies from stock vials ahead of time, the lesson will take approximately two three-hour timeslots. If the instructor prefers for the students to collect the flies, the lesson will require a third three-hour timeslot. The in-class learning time also depends on the length of time chosen to exercise the flies and the time between exercise and sacrificing. We are able to get a physiological response in flies from as little as 15 minutes of exercise, but one could exercise the flies as long as two hours.
Prerequisite Student Knowledge
Students should be comfortable using micropipettes in order to collect fly homogenate, prepare reagents, and to set up biochemical assays. Students should also have experience with spreadsheet organization to properly organize data tables. Students can be given a selection of papers on exercise physiology, exercise related metabolic pathways, and specifically fly exercise, to allow more informed insight into the system they are exploring and a deeper discussion of what potential results they may find (23,33,34).
Prerequisite Teacher Knowledge
Teachers should be comfortable preparing assay reagents and using a spectrophotometer; experience working with fruit flies would be advantageous. Teachers do not need to be experts in using Drosophila melanogaster in the laboratory, but should be comfortable with the organism. Detailed instructions on how to use flies as a research teaching-tool in the classroom are available through the Manchester Fly Facility (https://droso4schools.wordpress.com), and Dr. Michele Markstein has created a well-structured laboratory manual for working with Drosophila that is available through her website (https://marksteinlab.org/dwu/). Teachers do not need to be experts in metabolic pathways but should have a basic understanding of how exercise and other physiological stressors may affect aspects of physiology, such as lipid content, glycogen content, and enzyme activities.
SCIENTIFIC TEACHING THEMES
Active Learning
In this lesson, students can perform the experiments individually, but ideally they will work in groups of three to four, so that students can divide tasks, such as homogenization of flies and reagent preparation, across the group. At the beginning of the experiment, students can be informally quizzed with probing questions about the importance of using model organisms, as well as questions about the importance of abiding to ethics in research and anti-animal-research sentiments and protest. Students should be asked why we are using fruit flies and what other animal models they are familiar with for modeling exercise. Teachers can quiz the students on the drawbacks and benefits of various animal models, including flies, but also including model organisms like mice and rats. For example, when using fruit flies, we can easily study multiple genetic backgrounds, whereas in rats we generally only study one genetic background (although more are possible). Rats are, however, physiologically more similar to humans than flies. Teachers can lead discussions around the trade-offs, ethical and logistical, involved in different models. Students should be asked about their expectations for the results for the experiment. During the experiment, students will be performing standard laboratory procedures to gain hands-on laboratory research experience. Post-experiment, students will collect their data and perform data analysis and interpretation individually or in groups.
Assessment
Assessment can be done through a qualitative performance mark, in which students are evaluated during the lab period on their time management, data organization, pipetting ability, and overall preparedness for the lab. Assessment also includes asking pre-lab questions such as "why are flies used as model organisms?" and "what benefits are there to using flies for research?" Post-lab questions can also be asked such as "how does exercise effect the physiology of the fly?" and "is the assumed correlation between lipids and triglycerides correct?" Depending on the level of knowledge in biochemistry, the answers may vary in complexity: for example, a second-year student's answer may be simply observational, whereas an upper-year student may answer more in depth, suggesting biological pathways that would influence the change. The two experimental biochemistry pilot groups were only assessed for performance, since the purpose of the experiment was to collect results for an experimental biochemistry class. After the lab wet work has been completed and their data is assembled on spreadsheets, students can be evaluated on their ability to organize their collected raw data, statistically analyze their raw data, and present their data in appropriate bar graphs in order to compare the effects of fly line and exercise regime on their results. Assessments can also be made on students' written discussions of their results, the implications and limitations of these results, and future questions their results create.
Inclusive Teaching
The topic of physical exercise is relevant and engaging to a broad suite of students, as exercise affects everyone, but affects everyone differently, based on both genetic and environmental factors. The extent of variation between individuals is only just being appreciated. Exercise resistance, for example, is a new field of research, which looks at individuals who may be predisposed genetically or epigenetically to have a weak or absent metabolic response to exercise (24). There are only a handful of genes that are predicted to influence exercise response, and although these have been identified in some studies, follow up research has had limited success in verifying their roles (35), likely underscoring the variability in this system and the need for broader research. In this exercise, differences between individuals can be observed in how exercise affects fly lines and sexes differently. In fact, because of the ease of using a variety of fly lines and many individuals, the experiment can very effectively demonstrate how exercise has more complicated and diverse physiological effects than what many people may believe. The focus of the experiment is closely related to real-world problems, such as sedentary lifestyles, obesity, and cardiovascular complications, which could encourage more students to become engaged in an alternative hands-on learning environment, as opposed to a typical didactic lecture-based classroom (36,37). Working in small groups allows students to distribute tasks and share responsibility in their experiment, encouraging cooperation while maintaining individual responsibilities. Discussing questions relevant to the lesson in these groups can act as an interactive low-stress learning environment to students, that encourages multiple perspectives and ideas that creates an open learning environment to students.
LESSON PLAN
Pre-Class Preparations
Before the experiment, students should be given fly exercise papers (e.g. 20, 21, 22) to be read ahead of time. The instructor should maintain the fly lines on an appropriate fly food diet. We used a standard fly food diet consisting of cornmeal, yeast, agar, and corn syrup. Fly food recipes can be found on the BDSC website (https://bdsc.indiana.edu/information/recipes/index.html) or commercial food mixes can be purchased through on-line retailers like Amazon (https://www.amazon.ca or https://www.amazon.com). The fly food should be changed every 1.5-2 weeks by simply transferring the flies from the vial containing old food to a new vial containing fresh food. Students should be prepared to answer questions on the utility of flies as a model organism, the usage of multiple fly lines, and their own expectations for some of the physiological effects of exercise on fruit flies. To fit the experiment into two separate three-hour long sessions, we recommend that before the first session the instructor collects the flies required for the experiment. If students or instructors intend on exercising fruit flies for longer than 15 minutes, we recommend that the exercise periods are started before the first three-hour laboratory session begins, so that roughly the last ten minutes of the exercise period is performed during the laboratory session.
In-class lecture script
Day 1
To begin, the instructor will introduce the class to the fruit fly system, including the vials that flies are raised in and that will be used on the treadmill, and the concept of using negative geotaxis to exercise flies. The instructor will then ask conceptual questions about why we use model organisms to study biological processes and diseases as well as why, specifically, we use fruit flies. The instructor can then lead into questions about the fly model for exercise, and advantages (e.g. ease of use, multiple genetic backgrounds) and disadvantages (e.g. evolutionarily distant from humans, ectotherm) when compared with other model organisms. The instructor will then ask questions around the material that will be performed in the experiment. A lab protocol outlining the entire experiment, including instructions on the specific assays we performed in this example, is included in the supplementary materials (Supporting File S1: Fly Exercise Lab protocol). Prior to sacrificing the flies by freezing them in liquid nitrogen, the instructor can ask a question on the purpose of flash freezing (to halt metabolism at a specific moment and prevent degradation of metabolites and loss of enzyme activities). Flash freezing is performed by tapping flies through a funnel into a snap-cap microcentrifuge tube, and then dropping the microcentrifuge tube into liquid nitrogen.
After flash freezing the flies, the students will sort the flies into groups of five and place them into individual 1.5 mL microcentrifuge tubes and place the vials on ice. For the experiment, three biological replicates containing five flies each for each line, sex, and treatment is ideal. An example flow chart of experimental replicates can be seen in Figure 2. Three biological samples for each treatment will be set aside on ice for an organic lipid extraction and another three for various assays (instructors may elect to add and omit certain assays).

Figure 2. Experimental Replicate Flow Chart
Flies for the organic lipid concentration assay are treated separately from the other, aqueous, assays. For the organic extractions, one student will then take the fly samples set aside for the organic lipid extraction and weigh them. After weighing, the flies will be placed inside glass test tubes, left uncovered, and then incubated at 50˚C overnight to desiccate the samples (removing all water) to allow determination of sample dry weight, the first step in determination of lipid content.
While one student is weighing the flies for the organic lipid extraction, the remaining students in the group can be adding homogenization buffer to the remaining fly samples. For the assays we performed, we used 0.1 M TRIS-HCL, pH 7.4 homogenizing buffer at a homogenization concentration of one fly/100 μL homogenizing buffer. Different enzymatic and metabolite assays require different buffers for optimum results. Using a Dremel tool (Figure 3), flies are homogenized for ~5 seconds and returned to ice. Homogenates are then placed in a centrifuge at 4˚C for 5 minutes at 13,000 RPM to pellet fly solids. While avoiding the pellet, 300 μL of the homogenate is then transferred to a 96-well master plate, which will serve as the stock plate from which aliquots are removed to each individual aqueous assay. The master plate should be kept on ice to avoid enzyme degradation.

Figure 3. The Dremel Tool
Once the fly homogenate from each sample is transferred to the master plate (Figure 4), the students can begin assays. Note that enzymatic assays must be performed on day one, as even on ice enzymes will degrade and loose activity over time. Assays measuring metabolite content can be performed after a freeze thaw cycle. Once the enzymatic assays are finished, the master plate should be wrapped in parafilm and stored at -20˚C overnight.
Figure 4. Master Assay Plate
Day 2
To begin Day 2, instructors should take the frozen master plate out of the freezer at least one hour before the beginning of the second session and allow it to thaw in a refrigerator. As soon as students arrive for the beginning of the second period, the instructor should quiz the students on what types of assays they are performing that day, and what results they may expect. Students will then perform Day Two assays, consisting of a series of endpoint assays monitoring specific metabolite (e.g., glycogen and triglyceride--soluble lipid--concentration). Protocols on how to perform these assays can be found in the supplementary materials (Supporting File S1: Fly Exercise Lab protocol). Instructors may elect to change, add, or omit certain endpoint assays at their discretion.
At one point during the second session, one student will take the incubated test tubes for the organic lipid extraction, obtain the dry weight of each sample, and then add 1 mL of diethyl ether to each sample to extract lipids. The student should then cork the test tubes and leave them to incubate overnight at room temperature.
Day 3
On Day 3, the instructor should remove the ether from each organic lipid extraction test tube and then incubate the samples again at 50˚C overnight, uncovered to evaporate off any residual ether. Appropriate safety precautions should be taken while handling ether as it is flammable.
Day 4
On Day 4, the instructor or the students, depending on the students' availability, will take the dry weight of the flies in the lipid extraction experiment.
Post-Experiment
Once the organic lipid extraction is completed and the final weights of the flies are tallied, the data from the lipid extraction, the enzymatic kinetic assays, and the metabolite content assays can be organized into a spreadsheet (e.g., using Microsoft Excel).
An outline of the experiment and estimated times for completing each component is outlined below in Table 1.
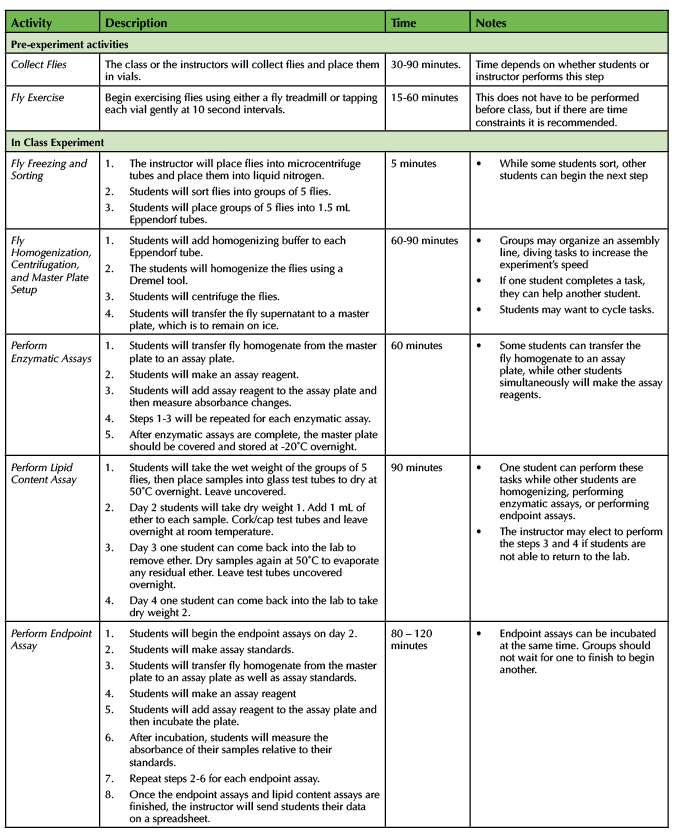
Table 1. Fly Exercise - Teaching timeline and description of steps of the experiment.
TEACHING DISCUSSION
We performed this experiment on two separate occasions with third year biochemistry students, as part of a semester-long Experimental Biochemistry course. The experiment could also be applied to molecular biology courses or kinesiology and exercise science courses, as long as students are taught proper laboratory techniques before they begin the experiment. We found that the experimental flexibility of this lesson allowed us to engage students in the thought process a researcher may go through when designing and carrying out an experiment, in a way which would not be typical in a standard classroom environment. We also found that students were heavily engaged in the subject matter and were interested in making predictions on metabolic reactions to exercise regimes.
Pre-Experiment Activities
Because we conducted the experiment twice, we were able to compare the engagement of students that either were, or were not, instructed to read background literature (e.g. 20, 21, 22) ahead of time. While the comparison was not replicated (we only tested two groups, not replicates of groups), we found that the students in the group that had previously read the papers were much more interested, engaged, and prepared to answer questions. Overall, the more prepared students had a more positive learning experience, so we conclude that the pre-experiment reading is an important step to gaining a more active and positive learning environment for the students. This aspect of the experiment is an important step for students to achieve the learning goals of understanding an experimental design setup and understanding the utility of model organisms in science. In the future to make this portion of the lesson more effective, we suggest assigning pre-experiment questions to answer to prime the students to the experiment they are about to perform.
In-class Experiment
One of our main learning goals in this experiment is to allow students to apply basic laboratory techniques, such as pipetting, solution making, standard curve construction, and applying laboratory skills to a research experiment. This experiment in fly exercise, specifically, also gives students the opportunity to work with living, complex organisms and to learn simple lab techniques, including how to exercise flies, how to transfer the flies from vials to Eppendorf tubes for freezing, and how to homogenize the flies using the Dremel tool without losing enzyme activity from heating up the homogenate. In both groups that we worked with, students responded well to the experiment and were keen to participate in an experiment that resembled a research experiment. Many students made positive remarks on how different an experiment designed to model a research environment is from a standard chemistry or biology lab in an undergraduate class. By working in groups, students were able to divide up tasks during the experiment, allowing less confident students to observe and choose tasks they were more comfortable with. We observed that students were at first hesitant to participate, but once they watched the instructors and fellow group members perform laboratory tasks, they were eager to engage in the exercise. Having students begin with sorting flies into 1.5 mL microcentrifuge tubes is a simple, low-risk activity a student can perform first to gain some early comfort in the experiment. We recommend that students do not begin with flash freezing the flies in liquid nitrogen, and that this task be performed by the instructor. Not only are undergraduate students often nervous about working with liquid nitrogen, but the process of quickly tapping flies into microcentrifuge tubes through a funnel and quickly capping the tube requires practice to successfully perform without flies escaping.
Since multiple assays were performed, students were able to cycle through different roles in the lab exercise so that every group member got the chance to participate in the experiment. For example, one student could be adding homogenizing buffer, while another homogenized the fruit flies with a Dremel tool, and another prepared enzymatic assay reagents. Then, those three students can cycle through those tasks, giving them each experience doing multiple lab activities. Having to organize these laboratory tasks between group members teaches group work practices and communication, as well as promoting work efficiency within the group.
Post Experiment
Another objective of the lesson was to have students organize, interpret and present their data in a graphical form. Once all the raw data was collected and assembled in a spreadsheet, the students were able to organize their results into fly line and exercise treatments. When we performed the experiment with two experimental biochemistry groups, the raw data was inputted into Excel, and the averages were taken from three biological replicates for each respective condition. Several bar graphs were made for each measurement taken, showing each fly line used, average activity or concentration, and post-exercise conditions. In our experiment, we included four different fly lines thathave different levels of motivation (tendency to continue physical exercise) (21), which demonstrates that physiological responses can vary across lines of organisms and that genetic background can play a key role in determining exercise response. An example graph can be found in Figure 5. The students were able to show that the bouts of exercise were enough to influence enzyme activity, but in a line-specific manner (Figures 5, 6, 7).
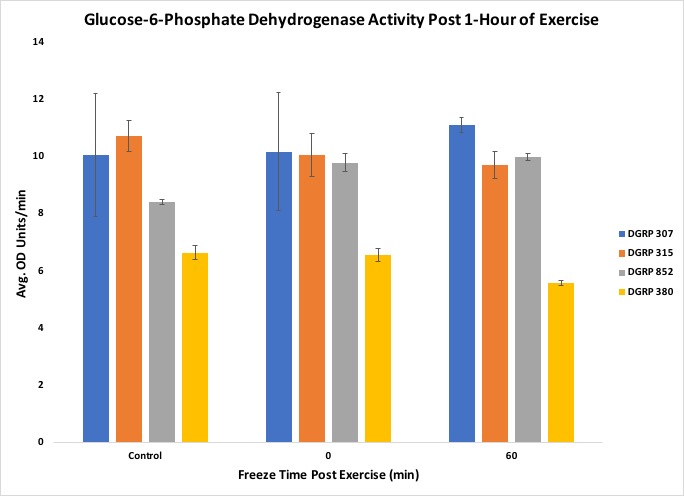
Figure 5. Results from the experiment showing Glucose-6-Phosphate Dehydrogenase activity
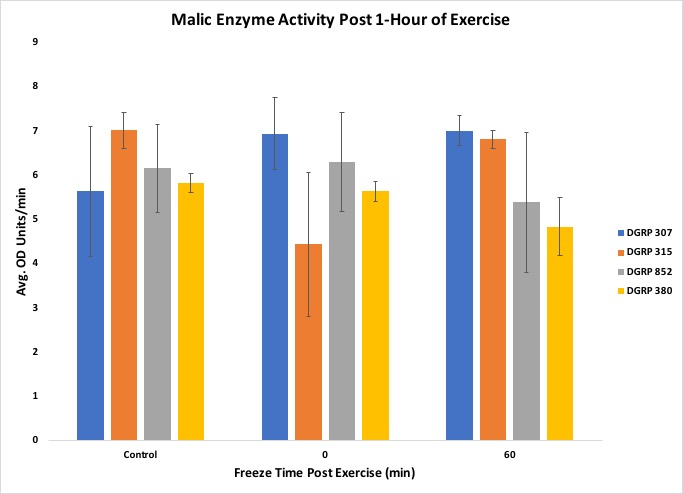
Figure 6. Results from the experiment showing Malic Enzyme activity
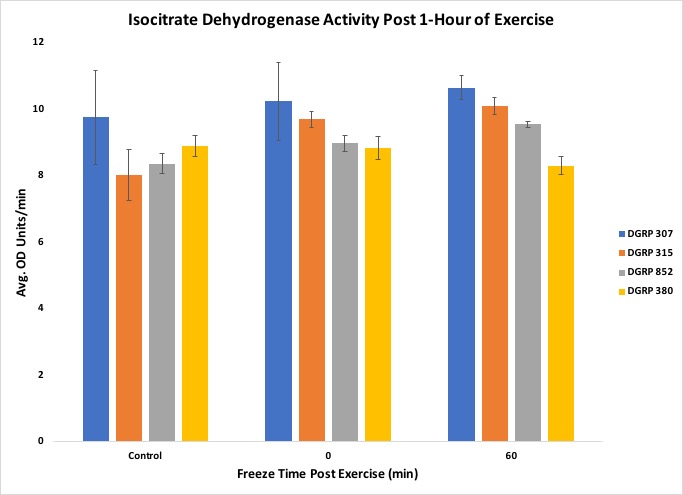
Figure 7. Results from the experiment showing Isocitrate Dehydrogenase activity
Further, the exercise regime affected triglyceride concentration in the low motivational line DGRP 307 more than the other lines (Figure 8), and organic lipid content showed a similar trend in response in both of the low motivational lines, DGRP 307 and DGRP 380 (Figure 9).
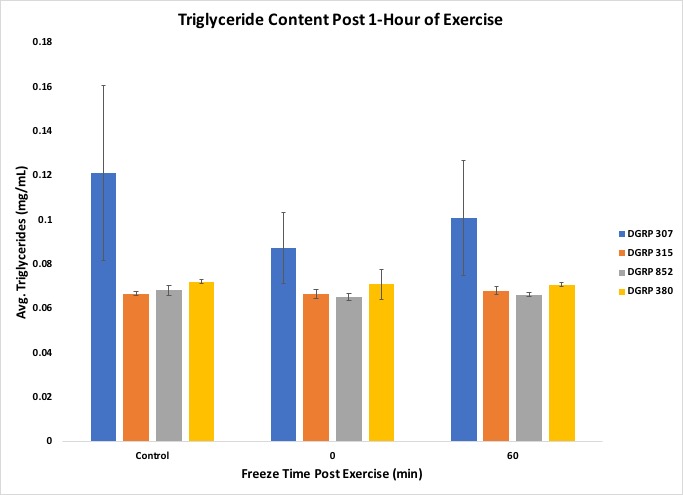
Figure 8. Results from the experiment showing Triglyceride content
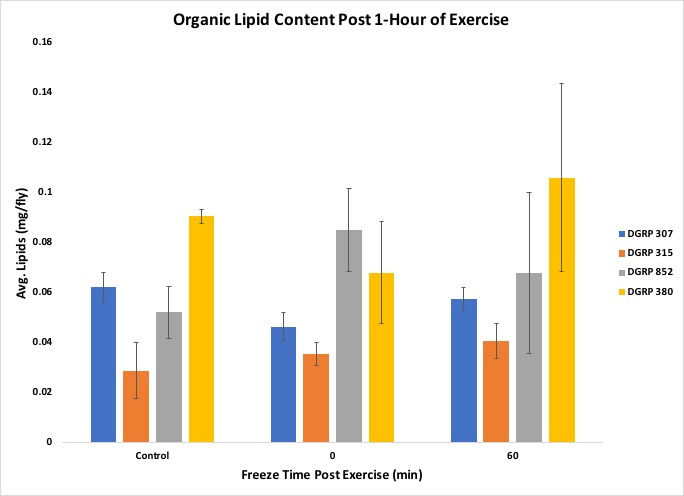
Figure 9. Results from the experiment showing Organic Lipid content
The students were also able to conclude that the correlation between triglyceride concentration and organic lipid content was line-specific, as shown by the DGRP line 307. Figures 5 through 9 include standard error to indicate the amount of variation across the samples. The suggested data layout is set up to allow calculation of group means and either standard deviation or standard error. Data analysis can easily be expanded to include chi-square tests or Analysis of Variance (ANOVA) and Tukey's HSD (honestly significant difference) post-hoc tests for multiple group comparisons. An ANOVA on our data indicated no statistically significant differences across the dataset, but, given past work with these lines, this result likely reflects the relatively small sample sizes that we used in this experiment. This result can, then, be used as a starting point for continuing the discussion on experimental reproducibility, statistical significance, and sample size.
In our exercise, students were instructed to graph their results to determine if there were any visible trends, as well as to try to gain insight into why responses occurred. Instructors should prime students with ideas on how exercise could affect different enzymatic activities and metabolite content. For example, we guided students with questions that could possibly be covered in their Discussion, such as a possible correlation between total lipid content and triglyceride (soluble, or mobilized lipid) content, and how this relationship may change with exercise and recovery time between exercise and freezing. The students were able to show with their graphs that the exercise response varied with genetic background, as each line had different activities or concentrations varying with time. The students had predicted that triglycerides would decrease with exercise but found that it only effected one low motivational line DGRP 307. Students can also create graphs that demonstrate the relationship between total lipid content and triglyceride content, and how this relationship may vary depending on the fly line, whether the flies exercised or not, and how long post exercise the flies were frozen. Students were also instructed to write a Discussion for their experiment. The post experiment assignment achieves the lesson goals of post-experimental analysis of raw data to gain insight into how exercise affects the physiology of flies, as well as that of students interpreting their raw data and presenting it in graphs.
Instructors may explore changes to their experimental procedure to fit their own expertise and resources. If instructors do not have access to a fly exercise machine (or the inclination to build one), they can use simple tap-down assays instead, in which instructors set a timer and have students tap down their vials every 10 seconds to induce exercise. This approach is more student labor-intensive, but should lead to similar results to the treadmill (22). Although we specifically chose fly lines (DGRP 852, 380, 315,307) that are known to have had physiological responses to exercise, instructors may elect to choose any fly line based off their availability (fly lines are available through biological supply companies). Students can choose beforehand what exercise regime they would like to expose the flies to (i.e. one continuous hour, or 15 minutes of exercise with 5 minutes of rest for one hour), and the amount of recovery time before freezing (i.e. immediately, 15-minutes, one hour). Instructors may elect to perform different enzymatic and endpoint assays depending on their budget and what reagents they have access to. This experiment is meant to be adapted to the type of questions about exercise the instructors and students want answered, as well as what budget the instructor wants to invest into the cost of the experiment.
SUPPORTING MATERIALS
- S1. Fly Exercise - Lab protocol. Includes additional information and instructions.
ACKNOWLEDGMENTS
We would like to acknowledge Kaitlyn Marshall-Bergeron, Nathan Limoko, Oluwadunsin Oguntade, Christian Blom, Jarod Milford, and Mitchell Slobodian, the students in CHMI3227 Experimental Methods in Biochemistry for their participation in the experiment.
References
- Krogh A. 1929. The progress of physiology. Am J Physiol Cell. 90(2), 243-251.
- Strange K. 2007. Revisiting the Krogh Principle in the post-genome era: Caenorhabditis elegans as a model system for integrative physiology research. J Exp Biol. 210: 1622-1631.
- Cutler DM, Glaeser EL, Shapiro JM, Cutler D. 2003. Why have Americans become more obese? J Econ Pers. 17(3): 93-118.
- Freeman PL, Maki JA, Thoemke KR, Lamm MH, Coffman CR. 2017. Evaluating the Quick Fix: Weight loss drugs and cellular respiration. CourseSource. https://doi.org/10.24918/cs.2017.17
- Karoly HC, Stevens CJ, Magnan RE, Harlaar N, Hutchison KE, Bryan AD. 2012. Genetic influences on physiological and subjective responses to an aerobic exercise session among sedentary adults. J Cancer Epidemiol. 2012: 540563. pmid:22899923.
- Bouchard C, Tremblay A, Nadeau A, Després JP, Thériault G, Boulay MR, Lortie G, Leblanc C, Fournier G. 1989. Genetic effect in resting and exercise metabolic rates. Metab Clin Exp. 38: 364-370.
- Reed LK, Baer CF, Edison AS. 2017. Considerations when choosing a genetic model organism for metabolomic studies. Curr Opin Chem Biol. 36, 7-14.
- Mohr SE. 2018. First in fly: Drosophila research and biological discovery. Harv. Uni. Press, Cambridge MA and London UK.
- Katz PS. 2016. 'Model organisms' in the light of evolution. Curr Biol. 26(14), R649-R650.
- Ankeny RA, Leonelli S. 2011. What's so special about model organisms? Stud Hist Philos Sci Part A. 42(2), 313-323.
- Leonelli S, Ankeny RA. 2013. What makes a model organism? Endeavour. 37(4), 209-212.
- Castelli-Gair J. 1998. The lines gene of Drosophila is required for specific functions of the Abdominal-B HOX protein. Development. 125(7), 1269-1274.
- Ormond KE, Mortlock DP, Scholes DT, Bombard Y, Brody LC, Faucett WA, Nanibaa'A G, Hercher L, Isasi R, Middleton A, Musunuru K. 2017. Human germline genome editing. Am J Hum Genet. 101(2), 167-176.
- Baumans V. 2004. Use of animals in experimental research: an ethical dilemma? Gene Ther. 11(S1), S64.
- Festing S, Wilkinson R. 2007. The ethics of animal research. EMBO Rep. 8(6), 526-530.
- Russell WMS, Burch RL, Hume CW. 1959. The principles of humane experimental technique. (Vol. 238) London: Methuen.
- Tannenbaum J, Bennett BT. 2015. Russell and Burch's 3Rs then and now: the need for clarity in definition and purpose. J Am Assoc Lab Anim. Sci. 54(2), 120-132.
- Pandey UB, Nichols CD. 2011. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharm Revs. 63(2): 411-436.
- Jennings BH. 2011. Drosophila: A versatile model in biology & medicine. Mater Today. 14(5): 190-195.
- Gargano JW, Martin I, Bhandari P, Grotewiel MS. 2005. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp Gero. 40: 386-395.
- Mendez S, Watanabe L, Hill R, Owens M, Moraczewski J, Rowe GC, Riddle NC, Reed LK. 2016. The TreadWheel: A novel apparatus to measure genetic variation in response to gently induced exercise for Drosophila. PLoS ONE: 11(10): e0164706. https://doi.org/10.1371/journal.pone.0164706
- Piazza N, Gosangi B, Devilla S, Arking R, Wessells R. 2009. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS ONE: 4(6): e5886. https://doi.org/10.1371/journal.pone.0005886
- Berlandi J, Lin F, Ambree O, Rieger D, Paulus W, Jeibmann A. 2017. Swing boat: Inducing and recording locomotor activity in a Drosophila melanogaster model of Alzheimer's disease. Front Behav Neurosci. 11: 159. doi: 10.3389/fnbeh.2017.00159.
- Rzezniczak TZ, Merritt TJ. 2012. Interactions of NADP-reducing enzymes across varying environmental conditions: a model of biological complexity. G3: Genes Genom Genet. 2(12), 1613-1623.
- Pollak N, Dolle C, Ziegler M. 2007. The power to reduce: pyridine nucleotides-small molecules with a multitude of functions. Biochem J. 402(2), 205-218.
- Ying W. 2008. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Sign. 10(2), 179-206.
- Agledal L, Niere M, Zieglar M. 2010. The phosphate makes a difference: cellular functions of NADP. Redox Rep. 15(1), 2-10.
- Morris SNS, Coogan C, Chamseddin K, Fernandez-Kim SO, Kolli S, Keller JN, Bauer JH. 2012. Development of diet-induced insulin resistance in adult Drosophila melanogaster. BBA- Mol Basis Dis. 1822(8), 1230-1237.
- Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. 2008. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 7(4), 478-490.
- Mackay TF, Richards S, Stone EA, Barbadilla A, Ayroles JF, Zhu D, Casillas S, Han Y, Magwire MM, Cridland JM, Richardson MF. 2012. The Drosophila melanogaster genetic reference panel. Nature. 482(7384), 173.
- Jumbo-Lucioni P, Bu S, Harbison ST, Slaughter JC, Mackay TF, Moellering DR, De Luca M. 2012. Nuclear genomic control of naturally occurring variation in mitochondrial function in Drosophila melanogaster. BMC Genom. 13(1), 659.
- Camus MF, Fowler K, Piper MW, Reuter M. 2017. Sex and genotype effects on nutrient-dependent fitness landscapes in Drosophila melanogaster. P Roy Soc B-Biol Sci. 284(1869), 20172237.
- Gabriel BM, Zierath JR. 2017. The limits of exercise physiology: from performance to health. Cell Metab. 25(5), 1000-1011.
- Egan B, Zierath JR. 2013. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 17(2), 162-184.
- Tanaka M, Wang G, Pitsiladis YP. 2016. Advancing sports and exercise genomics: moving from hypothesis-driven single study approaches to large multi-omics collaborative science. Physiol Genomics. 48, 173-174.
- Treagust DF, Duit R. 2008. Conceptual change: A discussion of theoretical, methodological and practical challenges for science education. Cul Stu Sci Edu. 3: 297-328. doi:/10.1007/s11422-008-9090-4.
- Felder RM, Brent R. 2005. Understanding student differences. J Eng Edu. 94(1), 57-72.
Article Files
Login to access supporting documents
Fly Exercise: A Simple Experiment to Test the Physiological Effects of Exercise on a Model Organism(PDF | 631 KB)
S1 Fly Exercise -Lab Protocol.docx(DOCX | 170 KB)
- License terms

Comments
Comments
There are no comments on this resource.