Design Your Own Flow Cytometry Experiment: A Four-Week Inquiry Laboratory
Editor: Shoshana D. Katzman
Published online:
Abstract
Due to its importance to the field, flow cytometry should be one of the hallmark methods a student learns in the lab portion of an undergraduate immunology course. Flow cytometry is a classic lab technique utilized within immunology and molecular biology research, as well as clinical laboratories to isolate cell populations from harvested tissue stained with a combination of antibodies to cell-specific markers. Despite its importance, instructions on how to complete lab exercises involving flow cytometry largely remain in primary literature articles without the level of detail necessary to teach the lab with ease, especially for those instructors without prior advanced flow cytometry knowledge. In this regard, this Lesson aims to provide clear, easy to follow instructions for how to successfully bring flow cytometry experiments to an undergraduate immunology, cell, or molecular biology teaching lab. In this Lesson, I describe a four-week lab module I teach in my upper-level undergraduate immunology course each fall. Students design and conduct their own experiment to address an immunological question of their choice using flow cytometry. Through this experience, they develop a greater understanding of the cellular makeup of the immune tissues they stain and analyze as the students work to answer a testable hypothesis and communicate their findings by writing a lab report and giving an oral presentation. Overall, this lab allows students to increase their knowledge of flow cytometry and what is necessary to conduct a realistic research experience to answer biological questions within immunology.
Citation
Dolence JJ. 2021. Design your own flow cytometry experiment: A four-week inquiry laboratory. CourseSource. https://doi.org/10.24918/cs.2021.19
Lesson Learning Goals
- Increase students’ knowledge of flow cytometry.
- Obtain a greater understanding of the cellular makeup of tissues important to immune responses.
- Develop and enhance practical skills important to work in a biological laboratory.
- Develop and expand scientific reasoning by:
- Asking a question and forming a testable hypothesis based on predicted outcomes.
- Designing scientific experiments that address the question/hypothesis.
- Critically analyzing and interpreting data and examining how data fits into prior research findings.
- Communicating scientific knowledge, findings, and processes.
- Obtain a greater appreciation of the complex and often enigmatic nature of scientific research.
Lesson Learning Objectives
Students will be able to:
- plan and conduct a flow cytometry experiment.
- develop an ability to work with a valuable model vertebrate within biological research.
- use appropriate lab techniques to isolate the following tissues: spleen, bone marrow, and thymus.
- generate single cell suspensions from isolated tissue in order to count cells using a hemocytometer.
- plate and stain specific numbers of cells with fluorescently-labeled antibodies.
- prepare stained cells to be analyzed by a flow cytometer.
- perform analysis of experimental data with flow cytometric analysis software (e.g., FlowJo, FCS Express).
- perform a literature search.
- communicate findings to an audience in both oral and written formats.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
Introduction
This four-week lab exercise is designed to introduce undergraduate students to the process of how real-world experimental design addresses questions within the field of immunology using flow cytometry. Students conduct a flow cytometry experiment based upon their own design, interpret their data, and present it to the class. This inquiry-driven research experience provides an important avenue for students to understand the complex nature of authentic science during their undergraduate education (1-4). Moreover, by using flow cytometry, a very common and powerful technique within immunology and other biomedical fields (5, 6), students learn what it is like to work in a research or clinical immunology lab. Students taking immunology at University of Nebraska at Kearney are interested in pre-health careers, biomedical science research, and clinical lab science. As the relationship between immunology and medicine grows ever closer (7), this experience not only helps inform career decisions, but importantly, gives students a glimpse into how flow cytometry can be used in a powerful way to give scientists and physicians the data necessary to design better treatments and improve patient outcomes. Providing students hands-on, inquiry-driven investigative experiences is important to include in curriculum centered around increasing critical thinking and problem solving designed to prepare students to enter into future careers and better understand our complex world (8).
I describe a laboratory activity that allows students (usually in groups of two) to integrate the immunological knowledge they have acquired in the lecture portion of the course with their own interests to allow them to design an experiment to address an immunological question they conceive. In my experience, I have found the most optimal projects to be ones that answer questions relating to the composition of the immune tissues (e.g., frequency of different T cells vs. B cells in bone marrow, spleen, and thymus; frequency of dendritic cells or macrophages in these tissues). Students are expected to independently conduct their designed experiment, analyze the data, and communicate their findings. The only part of the flow cytometry experiment the students do not participate in is collection of data on the flow cytometer. However, I demonstrate how to set up the machine as well as run a few samples with the students so they can visualize this process before running the rest of student samples because flow cytometer repair is expensive and the length of time to run samples surpasses normal lab time.
Student engagement in this activity is robust because they are allowed to explore a part of the immune system in which they are interested. This activity allows students a chance to see how data they see in papers and textbooks are generated. Since they design the experiment, the ownership of the projects gives the students a great sense of pride and I find this lab exercise to result in many positive outcomes. Here, I describe a laboratory activity using harvested immune tissue from mice to conduct a student-designed flow cytometry experiment that runs the whole gamut of scientific method from conception to communication of findings.
Introduction into Flow Cytometry
Flow cytometry is a hallmark technique within the field of immunology (6) and should therefore be a fundamental component of the lab portion of an undergraduate immunology course. It is also applicable to cell and molecular biology classes. Flow cytometry is a technique used to examine specific cell populations found within complex biological tissues (e.g., bone marrow, spleen, thymus, blood). Harvested tissues are processed into single cell suspensions and stained with a combination of fluorescently-labeled antibodies to cell-specific markers. Once stained, cells are processed through a flow cytometer, which allows for the elucidation of unique cell populations found within the sample. As the cells are run through the flow cytometer, they are individually exposed to lasers that cause bound fluorescent molecules (present due to antibody binding) to become excited and emit a signal that is read by the instrument. Each bound antibody will generate a signal as it passes through the machine, and collectively the signals emanating from the fluorescently-labeled cells are processed by the machine to allow the researcher to obtain a picture of the cellular composition of the tissue being analyzed (5, 6, 9). In addition to staining cell surface markers to elucidate specific cell populations within tissue, flow cytometry can also be used to detect intracellular events such as the phosphorylation of proteins (phosphoflow), calcium flux, and cytokine secretion (10-12). While this Lesson will focus on the aspect of flow cytometry that allows for the determination of different cell populations within organs using cell surface staining, flow cytometry has developed into a powerful technique because it allows for the measurement of rare populations where traditional biochemical analysis is extremely difficult or impossible.
Despite the importance of flow cytometry, instructions on how to complete lab exercises involving this technique largely remain in primary literature articles without the level of detail necessary to teach the lab with ease, especially for those instructors without prior advanced flow cytometry knowledge. In this regard, this Lesson aims to provide clear, easy to follow instructions for how to successfully bring flow cytometry experiments to an undergraduate immunology, cell, or molecular biology teaching lab. Moreover, this Lesson goes beyond simply learning flow cytometry, as students are tasked with generating a question that can be answered using flow cytometry. In this four-week long Lesson, students work in pairs to learn about flow cytometry by designing (Week 1) and conducting their own experiments (Week 2), analyzing their data (Week 3), and communicating their findings in a written lab report and oral presentation (Week 4) (Figure 1).
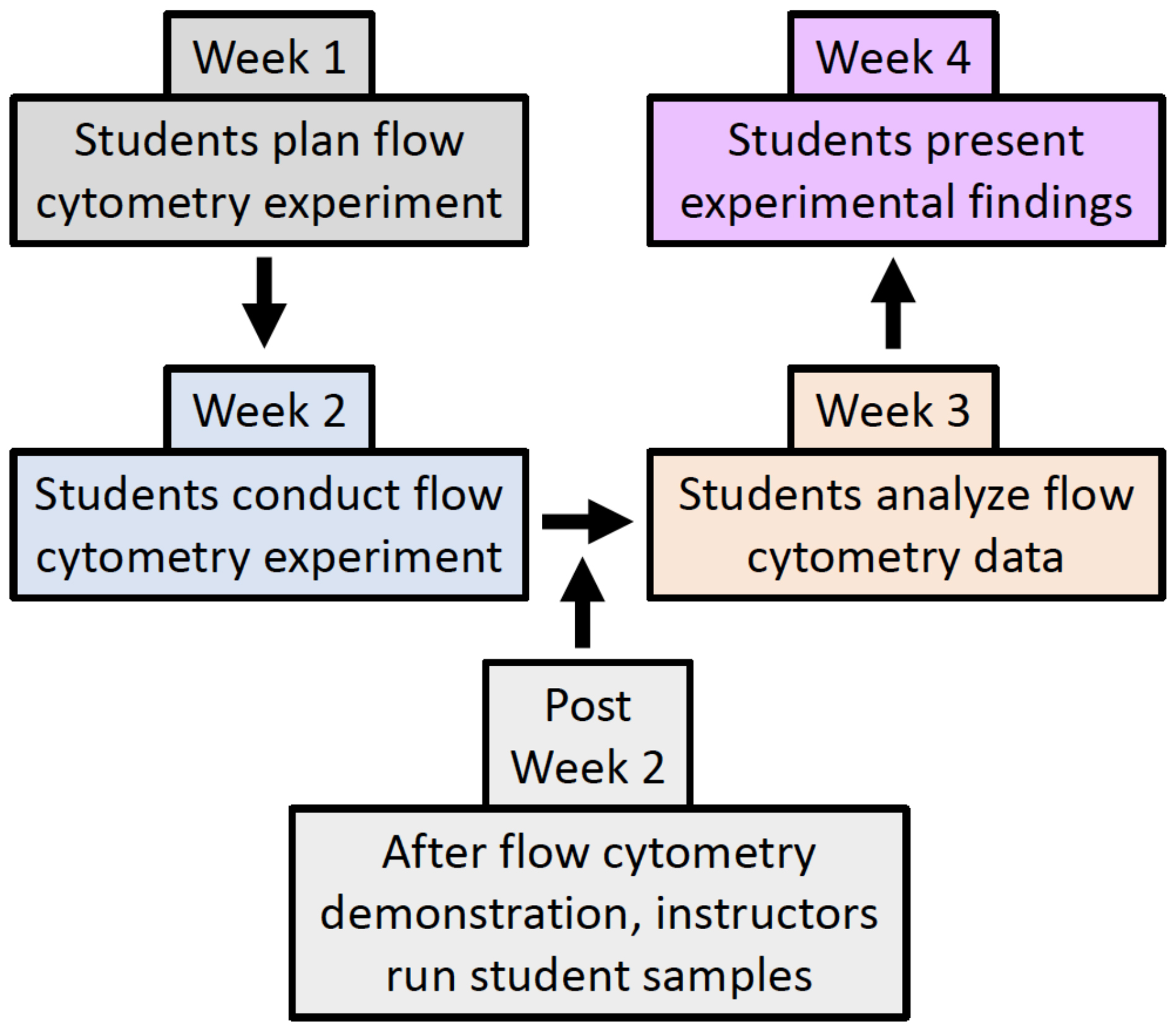
Previous publications have described the use of flow cytometry in undergraduate labs using either non-immune cells (Tetrahymena) or a half (8-week) to a full semester of activities (13-15). The inquiry-based lab activity described in this Lesson is unique in that it fits into a 4-week section of the curriculum of an immunology (or cell/molecular biology) laboratory in a class that also has a lecture component. It is designed to allow those with basic flow cytometry knowledge to guide students to conduct a discovery-based learning exercise using mouse tissue that will prove to be a meaningful part of their immunology experience.
Intended Audience
Participants in this Lesson were upper-level undergraduate students majoring in biology (general or health science emphasis), molecular biology, or medical laboratory science, as well as graduate students enrolled in the Master of Science in Biology program. This Lesson was taught in the last one-third of the semester in the laboratory component of a 400-level immunology course at a primarily undergraduate, public four-year institution. Students were required to enroll in both lecture and laboratory in the same semester. The number of students in each course laboratory section varied between 10 and 16 students, with a planned maximum capacity of 20 students. One section of lab is taught each semester the course is offered (it is offered every fall here at the University of Nebraska at Kearney).
Required Learning Time
The learning activities described in this Lesson are accomplished in 4-5 weeks of the course, depending how long the instructor gives the students between Week 3 - Data Analysis and Week 4 - Communicating Results. The laboratory class period is 2 hours 50 minutes in length, one day a week. Preparing for lab, data analysis, as well as preparing written lab reports and oral presentations require student out-of-class time. This Lesson was first taught during Fall of 2018 and to date has been run in three semesters, with one lab section each semester. A more detailed timeline is provided (Table 1).
Table 1. Design your own flow cytometry experiment lesson plan timeline. Weeks 1 through 4 are included.
|
Activity |
Description |
Estimated Time |
Notes |
|
Pre-Week 1 Preparation |
|||
|
Learn about flow cytometry (if necessary – instructor, necessary – students) |
|
1 hour |
|
|
Review cellular composition of immune tissues (if necessary – instructor, necessary – students) |
Evaluates the immune tissues (e.g., BM, spleen, thymus) students will harvest and decides which cells within these tissues to guide students towards staining to obtain the most optimal data to answer the question they develop during Week 1. |
30 minutes-1 hour |
During the lecture component of the course ahead of Week 1, instructors should review the cellular composition of immune tissues (typically, this is discussed early in an immunology course) to provide an overview of the different types of immune cells found in the different immune organs that the students will be harvesting in this experiment. Prior to Week 1, students should review this information to prepare for lab. |
|
Mini-lecture (instructor) |
Describe overall goals of design your own flow cytometry experiment lab. |
15-30 minutes in class |
Optimally, give this lecture during lecture component of course ahead of Week 1 to prepare students for the lab. Alternatively, start Week 1 with this lecture (after the quiz). |
|
Experimental Design (Week 1) |
|||
|
Quiz |
Give students quiz about the basics of flow cytometry. |
10-15 minutes |
Please see Supporting File S7. Flow Cytometry – Questions for Assessment of Student Learning for Week 1 quiz. |
|
Dry Lab |
Using knowledge students have acquired in the course, design flow cytometry experiments to answer immunological question of their choice. |
1-2 hours |
Depending on the expertise of the instructor and the particular students within lab groups, these experiments could take on various forms, but I have found ideal projects to ones that answer straightforward questions relating to the cellular composition of immune tissues (e.g., frequency of different T cells vs. B cells in BM, spleen, and thymus). These might seem like simple questions, but these experiments allow students to understand how information they commonly read about in their textbook or papers was discovered. Instructors are encouraged to use Supporting File S1. Flow Cytometry – Design Your Own Flow Cytometry Experiments Lab to guide Week 1 activities. |
|
Order materials |
Instructor reviews student-designed experiments and orders materials (e.g., flow cytometry antibodies, necessary buffers beyond standard buffer). |
30 min-1 hour |
I have found the optimal strategy is to have the students generate a list of the flow cytometry reagents they need me to order. I examine this list alongside the proposed experimental design with the students. This approach allows the instructor and the students to agree upon an experimental approach prior to Week 2. |
|
Flow Cytometry Experiment (Week 2) |
|||
|
Laboratory preparation (instructor AND students) |
|
1 hour |
Please see Supporting File S2. Flow Cytometry – Supplies and equipment necessary for flow cytometry experiments to assist in laboratory set up. Please see Supporting File S3. Flow Cytometry – Flow Cytometry Experiment Lab for detailed instructions on conducting the lab. To prepare for Week 2, I would encourage instructors to have the students edit this file to describe exactly what they plan to do. The more preparation the students do ahead of time, the smoother the lab will run. |
|
Quiz |
Give students quiz about flow cytometry experimental procedures. |
10 minutes |
Please see Supporting File S7. Flow Cytometry – Questions for assessment of student learning for Week 2 quiz. |
|
Mini-lecture |
Review experimental procedures prior to start of student work. |
15 minutes |
Please see Supporting File S3. Flow Cytometry – Flow Cytometry Experiment Lab for detailed instructions on conducting the lab. |
|
Laboratory Activities |
Conduct flow cytometry lab:
|
3.5 hours |
Please see Supporting File S3. Flow Cytometry – Flow Cytometry Experiment Lab for detailed instructions on conducting the lab. |
|
Post-laboratory activities |
|
2.5-4 hours, but it’s variable, please see notes. |
Time it takes to run samples depends on how many samples need to be processed as well the logistics of running the samples on your flow cytometer. Typically, it takes 30-45 minutes for me to run the samples from an individual student group. |
|
Data Analysis (Week 3) |
|||
|
Laboratory preparation (instructor AND students) |
|
Instructor: 1-2 hours Students: 15 minutes |
|
|
Dry Lab |
Students analyze Week 2 FCS files using FlowJo or other flow cytometry analysis software. |
1-2 hours |
|
|
Mini-lecture (instructor) |
Describe goals and expectations of written and oral assignments. |
15 min |
Please see Supporting file S8. Flow Cytometry – Lab Report Written Assignment, Supporting file S9. Flow Cytometry – Lab Report Written Assignment Grading Score Sheet, and Supporting file S10. Flow Cytometry – Guide to Lab Oral Presentations. |
|
Communicating Results (Week 4). For best results, give students a week between Weeks 3 and 4 to compile their findings. |
|||
|
Laboratory preparation (students) |
Each student group writes a lab report and prepares an oral presentation prior to Week 4 meeting. |
Variable |
|
|
Laboratory Activities |
Student groups present oral presentations and hand in written lab reports. |
1-2 hours, but it’s variable, please see notes. |
15 minutes is the recommended length for each student lab group presentation. Therefore, it takes roughly 1 hour for four presentations. Scale time expectations according to number of presentations. |
Prerequisite Student Knowledge
Prerequisites for the course for which this Lesson was designed include either completion or co-enrollment in Cell Biology or Biochemistry, including laboratories. This Lesson is taught in the last one-third of the course, so before the start of the laboratory activities described here, students have received and been assessed for the following content knowledge in the lecture component of the course: structural and functional aspects of the immune system; structure and function of antigen receptors (T-cell receptors [TCR] and B-cell receptors [BCR]) and antibody molecules; antigen-antibody interaction; antigen recognition and response; and development of T and B cells. Before conducting the described activities, students have learned how to handle mice, harvest and process murine tissue, cell counting, and flow cytometry in previous laboratory sessions of the course. Placing the Lesson towards the end of the course allows the students to have the content knowledge to design more thoughtful experiments and allows them to carry out these activities in a more independent fashion.
Prerequisite Teacher Knowledge
Instructors should have knowledge of basic immunology, as well as the skills necessary to carry out procedures in a typical immunology or molecular biology laboratory. Multiple supporting materials have been provided to assist in preparation for this lab activity. Supporting file S1. Flow Cytometry – Design Your Own Flow Cytometry Experiments Lab provides a guide for Week 1 activities. Supporting file S2. Flow Cytometry – Supplies and Equipment Necessary for Flow Cytometry Experiments is a list that can be used to set up your lab ahead of Week 2. Supporting file S3. Flow Cytometry – Flow Cytometry Experiment Lab provides details on how to isolate and process spleen, bone marrow, and thymus tissue. Further information on how to conduct these procedures is readily available (16-20). Knowing how to design and conduct flow cytometry experiments, as well as understanding how to analyze flow cytometry data is required and can be obtained via Supporting file S4. Flow Cytometry – Flow Cytometry Analysis Lab, as well as through immunology textbooks, literature, online videos, and other resources (Table 2 and (5, 6, 9, 21, 22)). Useful information on common antigens for students to pursue, along with where to purchase antibodies to detect these antigens including estimated costs can be found in Supporting file S2. Flow Cytometry – Supplies and Equipment Necessary for Flow Cytometry Experiments. Additionally, a basic understanding for how to compensate flow cytometry samples is required to demonstrate how to set up the flow cytometer and also to assist students with data analysis, depending on the quality of their staining. The process to compensate your samples, also known as compensation, using single color compensation samples is the process by which you correct for fluorescence spectral overlap by telling the flow cytometer what is positive for a particular antigen and what is negative for that same antigen. Nowadays, many flow cytometers have what is known as automatic compensation. Since establishing automatic compensation differs on each flow cytometer, instructors should be familiar with how to set it up on the machine they will be using ahead of the flow cytometer set-up demonstration to end Week 2. During the demonstration, instructors are encouraged to explain the basics of correcting for spectral overlap and why this is necessary in a flow cytometry experiment. Furthermore, FlowJo and other flow cytometry analysis softwares allow you to perform post-acquisition compensation (given the stains most students choose to complete, this is most likely unnecessary). If this becomes necessary to analyze student data and you are unfamiliar with this compensation option, I would recommend searching the website of the flow cytometry analysis software you are using for more information and guide the students accordingly. To assist you in learning how to analyze flow cytometry data, I have provided student example FCS files (Supporting file S5. Flow Cytometry – FCS files and Supporting file S6. Flow Cytometry – Catalog of example FCS files).
Table 2. Useful Resources to Learn Flow Cytometry.
|
Name |
Description |
|
Thermo Fisher Scientific videos: |
Thermo Fisher Scientific does a great job in these two videos explaining the basics of flow cytometry in the first video and going further in breaking down data collection, processing, and compensation in the second video. |
|
Biolegend Resources: |
|
|
Abcam provides an easy to understand introduction to flow cytometry as well as other resources and links on the website to help you learn about flow cytometry. |
|
|
FlowJo Basic Tutorial (click download link under FlowJo Basic Tutorial). |
Beyond what is written in Supporting File S4. Flow Cytometry – Flow Cytometry Analysis Lab, FlowJo’s Basic Tutorial lays out in six lessons how to use FlowJo using a detailed approach. The tutorial uses pictures from FlowJo which make it straightforward to follow. |
|
Scientific literature:
|
|
Scientific Teaching Themes
Active Learning
This exercise provides students with quintessential active learning: an ability to do undergraduate research within a structured laboratory environment. Furthermore, written lab reports and oral presentations by student pairs help strengthen written and oral communication skills.
Assessment
Quizzes and Exams
At the beginning of Week 1 and Week 2, a quiz is used to test each student’s understanding of flow cytometry (Week 1) and experimental procedures (Week 2). On exams following completion of these laboratory activities, students are asked questions in which they must outline a flow cytometry experiment and analyze flow cytometry data. The quiz and exam questions, along with how I use them in this Lesson are provided (Supporting file S7. Flow Cytometry – Questions for assessment of student learning).
Written Lab Reports and Oral Presentations
Each student pair must write a lab report and present a 15-minute oral presentation over the experiment they conducted, presenting their work as a study. The reports are modeled after a primary research article with an abstract, introduction, materials and methods, results, discussion, and reference section. The lab report instructions (Supporting file S8. Flow Cytometry – Lab Report Written Assignment) and score sheet (Supporting file S9. Flow Cytometry – Lab Report Written Assignment Grading Score Sheet) are provided to students before the start of the lab activities described in this Lesson. The score sheet provides a concise way to provide feedback from instructor to student pairs. The oral presentation guide (Supporting file S10. Flow Cytometry – Guide to Lab Oral Presentations) describes how to assemble the slideshow, giving details at a slide-by-slide level. This guide also provides expectations and the score sheet used to provide feedback on their presentation.
These two assignments (report and presentation) provide students with an opportunity to communicate their understanding of the background necessary to address the question they asked, the laboratory procedure they followed in their experimental design, the results of their study, and finally, an ability to put the data into proper context. Giving students the ability to communicate by these means is not only critical to create authentic research experiences, but it allows them to obtain greater levels of confidence in their ability to write and speak scientifically. Indeed, students have commented on how the two end of lab assignments helped them to better understand how real research is communicated.
Inclusive Teaching
This Lesson provides students with an inquiry-driven activity, and as designed, each student pair is presented with an opportunity to design an experiment to test a different question. At the end of the four-week lab exercise, each student pair shares their data in the form of an oral presentation, making each group’s contribution of equal importance. Using this format overcomes significant barriers and gives students an ability to see themselves and their peers as scientists (23). Each student pair is required to adjust the given protocol to carry out the experiment they design, troubleshooting any problems they encounter as if they were working in a research laboratory. This approach encourages the less-vocal students to actively participate in figuring out how to successfully complete the laboratory exercises (24). Students help each other, which leads to minimal instructor intervention. For both the written lab report and in-class oral presentation, students continue to work with their lab partner, which functions to reduce writing and presentation anxiety. An additional element that enhances lab performance is how the students pair up with each other. At the beginning of the semester, students are free to choose their partner. Since this lab activity is towards the end of the semester, the student pairs have worked together in many lab exercises and have developed an excellent rapport with each other. Furthermore, every student has access to the same time and resources throughout the activity ensuring that no students have a preparation advantage. To increase the inclusivity of this Lesson, instructors who do not have the resources at their institution or training to complete this full Lesson are encouraged to read the Potential Modifications section below to see how the Lesson can be adjusted to fit their circumstance.
Lesson Plan
This lesson is designed for four consecutive 3-hour lab periods with a week off between Weeks 3 and 4 (Figure 1 and see Table 1 for the four-week timeline to visualize how to progress through the lesson). The purpose of this lesson is to have students design and conduct their own experiment to address an immunological question of their choice using flow cytometry to examine the murine immune system. Following completion of lab activities, the students will communicate their findings in two formats: written lab reports and oral presentations.
Pre-Class Preparation
Instructors are encouraged to familiarize themselves with flow cytometry and the capabilities of the flow cytometer at their institution. Instructors are encouraged to introduce the students to flow cytometry either in lecture or assign materials for students to learn about flow cytometry during out-of-class time. Background information about flow cytometry including websites and optional videos are listed in Table 2. To motivate students to learn flow cytometry in preparation for Week 1, a quiz is provided (Supporting file S7. Flow Cytometry - Questions for assessment of student learning). Before Week 1, instructors and students should also review the cellular composition of immune tissues, such as bone marrow, spleen, and thymus in order to best select answerable questions during the time allotted for experimental design. Immunology textbooks, as well as recent reviews, are a good place to acquire such information (21, 22, 25-28).
Week 1: Experimental Design
Students are asked to design a flow cytometry experiment to address a question relevant to immunology. Students work in pairs to conduct this activity. The model organism used is a mouse. The laboratory handout (Supporting file S1. Flow Cytometry – Design Your Own Flow Cytometry Experiments Lab) guides the students through the process of selecting a question to answer, along with the broader outline of this multi-week lab activity. Since flow cytometry allows you to interrogate cell populations using antibodies to antigens found on the surface of cells, I have found ideal projects to be ones that answer straightforward questions relating to the composition of the immune tissues (e.g., frequency of different T cells vs. B cells in bone marrow (BM), spleen, and thymus; frequency of dendritic cells or macrophages in these tissues). I encourage students to choose an experimental question that leads them to use a maximum of six colors for several reasons. One is logistical: six colors represents the capacity of our Sony flow cytometer. Second, less is often more: choosing fewer colors, even just two, allows the students to be confident in their investigation of a few cell types rather than trying to answer too many questions with more colors (the caveat being it depends on how many cell surface markers are necessary to examine the particular cell they are interested in). Third, keeping the analysis to fewer colors reduces compensation issues. Instructors who have access to flow cytometers capable of detecting a high number of colors (e.g., many BD systems, Attune NxT, Cytek’s Aurora system) will have to make their own determination about the maximum number of colors students should use to conduct their experiment. Once the students have chosen a project, they need to provide the instructor a list of reagents (e.g., fluorescently-labeled flow cytometry antibodies) to obtain for them to complete the project. Alternatively, instructors could provide students a list of flow cytometry antibodies and have the students use this list to design their projects (example lists are provided in Supporting file S2. Flow Cytometry – Supplies and Equipment Necessary for Flow Cytometry Experiments).
Week 2: Experiment
The student pairs are asked to carry out the flow cytometry project they planned during Week 1. Instructors can use the supply list (Supporting file S2. Flow Cytometry – Supplies and Equipment Necessary for Flow Cytometry Experiments) to assist in preparation for this week’s lab. The laboratory handout (Supporting file S3. Flow Cytometry – Flow Cytometry Experiment Lab) provides the students with detailed instructions for how to harvest spleen, BM, and thymus tissue, generate single cell suspensions, count, plate, and stain cells from these tissues to conduct their flow cytometry experiment. To insure students understand the Week 2 experimental procedures, a quiz should be assigned at the beginning of lab (Supporting file S7. Flow Cytometry – Questions for assessment of student learning). Once sample processing is complete, the students transfer the stained cells to tubes appropriate to run on your flow cytometer (e.g., 5 mL polystyrene or polypropylene tubes, 1.5 mL microcentrifuge tubes) covered with aluminum foil to protect from light, and placed in a 4°C refrigerator until samples are run on the flow cytometer by the instructor. In the protocol I provide (Supporting file S3. Flow Cytometry – Flow Cytometry Experiment Lab), cells are alive, not fixed, and therefore need to be analyzed on the flow cytometer the same day. If same day analysis is not possible, students can fix their samples with 325 µL 4% paraformaldehyde during the final resuspension step. Once fixed, covered, and protected from light in a 4°C refrigerator, cells can be safely analyzed up to a few days later without losing fluorescent signal.
Week 3: Data Analysis
Students are asked to analyze and interpret the data collected from the flow cytometry experiment they completed during Week 2. The laboratory handout (Supporting file S4. Flow Cytometry – Flow Cytometry Analysis Lab) provides students with detailed instructions for how to download their flow cytometry files (FCS files) as well as FlowJo analysis software. FlowJo offers a complementary 30-day trial of their software and this allows each student to obtain experience using the software. Supporting file S4. Flow Cytometry – Flow Cytometry Analysis Lab also provides a step-by-step guide for how to gate and analyze their stains using FlowJo, with examples to guide them. Instructors should inform their students about the location of FCS files so the students know where to download their data files. Instructors should also familiarize themselves with how to gate and analyze flow cytometry data before Week 3. To assist, two sets of student example FCS files have been provided (Supporting file S5. Flow Cytometry – FCS files; Supporting file S6. Flow Cytometry – Catalog of example FCS files). Figure 2 depicts the analysis of a four-color B and T cell stain conducted by students on splenic and thymic tissue. While I use FlowJo, other analysis software such as FCS Express will work. Regardless of the software package used, the basics of gating and analysis are very similar and instructors should review how the flow cytometric data analysis software they plan to use works in order to best guide their students.

Week 4: Communicating Results via Written Lab Reports and Oral Presentations
Following Week 3’s data analysis lab, students are asked to communicate their experimental findings by completing two assignments: a written lab report and an oral presentation. The laboratory handout (Supporting file S8. Flow Cytometry – Lab Report Written Assignment) gives clear and concise instructions for how each student pair should write their lab report and the score sheet (Supporting file S9. Flow Cytometry – Lab Report Written Assignment Grading Score Sheet) informs them on how it will be graded. For the oral presentation, the students are given a guide to walk them through the process of putting together and presenting their data in an oral format with slides (Supporting file S10. Flow Cytometry – Guide to Lab Oral Presentations). The guide explains at a slide-by-slide level how to assemble the slideshow, as well as laying out the expectations and the grading score sheet is used to provide assessment and feedback for their presentation. To give the students time to interpret their findings and complete these two final activities, I give the students two weeks between Weeks 3 and 4. Since I do this lab module towards the end of fall semester, Thanksgiving week serves as a natural break in the schedule to allow this to occur. On the final lab meeting of the semester, student pairs hand in their lab reports and give their oral presentations.
Teaching Discussion
I describe a four-week laboratory module that provides undergraduate immunology students with an opportunity to learn about flow cytometry through designing an experiment, the hands-on experience of conducting the experiment, analyzing and interpreting their own data, and finally, communicating their findings in both written and oral formats. Furthermore, this inquiry-driven activity gives the students a more realistic view of the nature of scientific research, which at times can be complex and enigmatic (4). Such knowledge is incredibly valuable and often difficult to achieve in an undergraduate teaching laboratory (4, 8).
Tips for Student Success
As the students plan their experiments (Week 1), they can get stuck and be unsure of what to ask. At these times, the instructor can remind the students that the novelty of the question is not the goal of this lab, but understanding the process of designing an experiment to address a question, any question, even if the answer is known, is the purpose. The students gain incredible value by seeing data that illustrates a figure they have seen in a paper or the textbook. Therefore, in such cases, I try to point students in a direction to discover simple immunological principles such as frequency of naive vs. memory T cells in BM, spleen, or thymus or naive vs. recirculating B cells in BM or spleen. Every time I do this Lesson, I am reminded of how powerful it is for students to discover simple known truths with their own hands, instead of just reading about them.
During the experimental part of the Lesson (Week 2), students are commonly confused at the times in which they must do calculations: cell counting, plating the cells, and determining how much fluorescent antibody to add during the antibody staining step. It is important for the instructor to walk through these specific steps very deliberately to ensure students understand how to correctly accomplish these parts of the experiment. During these parts of the protocol, I continually move from group to group asking if the students have any questions and working with them to resolve any confusion.
In my course, students have previously used FlowJo to analyze flow cytometry data earlier in the course. While this experience is instructive, students still have questions surrounding the gating of cell populations. Instructors need to have a solid understanding of how to gate and interpret flow cytometry data. To assist, several supporting materials are available to the instructor. The lab handout describing Week 3 activities (Supporting file S4. Flow Cytometry – Flow Cytometry Analysis Lab) is helpful in guiding the gating and data analysis process. Moreover, I provided sample FCS files (Supporting file S5. Flow Cytometry – FCS files) cataloged to make it easier to follow (Supporting file S6. Flow Cytometry – Catalog of Example FCS files). This practice data provides an ideal way for the students (and if necessary, the instructor) to learn the intricacies of analyzing multi-color flow cytometry data. If this Lesson is the only flow cytometry you are doing in your immunology course, it is especially important to have the students download and analyze the sample FCS files provided to understand basic gating strategy and analysis before examining their data. With FlowJo (or other analysis software), the more experience the students get, the better the analysis and subsequent communication of the data during Week 4.
Overcoming Limitations
Flow cytometry is a fundamental technique within the field of immunology (6), and many colleges and universities have at least one instrument that can be used for this Lesson. This Lesson can be carried out with any flow cytometer. If your institution doesn’t have one, instructors are encouraged to find a nearby institution and request use of their flow cytometer (if you take this route, be sure to fix your samples with 4% paraformaldehyde as described above). For teaching purposes, many institutions will either waive or reduce fees they will charge to use their flow cytometer. The price of flow cytometry reagents, in particular antibodies, can be costly. If this is an issue, contact the company (I use BioLegend, as they are very helpful in this regard) and get in contact with a technical application scientist or a customer service representative and request a discount. Under most scenarios, they will honor this request and offer you steep discounts on antibodies and other reagents (in my experience, these can go up to 50% off list price). Once purchased, antibodies can be stored in a 4°C refrigerator for many years of use for teaching purposes. Other reagents for flow cytometry are relatively inexpensive and easy to prepare. Materials necessary to conduct this Lesson are listed in Supporting file S2. Flow Cytometry – Supplies and equipment necessary for flow cytometry experiments.
Maintaining mouse colonies is expensive, especially if you are only using mice for this Lesson. For this Lesson, I use excess mice from my breeding colony. If this is not an option, instructors are encouraged to ask colleagues at the institution who have a breeding colony if they could use spare mice to conduct this Lesson. Since each student pair only needs one mouse, you will not need to acquire many mice. If this is not possible, mice could be purchased from mouse vendors such as Jackson Laboratory, Taconic Biosciences, Charles River, or even from local pet stores, similar to published immunological studies (29, 30). Instructors must follow the regulations and guidance provided by their institution’s animal use and care committee (IACUC) in conducting all mouse experiments. For instance, our IACUC protocol allows students to perform euthanasia in a teaching lab setting (Supporting file S3. Flow Cytometry – Flow Cytometry Experiment Lab provides more information), but this will not be allowed at every institution and instructors need to be sure they are following the guidelines put forth by their local IACUC.
Student Perception of Lesson
Student feedback is very favorable. One student remarked, “When I first read we were doing this, I was wondering how it would work. It seemed like a lot of work, but after going through it, I learned a lot and I am very happy to see how the process of generating this kind of data is conducted.” Many student comments reflect an incredibly positive experience. They enjoyed the freedom to investigate their own questions and the ability to visualize how flow cytometry data is generated from start to finish. This point is important due to widespread prevalence of flow cytometry plots across the immunology literature. Students also remarked about how working in pairs cuts down on the anxiety of the four-week experience.
Potential Modifications
Instructors could expand the lab activity by 1-2 weeks to allow student pairs to react to their analyzed data and design a new experiment to further address their question. Making this extension would allow the Lesson to be even better research experience. Another way this Lesson could be adjusted is by allowing the students to compare wildtype mice to mutant mice the instructor has access to in their research lab or at their institution. This addition would allow students to take part in an even more authentic research experience as they examined whether the cell populations being investigated were impacted in the mutant mouse in question. Alternatively, while this lesson was originally designed as an investigative lab for immunology students, instructors could use the Week 2 and 3 activities as a more directed two-week lab exercise where specific cell markers chosen by the instructor are investigated in an immunology or cell/molecular biology class where students learn about flow cytometry. Instructors could choose to forgo mice and stain cell lines they are familiar with if these are more readily available at their institution. If the cell line route is chosen, I would suggest having the students stain two or three cell lines so they can compare surface marker expression across multiple cell lines. Additionally, instructors could guide the students in running their own samples. Instructors will need to decide on how they will conduct the processing samples part of the flow cytometry experiment. Operating flow cytometers can be challenging for novice users, repairs expensive, and running the samples will likely exceed scheduled lab time. For these reasons, I demonstrate how to set up the machine and then run a few samples to allow the students to get a feel for how flow cytometry data is collected (before running the rest of the samples after lab). For instructors that do not have access to a flow cytometer or do not have the resources (e.g., mice, cell lines, or money for reagents) to complete this Lesson, a “dry” lab using the FSC files provided (Supporting file S5. Flow Cytometry – FCS files and Supporting file S6. Flow Cytometry – Catalog of Example FCS files) could be conducted to give students a valuable experience in how flow cytometry data is analyzed. These students might not be able to do the full Lesson, but the experience of analyzing the flow cytometry data and generating a figure from the data is an important educational experience for an Immunology student. At the discretion of the instructor, this option could also be employed for any student who cannot participate in animal work for personal reasons.
Overall, this Lesson is incredibly effective at exposing students to the scientific process from start to finish. They generate and carry out their own experiments and communicate their data to the class. When the students present their work during Week 4, their excitement and pride in what they have accomplished is notable. The instructions I give them to be open about what worked and what could be improved for future experiments in both their lab reports and oral presentations allows them to develop a greater understanding of how scientific projects change through time.
SUPPORTING MATERIALS
Supporting file S1. Flow Cytometry – Design Your Own Flow Cytometry Experiments Lab
Supporting file S2. Flow Cytometry – Supplies and Equipment Necessary for Flow Cytometry Experiments
Supporting file S3. Flow Cytometry – Flow Cytometry Experiment Lab
Supporting file S4. Flow Cytometry – Flow Cytometry Analysis Lab
Supporting file S5. Flow Cytometry – FCS files
Supporting file S6. Flow Cytometry – Catalog of Example FCS Files
Supporting file S7. Flow Cytometry – Questions for Assessment of Student Learning
Supporting file S8. Flow Cytometry – Lab Report Written Assignment
Supporting file S9. Flow Cytometry – Lab Report Written Assignment Grading Score Sheet
Supporting file S10. Flow Cytometry – Guide to Lab Oral Presentations
Acknowledgments
I would like to thank the University of Nebraska at Kearney, Department of Biology for their unwavering support of my teaching. I would also like to thank the students who participated in this Lesson as it was being developed—you are the reason I do this each and every day. Finally, I am grateful to Dr. Kimberly Carlson and McKenna Vininski for their helpful advice in editing this manuscript.
References
- Luckie DB, Maleszewski JJ, Loznak SD, Krha M. 2004. Infusion of collaborative inquiry throughout a biology curriculum increases student learning: a four-year study of "Teams and Streams". Adv Physiol Educ 28:199-209. doi: 10.1152/advan.00025.2004
- Park Rogers MA, Abell SK. 2008. The design, enactment, and experience of inquiry-based instruction in undergraduate science education: A case study. Science Education 92:591-607. doi: 10.1002/sce.20247
- Spell RM, Guinan JA, Miller KR, Beck CW. 2014. Redefining authentic research experiences in introductory biology laboratories and barriers to their implementation. CBE Life Sci Educ 13:102-10. doi: 10.1187/cbe.13-08-0169
- Brownell SE, Kloser MJ. 2015. Toward a conceptual framework for measuring the effectiveness of course-based undergraduate research experiences in undergraduate biology. Studies in Higher Education 40:525-544. doi: 10.1080/03075079.2015.1004234
- McKinnon KM. 2018. Flow Cytometry: An Overview. Curr Protoc Immunol 120:5 1 1-5 1 11. doi: 10.1002/cpim.40
- Cossarizza A, Chang HD, Radbruch A, Akdis M, Andra I, Annunziato F, Bacher P, Barnaba V, Battistini L, Bauer WM, Baumgart S, Becher B, Beisker W, Berek C, Blanco A, Borsellino G, Boulais PE, Brinkman RR, Buscher M, Busch DH, Bushnell TP, Cao X, Cavani A, Chattopadhyay PK, Cheng Q, Chow S, Clerici M, Cooke A, Cosma A, Cosmi L, Cumano A, Dang VD, Davies D, De Biasi S, Del Zotto G, Della Bella S, Dellabona P, Deniz G, Dessing M, Diefenbach A, Di Santo J, Dieli F, Dolf A, Donnenberg VS, Dorner T, Ehrhardt GRA, Endl E, Engel P, Engelhardt B, Esser C, et al. 2017. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur J Immunol 47:1584-1797. doi: 10.1002/eji.201646632
- Haidaris CG, Frelinger JG. 2019. Inoculating a New Generation: Immunology in Medical Education. Front Immunol 10:2548. doi: 10.3389/fimmu.2019.02548
- AAAS. 2011. Vision and Change in Undergraduate Biology Education: A Call to Action. Washington, DC. www.visionandchange.org.
- Adan A, Alizada G, Kiraz Y, Baran Y, Nalbant A. 2017. Flow cytometry: basic principles and applications. Crit Rev Biotechnol 37:163-176. doi: 10.3109/07388551.2015.1128876
- Krutzik PO, Nolan GP. 2003. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A 55:61-70. doi: 10.1002/cyto.a.10072
- June CH, Abe R, Rabinovitch PS. 2001. Measurement of intracellular calcium ions by flow cytometry. Curr Protoc Cytom Chapter 9:Unit 9 8. doi: 10.1002/0471142956.cy0908s02
- Jung T, Schauer U, Heusser C, Neumann C, Rieger C. 1993. Detection of intracellular cytokines by flow cytometry. J Immunol Methods 159:197-207. doi: 10.1016/0022-1759(93)90158-4
- Boothby JT, Kibler R, Rech S, Hicks R. 2004. Teaching phagocytosis using flow cytometry. Microbiol Educ 5:36-41. doi: 10.1128/jmbe.v5.76
- Ott LE, Carson S. 2014. Immunological tools: engaging students in the use and analysis of flow cytometry and enzyme-linked immunosorbent assay (ELISA). Biochem Mol Biol Educ 42:382-97. doi: 10.1002/bmb.20808
- Tueller JA, Whitley KV, Weber KS. 2020. A full semester flow cytometry course improves graduate and undergraduate student confidence. Biochem Mol Biol Educ 48:99-107. doi: 10.1002/bmb.21318
- Amend SR, Valkenburg KC, Pienta KJ. 2016. Murine Hind Limb Long Bone Dissection and Bone Marrow Isolation. J Vis Exp doi:10.3791/53936
- Liu X, Quan N. 2015. Immune Cell Isolation from Mouse Femur Bone Marrow. Bio Protoc 5.
- JoVE. 2020. Sterile Tissue Harvest. https://www.jove.com/science-education/10298/sterile-tissue-harvest. Accessed June 16, 2020.
- Bedoya SK, Wilson TD, Collins EL, Lau K, Larkin J, 3rd. 2013. Isolation and th17 differentiation of naive CD4 T lymphocytes. J Vis Exp doi:10.3791/50765:e50765
- Xing Y, Hogquist KA. 2014. Isolation, identification, and purification of murine thymic epithelial cells. J Vis Exp doi:10.3791/51780:e51780
- Punt J, Stranford S, Jones P, Owen J. 2018. Kuby Immunology, Eighth Edition. WH Freeman.
- Murphy KM, Weaver C. 2016. Janeway's Immunobiology, 9th Edition. W W Norton & Company.
- Bangera G, Brownell SE. 2014. Course-based undergraduate research experiences can make scientific research more inclusive. CBE Life Sci Educ 13:602-6. doi: 10.1187/cbe.14-06-0099
- Tanner KD. 2013. Structure matters: twenty-one teaching strategies to promote student engagement and cultivate classroom equity. CBE Life Sci Educ 12:322-31. doi: 10.1187/cbe.13-06-0115
- Bronte V, Pittet MJ. 2013. The spleen in local and systemic regulation of immunity. Immunity 39:806-18. doi: 10.1016/j.immuni.2013.10.010
- Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu Y, Wang G, Zou W. 2012. Bone marrow and the control of immunity. Cell Mol Immunol 9:11-9. doi: 10.1038/cmi.2011.47
- Rothenberg EV. 2014. Transcriptional control of early T and B cell developmental choices. Annu Rev Immunol 32:283-321. doi: 10.1146/annurev-immunol-032712-100024
- Shah DK, Zuniga-Pflucker JC. 2014. An overview of the intrathymic intricacies of T cell development. J Immunol 192:4017-23. doi: 10.4049/jimmunol.1302259
- Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, Sekaly RP, Jenkins MK, Vezys V, Haining WN, Jameson SC, Masopust D. 2016. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532:512-6. doi: 10.1038/nature17655
- Huggins MA, Sjaastad FV, Pierson M, Kucaba TA, Swanson W, Staley C, Weingarden AR, Jensen IJ, Danahy DB, Badovinac VP, Jameson SC, Vezys V, Masopust D, Khoruts A, Griffith TS, Hamilton SE. 2019. Microbial Exposure Enhances Immunity to Pathogens Recognized by TLR2 but Increases Susceptibility to Cytokine Storm through TLR4 Sensitization. Cell Rep 28:1729-1743 e5. doi: 10.1016/j.celrep.2019.07.028
Article Files
Login to access supporting documents
Dolence-Design Your Own Flow Cytometry Experiment.pdf(PDF | 214 KB)
S1. Flow Cytometry - Design Your Own Flow Cytometry Experiments Lab.docx(DOCX | 27 KB)
S2. Flow Cytometry - Supplies and equipment necessary for flow cytometry experiments.docx(DOCX | 22 KB)
S3. Flow Cytometry - Flow Cytometry Experiment Lab.docx(DOCX | 120 KB)
S4. Flow Cytometry - Flow Cytometry Analysis Lab.docx(DOCX | 8 MB)
S5. Flow Cytometry-FCS files.zip(ZIP | 56 MB)
S6. Flow Cytometry - Catalog of Example FCS Files.docx(DOCX | 15 KB)
S7. Flow Cytometry - Questions for Assessment of Student Learning.docx(DOCX | 6 MB)
S8. Flow Cytometry - Lab Report Written Assignment.docx(DOCX | 21 KB)
S9. Flow Cytometry - Lab Report Written Assignment Grading Score Sheet.docx(DOCX | 15 KB)
S10. Flow Cytometry - Guide to Lab Oral Presentations.docx(DOCX | 22 KB)
- License terms

Comments
Comments
There are no comments on this resource.