SEAS CURE: Exploring Coral Biology Across Scales
Editor: Joseph Dauer
Published online:
Abstract
Complex biological concepts (like symbiosis and coral biology) that span multiple scales and cross disciplinary boundaries are often difficult for students to understand. This complexity is compounded by the challenges inherent to teaching and learning the process of science, especially at the undergraduate level. To address these issues, we developed the Symbiotic Exaiptasia-Algae System, or SEA System, which leverages the model anemone Exaiptasia diaphana (often used as a proxy for corals in research laboratories) along with its dinoflagellate symbionts. The SEA System represents a cost-effective, tractable platform for students to explore symbiosis and coral biology in the laboratory. We provide lesson plans for both a Preliminary Laboratory Activity (PLA) and multiple Authentic Research Experiences (AREs) that are accompanied by detailed, user-friendly protocols. Collectively, these resources support a versatile course-based undergraduate research experience (CURE) that instructors can implement in one or multiple laboratory sessions of biology courses at any level. The SEAS CURE allows students to learn about biological concepts from molecular to ecological scales and to engage in authentic research. By emphasizing both concepts and competencies, this holistic and inclusive approach facilitates the teaching and learning of science in undergraduate biology courses.
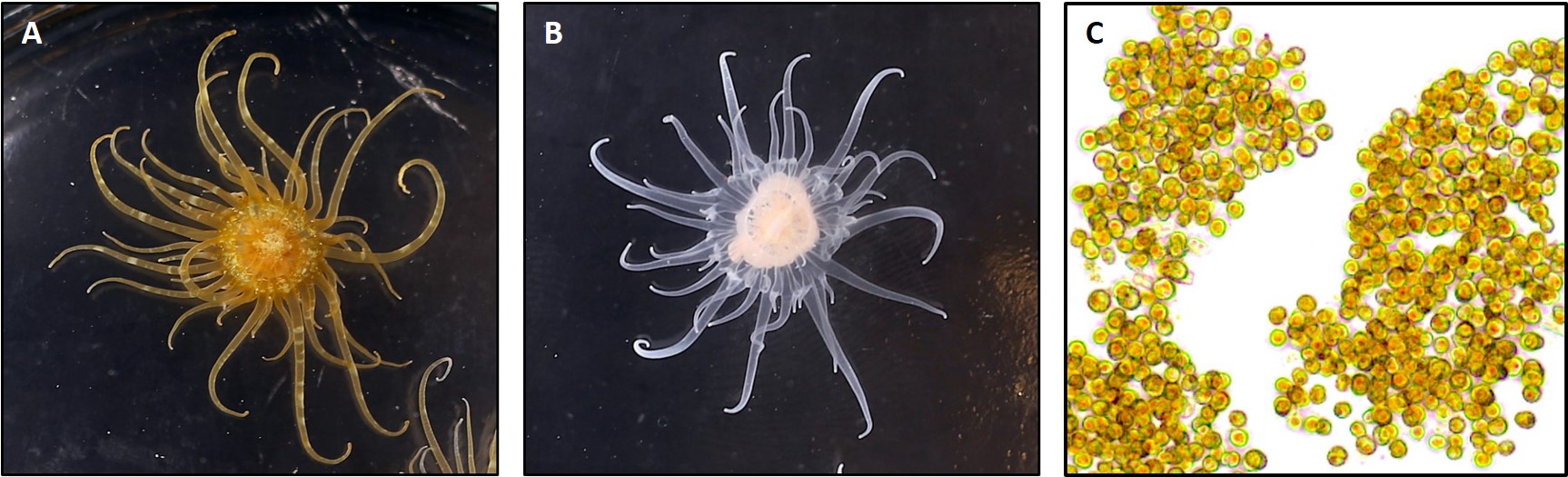
Primary Image: The SEA System partners: symbiotic anemones, aposymbiotic anemones, and dinoflagellate symbionts.
Citation
Poole AZ, Mitchell G, Roark AM, Schwarz J. 2022. SEAS CURE: Exploring Coral Biology Across Scales. CourseSource 9. https://doi.org/10.24918/cs.2022.38Lesson Learning Goals
- Use a model research system to study coral biology and symbiosis.
- Develop a synthetic understanding of the interactions among fundamental biological components of a complex system across multiple scales, from molecular to ecological.
- Develop proficiency with common laboratory techniques.
- Engage in authentic scientific research.
- Productively participate in science as a member of a collaborative team.
- Professionally communicate scientific findings.
Lesson Learning Objectives
- Describe structural and functional features of cnidarian hosts and their algal symbionts.
- Explain connections among levels of biological organization by describing how events occurring at molecular and cellular levels can manifest at other levels, such as ecological levels.
- Use techniques including cell culture, animal husbandry, and microscopy to empirically demonstrate how various factors influence the relationship between cnidarian hosts and their algal symbionts.
- Make and test predictions about the effects of these factors on the health of reef ecosystems by designing, conducting, and analyzing the results of novel experiments.
- Describe and demonstrate behaviors and practices that contribute to inclusive and productive group work.
- Generate written or oral reports of scientific findings.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
Introduction
Coral Reefs: Symbiosis and Environmental Change
The coral reef ecosystem is often used in biology courses to demonstrate fundamental principles that span organismal to biosphere levels. Corals provide a compelling example for teaching physiological, cellular, and molecular biology, because both the physical and trophic structures of the reef ecosystem originate from a symbiotic relationship between photosynthetic algal symbionts and cnidarian hosts, including corals and sea anemones (1). In this symbiotic relationship, algae live within membrane-bound compartments inside the host cells (2). The animal host and algal symbiont engage in exchange and recycling of metabolic products and nutrients that facilitate their ability to inhabit an otherwise nutrient-poor environment (2–4). As a result, corals serve as the trophic foundation for the reef ecosystem and are able to grow at rates sufficient to create the three dimensional structure in which other organisms can live. The symbiotic partnership serves as the basis for an emergent property generated through symbiosis, the coral reef.
Coral reefs also serve as a powerful case study for the impact of anthropogenic disturbances on ecological systems, such as rising sea water temperature, ocean acidification, and pollution (5). When exposed to environmental stressors such as elevated sea water temperatures, the symbiotic relationship breaks down at a cellular level and the symbiotic algae are expelled from host cells, a phenomenon called bleaching (6). Without the contribution of symbionts, bleached corals cannot obtain sufficient nutrition to meet their metabolic requirements, resulting in reduced growth rates and increased susceptibility to disease (5). The decline in coral health due to bleaching is manifested at the ecosystem level as a decline in the biodiversity and ecosystem function of coral reefs.
Understanding the dynamics of the symbiotic relationship and its connection to coral reef decline requires an interdisciplinary approach that encompasses multiple scales, from molecular to ecosystem science. Students often struggle to develop a synthetic understanding of these connections without the opportunity to explore the symbiotic relationship directly in a field or laboratory experience. The difficulty inherent in studying such complex biological processes across scales, especially for novice scientists, is complicated by the logistical challenge of rearing and manipulating corals in a laboratory environment.
To address these issues, we developed an experimental system (the Symbiotic Exaiptasia-Algae System, or SEA System) and a set of authentic research experiences (AREs) to promote meaningful and authentic engagement of undergraduate students in the study of coral symbiosis and biology across multiple biological subdisciplines and scales. The SEA System teaching platform helps students make a compelling personal connection with the real world problem of coral reef decline. In the laboratory, students observe the structure and function of both host and symbiont cells, explore the physiological underpinnings of the symbiosis, investigate the impacts of anthropogenic disturbance on the symbiotic partnership, and engage in other learning activities that cross scales of biological organization. By developing an understanding of the animal and algal partners in the symbiotic relationship, the cellular structure of the symbiosis, and the physiological interactions among the partners, students learn how events occurring at molecular and cellular levels cascade through biological systems and become manifested at ecological levels.
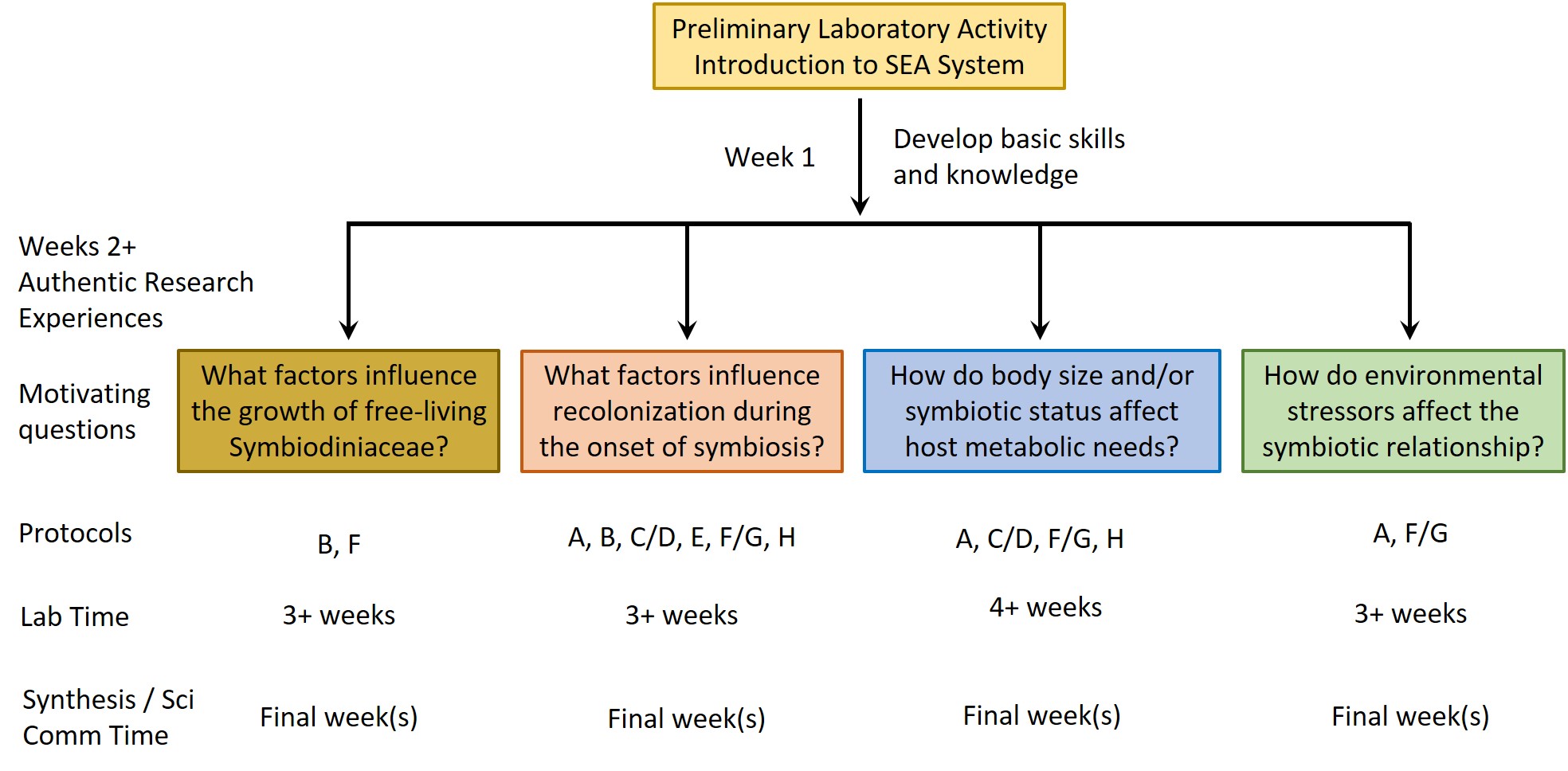
In the SEA System teaching platform, we utilize a symbiotic sea anemone, Exaiptasia diaphana, along with its dinoflagellate symbionts (Figure 1) and a series of standardized protocols to create a tractable, versatile, and easily accessible experimental system in which sea anemones can be used as a model to study underlying biological processes related to coral symbiosis. Our lesson activities are scaffolded, such that students first learn the basic biology of the system via a Preliminary Laboratory Activity (PLA) and then explore their own questions using a series of modular Authentic Research Experiences (AREs). Collectively, the PLA and AREs make up the SEA System Course-Based Undergraduate Research Experience (SEAS CURE).
Course-Based Undergraduate Research Experiences (CUREs)
The CURE presented here addresses several of the core concepts and competencies presented in the Vision and Change in Undergraduate Biology Education report (7). In particular, our CURE allows students to gain expertise in the core concept that “living systems are interconnected and interacting” across multiple functional scales. It helps students tap into the interdisciplinary nature of science to connect seemingly disparate disciplines, for example using cell biology techniques such as microscopy to explore environmental factors that promote bleaching. As opposed to the predictable outcomes of “cookbook” laboratory exercises, our CURE allows students to address novel, literature-driven scientific questions with relevance to the real world problems of climate change and coral bleaching. Additionally, it allows students to learn science in a way that reflects how research is conducted by experts in the fields of coral biology and symbiosis. Because students collect, manage, and analyze real data, they practice quantitative reasoning by using quantitative evidence to make arguments and draw conclusions about their hypotheses. Lastly, our CURE promotes effective scientific communication skills and collaboration within laboratory groups. Overall, our CURE engages undergraduates in authentic research experiences that promote scientific literacy.
CUREs within biology education have been shown to have numerous positive impacts on both students and faculty (8). First, CUREs increase student content knowledge and ability to analyze and interpret scientific data (9, 10). Furthermore, CUREs impact students’ attitudes towards scientific research and their identities as scientists. Specifically, students engaged in CURE activities demonstrate gains in self-confidence in performing research-related tasks, interest in pursuing future research opportunities, and ability to “think like a scientist” (9, 11). CUREs have also been shown to impact student-instructor interactions. Specifically, CURE learning environments, in comparison to non-CURE classes, promote more interactive behaviors including one-on-one dialogue and asking questions (12). Lastly, CUREs provide multiple benefits for faculty including the integration of research and teaching, increased enjoyment and fulfillment in their teaching, and assistance with promotion and tenure (13).
Despite their many benefits, CUREs are challenging to incorporate within a biology curriculum. CUREs represent a marked departure from traditional biology laboratories and therefore require a large time investment from faculty both in development and implementation. In addition, CUREs often involve increased costs to provide a research-intensive laboratory experience. Lastly, CUREs can be complicated to run and manage, especially given the degree of uncertainty inherent to open-ended laboratory projects (13). Thus, for biology CUREs to become more widely used, well-designed, cost-effective laboratory platforms and systems with accompanying teaching exercises are essential. In response to this need, we designed a teaching platform and accompanying materials that can be used to explore questions rooted in the real world problem of climate change and coral reefs and to help students explore biology from molecular to ecological scales. The organisms and tools necessary to use this platform are accessible to any type of biologist and provide low-tech, inexpensive options to integrate research into the classroom.
Using a Model System to Study Biology Across Scales: Exaiptasia diaphana
The pale anemone (Exaiptasia diaphana, formerly known as Aiptasia pallida) is a charismatic, versatile, and tractable model system commonly used in research laboratories to study questions related to coral symbiosis as well as cnidarian ecology and physiology. Like corals, this species is facultatively symbiotic with intracellular (endosymbiotic) dinoflagellate algae in the family Symbiodiniaceae. Anemones can be reared in the laboratory in the symbiotic condition, and they can also be experimentally bleached and reared in an aposymbiotic (lacking symbionts) condition. Similarly, the symbionts can be extracted from the host and grown in culture, and symbionts can also be used to recolonize aposymbiotic anemones. The combination of host and symbiont represents a tractable system to explore questions related both to the formation of the symbiotic condition (by bringing the two partners together) and to the disassociation of the symbiotic condition (by exposing the symbiotic anemones to conditions that provoke bleaching). Collectively, the holobiont (host + symbionts) allows for the simultaneous teaching of animal and “plant” biology across multiple scales and subdisciplines of biology (e.g., cell biology, physiology, ecology).
In the SEA System, educators leverage an experimental system widely used in research laboratories that we have adapted for teaching purposes. The SEA System consists of the living organisms (the symbiotic partners in the three symbiotic states: symbiotic anemone, aposymbiotic anemone, and free-living symbionts) and a set of protocols that can be deployed to rear the organisms and experimentally explore the symbiotic relationship and questions relating to the cellular biology, physiology, or ecology of the organisms living separately or together in symbiosis.
The SEA System is an attractive teaching model because the organisms are easy to maintain in a laboratory setting using basic equipment. Both host anemones and their algal symbionts can be propagated indefinitely (by asexual reproduction) in small dishes on a benchtop or in a standard growth chamber, with light provided by fluorescent bulbs. Symbiotic anemones are commercially available from suppliers like Wards and Carolina Biological, and Symbiodiniaceae symbionts are available through the National Center for Marine Algae at Bigelow Marine Laboratory. Standardized clone lines of anemones and algal symbionts can also be acquired from several research laboratories. As invertebrates, E. diaphana anemones can be studied using basic laboratory equipment and techniques without the need for animal research permits. For these reasons, the SEA System is a highly amenable platform with which undergraduate students can readily conduct authentic research at little cost.
One of the compelling learning outcomes of the SEA System is that students learn how to connect what is happening at the ecological scale (global coral bleaching) to the mechanistic biological processes occurring at the physiological and cellular levels. The suggested readings and videos provided in a supporting file create a scaffold for crossing scales, and this scaffold can then serve as the basis to support student learning of the different scales at which the symbiotic partnership is constructed and maintained. One of the most powerful elements of the SEA System is the ability to use the lesson plans and AREs in a wide variety of courses that span many levels of biological organization, including Genetics, Cell Biology, Plant Physiology, Animal Structure and Diversity, Ecology, and Research Methods courses. Instructors can use pre- and post-ARE assessments to help students identify what they have learned across multiple scales of biology (examples are provided as supporting files).
Undergraduate research and education are well supported by the Exaiptasia research community as a whole. A key online research resource for Exaiptasia investigators includes a page about the Aiptasia Education Network, a group of educators (including the authors of this manuscript) from primarily undergraduate institutions who are committed to using the SEA System in both the research laboratory and the classroom. Investigators in this group have used the AREs described here as a launching point for undergraduate research projects that have been presented by students at regional and national conferences, including the annual meeting of the Society for Integrative and Comparative Biology.
Intended Audience
These lesson activities were developed for and have been successfully implemented by faculty in their teaching laboratories associated with undergraduate biology courses at all levels, from introductory to advanced, including both majors and non-majors courses at both liberal arts colleges and research universities. The scope and duration of each activity can be modified to suit the needs, interests, abilities, and skill levels of the instructors and students. The materials required are readily available in most college or university teaching laboratories.
Required Learning Time
We provide a PLA that introduces students to the symbiotic system consisting of host anemones and their symbiotic dinoflagellate partners. This activity is intended to be completed during one three-hour laboratory session. We also provide ideas for AREs that leverage the symbiotic partners and accompanying protocols and that can be implemented in one or multiple three-hour laboratory sessions, depending on the course goals and intended outcomes. For example, an instructor could use the PLA along with one of the AREs to teach experimental design over the course of several weeks in an introductory or research methods course. Alternatively, an instructor could use the PLA along with any or all of the AREs in an introductory, cell, physiology, ecology, or specialized biology course over the course of several weeks to an entire semester. The AREs can even be expanded into capstone research projects.
Prerequisite Student Knowledge
The knowledge and skills required to complete the PLA and the associated AREs depend on the level of the course in which they are implemented. When used in an introductory biology course, these activities require minimal prerequisite knowledge or skills, although familiarity with simple laboratory equipment (including balances, pipets, and microscopes) and computers will be helpful. At the introductory level, the activities will serve to reinforce concepts relating to eukaryotic cells, symbiosis, and the relationship between structure and function. In courses beyond the introductory level, familiarity with reading scientific literature and writing laboratory reports will be beneficial.
Prerequisite Teacher Knowledge
Prerequisite teacher knowledge varies from minimal to some level of specialized knowledge. Because the PLA and AREs encompass many subdisciplines of biology, instructors can implement those elements that align best with their knowledge, experience, interests, and course goals. If instructors have never worked with Exaiptasia diaphana or Symbiodiniaceae, some investment of time will be required to become familiar with culturing and maintaining the anemone and algal cultures. We provide a foundational reading list of papers to help instructors become familiar with the field, including questions related to coral reefs and climate change, basic physiology and cell biology of the symbiosis, and the use of model systems to study cnidarian symbiosis.
Scientific Teaching Themes
Active Learning
CUREs are exemplars of active learning in that they require students to conduct scientific research in a collaborative setting. By engaging with the SEA System, students achieve specific learning objectives and goals that address the active process of science. Furthermore, the focus on real world problems such as climate change and coral bleaching enhances student engagement and encourages students to make personal connections to their coursework. While the instructor will do some lecturing to provide students with the necessary background knowledge for working with the SEA System, carrying out laboratory techniques, and analyzing data, AREs are primarily student-driven.
Assessment
A variety of formative and summative assessments can be used to evaluate both cognitive and affective student learning gains associated with implementation of the SEAS CURE, depending on the course goals developed by the instructor. For example, formative cognitive assessments developed for use with the PLA are included within the PLA handout. Additionally, for each ARE, formative assessments can include discussions, homework assignments, quizzes, and informal conversations with individual student groups. Summative cognitive assessments can include traditional assignments such as exams, laboratory reports, oral presentations, and posters, or more creative final projects such as blog posts or videos. Affective gains relating to attitude, motivation, and self-efficacy as well as perceived competencies can be assessed through critical reflection and surveys. Lastly, peer evaluations can be utilized to promote self-reflection and to assess the dynamics of collaborative teams. Examples of formative and summative assessments and rubrics that can be implemented to evaluate cognitive and affective gains associated with the AREs are provided as supporting files.
Inclusive Teaching
Importantly, CUREs provide opportunities for students to engage in authentic research during the academic year, unlike traditional summer research projects (e.g., Research Experiences for Undergraduates, or REUs). CUREs thereby remove a barrier to access that is difficult for some students, particularly those from underserved groups, to overcome. Like all CUREs, the SEAS CURE thus provides an excellent opportunity to include all students in authentic research experiences, at levels ranging from introductory to advanced (14).
The fate of corals in a warming world is a topic about which few students know much from their own experience or their prior academic work. However, once they begin learning about the ecosystem services of corals and the human dimensions of coral reef decline, almost all students find the issue compelling and urgent. Students quickly grasp that the complexity of this work demands skills and perspectives from a broad range of disciplines. They also recognize that the diversity required to solve large global challenges encompasses not only scientific disciplines but also identities such as gender, ethnicity, religion, socioeconomic status, personality, and approach to problem-solving (15). In each of the AREs described here, students engage in making connections across ecological, organismal, physiological and cellular scales to address impacts of climate change and other anthropogenic threats to the ecosystem services of corals.
To create and empower inclusive research teams, instructors must be intentional about demonstrating the value of diversity in teamwork and must make the practice of collaborative work (including effective communication, conflict management, and consensus building) a key learning objective for the course (16). A core competency in the Vision and Change in Undergraduate Biology Education report is the “ability to communicate and collaborate with other disciplines.” Both faculty and students should be intentional and reflective about engaging in teamwork, first by making the collaborative process a valued component of each student’s individual efforts and second by implementing teaching approaches that support students in developing inclusive and equitable collaboration skills. An excellent resource for instructors wishing to cultivate inclusivity and equity in teamwork is available at the Eberly Center at Carnegie Mellon University. This resource offers suggestions for teaching approaches that support students as they form a team and work together through a project (e.g., how to structure teamwork in a way that ensures interdependence among the students, how to create accountability of each individual while working in a team). Valuing the collaborative process is key to cultivating inclusivity and equity because doing so allows students to see for themselves that complex problems require diverse skills, perspectives, and contributions.
When implementing CUREs, work should be scaffolded so that students can establish an initial base of knowledge and skills and then build upon that foundation as they engage in the research process. For this reason, we encourage instructors to start with the PLA that introduces students to the SEA System. While familiarizing students with background information about the SEA System, instructors can use the simple laboratory activities embedded within the PLA to help students develop and practice the skills they will need when they begin developing their own project ideas (e.g., culturing the symbiotic partners). Further, by beginning with a broad introduction to the SEA System, instructors scaffold subsequent work, allowing students to tackle problems that would otherwise be intractable. Scaffolding is vital to an inclusive classroom as it provides instructors with a method for adapting their teaching to the individual needs of students (17).
Lesson Plan
The SEA System leverages the three states of the symbiotic relationship between anemones and their dinoflagellate symbionts along with standardized protocols (see Supporting Files S1-S8 for protocols plus Supporting Files S9, S10 for supporting images) to study symbiosis and coral biology (Figure 2). Instructors should begin with the PLA (student handout provided as S11. SEAS CURE - Preliminary Laboratory Activity Handout; list of required materials provided as S12. SEAS CURE - Preliminary Laboratory Activity Supplies; suggested “script” for use by instructors as they introduce the PLA provided as S13. SEAS CURE – Teaching Script Outline). The PLA will introduce students to the SEA System and allow them to learn background information and skills required for subsequent study. This scaffolding empowers students to tackle problems that arise during subsequent weeks.
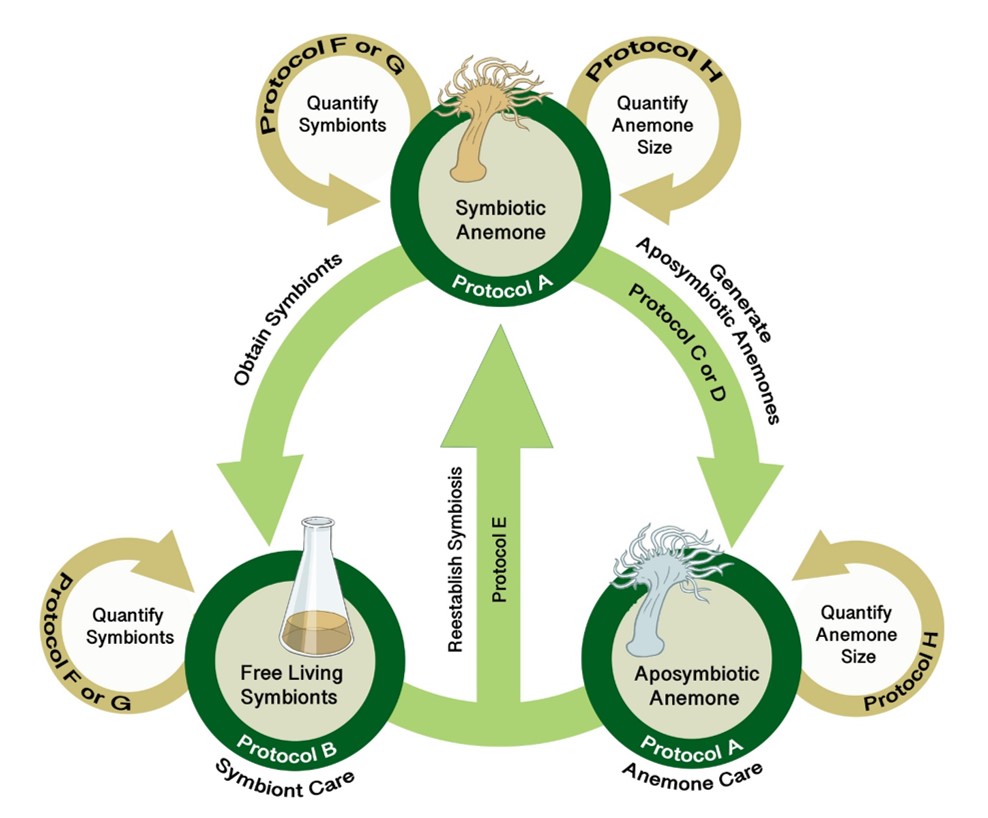
After completing the PLA, instructors can choose (or develop) one or more AREs. These AREs all require the use of various protocols (see Supporting Files S1-S8 for protocols plus Supporting Files S9, S10 for supporting images) for working with Exaiptaisa diaphana and its symbionts. Instructors can mix and match these protocols as necessary to address specific AREs (Figure 3). A list of relevant resources about the biology underlying the SEA System can be found in a supporting file (S14. SEAS CURE - Foundational Resources About Coral-Algal Symbiosis), and a list of relevant resources about scientific communication can be found in a different supporting file (S15. SEAS CURE - Readings About Scientific Communication). These resources can be provided to students as they proceed through the various components of the SEAS CURE, as appropriate. Alternatively, if time and resources are limited, instructors could implement the PLA or the PLA in combination with modified versions of each ARE.
Week 1 of the SEAS CURE: Preliminary Laboratory Activity (PLA) - Introduction to the SEA System
In the first week of the SEAS CURE, students engage in a series of activities to become familiar with the SEA System and with terminology, biology, and laboratory techniques for working with the sea anemone Exaiptasia diaphana and its dinoflagellate symbionts. Students learn to use light (and optional fluorescence) microscopy to observe the partners separately and in symbiosis, and they practice basic techniques for maintaining and working with the organisms. Students also learn how to quantify the abundance of symbionts living within the host using a hemocytometer and a microscope. Collectively, all of the PLA exercises can be completed in a single three-hour laboratory session using the materials listed in a supporting file (S12. SEAS CURE - Preliminary Laboratory Activity Supplies). The PLA requires the use of Protocols A, B, C/D, and F/G. An example of a student work station required for the PLA is shown in Figure 4, and a PLA handout that can be provided to students is available in a supporting file (S11. SEAS CURE - Preliminary Laboratory Activity Handout).
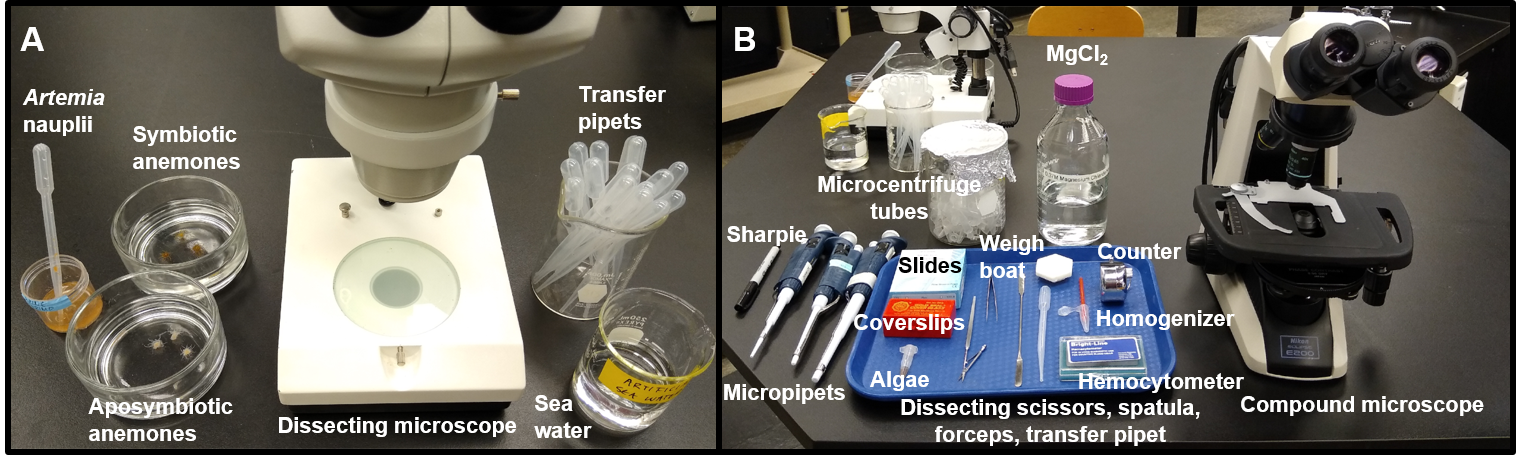
PLA Learning Objectives and Required Equipment
-
Understand the morphology and behavior of sea anemone hosts and Symbiodiniaceae symbionts in different stages of symbiosis: dissecting, compound, and fluorescence microscopes
-
Observe and compare the three symbiotic states using microscopy
-
Symbiotic anemones (holobiont): dissecting microscope
-
Aposymbiotic anemones: dissecting microscope
-
Cultured Symbiodiniaceae cells: compound and/or fluorescence microscope
-
-
Observe anemone feeding behavior: dissecting microscope
-
-
Examine symbionts living within tentacles by comparing tentacle clips of symbiotic and aposymbiotic anemones: compound and/or fluorescence microscope
-
Quantify the density of symbionts living within their host: hemocytometer and compound microscope
Weeks 2+ of the SEAS CURE: Authentic Research Experiences (AREs)
Once students have completed the PLA, they will have developed a good understanding of the symbiotic relationship between anemones and their dinoflagellate symbionts and will have learned the basic husbandry techniques to care for the organisms. In subsequent weeks of the SEAS CURE, students can pursue AREs using the SEA System. Below, we describe four authentic research questions related to dinoflagellate physiology, host physiology, or aspects of the symbiosis that leverage some or all of eight protocols (Figure 3 and Supporting Files S1-S8). Within each ARE, multiple treatment options are presented to allow instructors the flexibility to tailor experiments to the interests of their students and to the equipment and reagents available. Paired pre- and post-ARE assessments that can be implemented as students develop and then conduct their AREs are available as supporting files (Supporting Files S16, S17). These assessments were designed to be comprehensive in scope and include questions about the biology of the SEA System, motivation and relevance, experimental design, data analysis, broader implications, and personal reflections. Instructors may opt to use or modify some or all of the questions as they see fit based on their course goals.
-
What factors influence the growth of free-living Symbiodiniaceae? Students design experiments to explore how different factors (e.g., quantity or quality of light, temperature, or chemical treatment) influence symbiont growth in culture.
-
Environmental factors: quantify symbiont density in populations exposed to various growth conditions
-
Determine the population density of a population of cultured symbionts (Protocol F)
-
Split the culture into groups (Protocol B) and maintain the cultures for 5-7 days under various growth conditions (e.g., bright vs. dim light, white vs. red light, 25 °C vs. 33 °C)
-
Continue to monitor the population density of each culture every other day (Protocol F)
-
-
Chemical treatment: quantify symbiont density in populations exposed to various chemicals
-
Determine the population density of a population of cultured symbionts (Protocol F)
-
Split the culture into groups (Protocol B) and maintain the cultures for 5-7 days while manipulating media components to which symbionts are exposed (e.g., high vs. low phosphate, presence or absence of herbicides)
-
Continue to monitor the population density of each culture every other day (Protocol F)
-
-
-
What factors influence recolonization during the onset of symbiosis? Students design experiments to explore how different factors (e.g., symbiont type, environment, or chemical treatment) influence recolonization during the onset of symbiosis.
-
Host-symbiont specificity: design experiments to determine how well different symbiont types recolonize aposymbiotic anemones
-
Prepare aposymbiotic anemones (Protocol C/D)
-
Culture and prepare symbionts (Protocols B and F)
-
Recolonize aposymbiotic anemones with different symbiont types (Protocol E)
-
Maintain the anemones for the desired period of time (days to weeks, Protocol A)
-
Quantify symbiont numbers in host anemones (Protocols F/G and H1)
Note: In the early stages of recolonization (at least the first several days), too few symbionts are present to quantify using cell counts (Protocol F); therefore, use of a fluorescence microscope will be required (Protocol G)
-
-
Environmental factors: design experiments to determine how exposure to elevated temperature, ultraviolet light, salinity, or other environmental factors influences recolonization of aposymbiotic anemones
-
Prepare aposymbiotic anemones (Protocol C/D)
-
Culture and prepare symbionts (Protocols B and F)
-
Recolonize aposymbiotic anemones (Protocol E)
-
Maintain the anemones for the desired period of time (days to weeks, Protocol A) while manipulating environmental factors to which anemones are exposed
-
Quantify symbiont numbers in host anemones (Protocols F/G and H1)
Note: In the early stages of recolonization (at least the first several days), too few symbionts are present to quantify using cell counts (Protocol F); therefore, use of a fluorescence microscope will be required (Protocol G)
-
-
Chemical treatment: design experiments to determine how exposure to chemicals that inhibit or promote certain cellular pathways or enzymes influences recolonization to determine whether they are involved in this process.
-
Prepare aposymbiotic anemones (Protocol C/D)
-
Culture and prepare symbionts (Protocols B and F)
-
Recolonize aposymbiotic anemones (Protocol E)
-
Maintain the anemones for the desired period of time (days to weeks, Protocol A) while manipulating media components to which anemones are exposed
-
Quantify symbiont numbers in host anemones (Protocols F/G and H1)
Note: In the early stages of recolonization (at least the first several days), too few symbionts are present to quantify using cell counts (Protocol F); therefore, use of a fluorescence microscope will be required (Protocol G)
-
-
-
How do body size and/or symbiotic status affect the metabolic needs of the host? Students design experiments to test whether anemones that differ in body mass and/or symbiotic status feed at different rates.
-
Body size: quantify the feeding rate of anemones that differ in body mass (Note: body mass can be determined as wet mass or total protein content)
-
Determine anemone wet mass using an analytical balance (Protocol H1)
-
Feed anemones (Protocol A) under a dissecting microscope and determine the rate at which Artemia nauplii are consumed
-
Optional: quantify anemone protein content (Protocol H2) 48 hours after feeding
-
-
Symbiotic status: quantify the feeding rate of anemones that differ in symbiont density (Note: symbiont density can be categorical [aposymbiotic vs. symbiotic] or continuous [quantified by tentacle squash])
-
Generate partially or completely aposymbiotic anemones (Protocol C/D)
-
Feed aposymbiotic and symbiotic anemones (Protocol A) under a dissecting microscope and determine the rate at which Artemia nauplii are consumed
-
Quantify symbiont density using tentacles (Protocol F/G)
-
-
Body size and symbiotic status: quantify the feeding rate of anemones that differ in both body mass and symbiotic status (Note: body mass can be determined as wet mass or total protein content, and symbiont density can be categorical [aposymbiotic vs. symbiotic] or continuous [quantified by tentacle squash])
-
Generate partially or completely aposymbiotic anemones (Protocol C/D)
-
Determine anemone wet mass using an analytical balance (Protocol H1)
-
Feed anemones (Protocol A) under a dissecting microscope and determine the rate at which Artemia nauplii are consumed
-
Determine symbiont density using tentacles (Protocol F/G)
-
Optional: quantify anemone protein content (Protocol H2) 48 hours after feeding
-
-
-
How do environmental stressors affect the symbiotic relationship? Students design experiments to explore how the symbiosis is affected by anthropogenic factors such as rising sea water temperature or chemical pollution.
-
Thermal stress (bleaching): design experiments to test the effects of elevated temperature (32-33 °C compared to the control temperature of 25 °C) on symbiont density over time
-
Maintain symbiotic anemones for the desired period of time (days to weeks, Protocol A) while manipulating temperatures to which anemones are exposed and monitoring anemone behavior/health
-
Quantify symbiont numbers in host (Protocol F/G)
Note: when anemones become bleached, too few symbionts may be present to quantify using cell counts (Protocol F); therefore, use of a fluorescence microscope may allow for more accurate assessment (Protocol G)
-
-
Other environmental stressors: design experiments to test the effects of chemical contaminants, nitrogen or other nutrients, ultraviolet light, or other environmental factors of interest
-
Maintain symbiotic anemones for the desired period of time (days to weeks, Protocol A) while manipulating conditions to which anemones are exposed and monitoring anemone behavior/health
-
Quantify symbiont numbers in host (Protocol F/G)
Note: when anemones become bleached, too few symbionts may be present to quantify using cell counts (Protocol F); therefore, use of a fluorescence microscope may allow for more accurate assessment (Protocol G)
-
-
Final Week(s) of the SEAS CURE: Scientific Communication and Reflection
Depending on the duration of the ARE(s) implemented, instructors may wish to dedicate an entire week or even multiple weeks to communicating results and reflecting on the process of science. Students may present their findings in many ways, from traditional research papers and presentations (PowerPoint or posters) to more creative modalities like blog posts and videos. A short list of suggested readings about scientific communication can be found in a supporting file (S15. SEAS CURE - Readings About Scientific Communication); these resources can be provided to students as appropriate. Examples of rubrics that can be used to assess scientific communication are available as supporting files (Supporting Files S18, S19). Additionally, students should be given time to reflect on their experiences. The value of CUREs is not entirely in the data generated; often results are dubious or of little scientific import. Instead, CUREs provide students a way to learn about the scientific process and reflect on the process of working collaboratively as a research team. Critical reflection is essential for reinforcing this learning (18). The paired pre- and post-ARE assessments available as supporting files (Supporting Files S16, S17) include some recommended reflection questions. Lastly, students can use a peer evaluation form to assess their laboratory group members; this form is provided as a supporting file (S20. SEAS CURE - Peer Evaluation Form).
Timeline
In Tables 1-4, we provide example timelines for each of the four AREs. All AREs begin with the same introductory session (Session #1), during which students complete the PLA. Subsequent sessions allow students to design and conduct their own authentic research projects. All AREs are intended to be completed during four or more laboratory sessions, each lasting no more than three hours, although the duration of each ARE can easily be modified. Instructors may wish to provide students with relevant literature prior to the first or second laboratory session. A short list of suggested readings appropriate for this purpose can be found in a supporting file (S14. SEAS CURE - Foundational Resources About Coral-Algal Symbiosis). In select cases, students or instructors may also need to complete some simple tasks outside of laboratory time. The final laboratory session of each ARE provides the opportunity for students to communicate their findings and reflect on their experiences. A short list of suggested readings about scientific communication can be found in a supporting file (S15. SEAS CURE - Readings About Scientific Communication). Within each table, the “Notes and Relevant Protocols” column contains information that will help instructors prepare for each session (“Teacher preparation”) as well as a list of the activities that students will complete (“During laboratory” and “Outside of laboratory time”).
Table 1. Lesson timeline for implementing Authentic Research Experience #1 (parts a and b). This example lesson spans four or more laboratory sessions plus additional instructor preparation time. Note that instructors may wish to collect data for more than one week; if so, laboratory time during intervening weeks can be used for writing workshops.
| Laboratory Session # | Description | Estimated Time | Notes and Relevant Protocols |
|---|---|---|---|
|
Preliminary Laboratory Activity Note: this activity utilizes Protocols A, B, C/D, and F/G |
|||
| Session #1 |
Pre-laboratory lesson |
15 min |
Teacher preparation:
During laboratory:
|
|
Observing anemones and symbionts |
45 min |
||
|
Observing anemone feeding behavior |
20 min |
||
|
Observing tentacles |
20 min |
||
|
Homogenizing anemones |
20 min |
||
|
Quantifying symbiont density |
40 min |
||
|
Discussion |
20 min |
||
|
Authentic Research Experience #1 (Parts a and b): What factors influence the growth of free-living Symbiodiniaceae? Note: this activity utilizes Protocols B and F |
|||
|
Session #2 |
Pre-laboratory lesson and demonstration of algal culture techniques |
20 min |
Teacher preparation:
During laboratory:
|
|
Discussion of relevant literature (optional) |
30 min |
||
|
Discussion of experimental design and selecting treatments |
30 min |
||
|
Starting algal cultures (1x106 cells/10 mL culture) and applying treatments |
30 min |
||
|
Outside of laboratory time throughout intervening week(s) |
Quantifying symbiont density of algal cultures |
30-60 min |
Teacher preparation:
Outside of laboratory time:
|
|
Session #3 |
Pre-laboratory lesson |
10 min |
During laboratory:
|
|
Analyzing data |
30 min |
||
|
Generating figures |
30 min |
||
|
Discussion |
20 min |
||
|
Writing workshop |
60 min |
||
|
Session(s) #4+ |
Scientific communication and reflection |
As needed |
During laboratory:
|
Table 2. Lesson timeline for implementing Authentic Research Experience #2 (part b). This example lesson spans five or more laboratory sessions plus additional instructor preparation time.
| Laboratory Session # | Description | Estimated Time | Notes and Relevant Protocols |
|---|---|---|---|
|
Preliminary Laboratory Activity Note: this activity utilizes Protocols A, B, C/D, and F/G |
|||
|
Session #1 |
Pre-laboratory lesson |
15 min |
Teacher preparation:
During laboratory:
|
|
Observing anemones and symbionts |
45 min |
||
|
Observing anemone feeding behavior |
20 min |
||
|
Observing tentacles |
20 min |
||
|
Homogenizing anemones |
20 min |
||
|
Quantifying symbiont density |
40 min |
||
|
Discussion |
20 min |
||
|
Authentic Research Experience #2 (Part b, testing salinity): What factors influence recolonization during the onset of symbiosis? Note: this activity utilizes Protocols A, B, C/D, E, F/G, and H1 |
|||
|
Session #2 |
Pre-laboratory lesson |
15 min |
Teacher preparation:
During laboratory:
|
|
Preparing dinoflagellates |
60 min |
||
|
Discussion of experimental design and selecting treatments |
30 min |
||
|
Preparing sea water of varying salinities |
30 min |
||
|
Introducing dinoflagellates to anemones |
20 min |
||
|
Outside of laboratory time throughout intervening week(s) |
Maintaining anemones |
30-60 min, 3/7X per week |
Teacher preparation:
Outside of laboratory time:
|
|
Session #3 |
Pre-laboratory lesson |
20 min |
Teacher preparation:
During laboratory:
|
|
Weighing anemones |
15 min |
||
|
Quantifying symbiont density in anemones |
90 min |
||
|
Analyzing data |
45 min |
||
|
Session #4 |
Pre-laboratory lesson |
10 min |
During laboratory:
|
|
Analyzing data |
60 min |
||
|
Generating figures |
30 min |
||
|
Discussion |
20 min |
||
|
Writing workshop |
60 min |
||
|
Session(s) #5+ |
Scientific communication and reflection |
As needed |
During laboratory:
|
Table 3. Lesson timeline for implementing Authentic Research Experience #3 (part c). This example lesson spans five or more laboratory sessions plus additional instructor preparation time.
| Laboratory Session # | Description | Estimated Time | Notes and Relevant Protocols |
|---|---|---|---|
|
Preliminary Laboratory Activity Note: this activity utilizes Protocols A, B, C/D, and F/G |
|||
|
Session #1 |
Pre-laboratory lesson |
15 min |
Teacher preparation:
During laboratory:
|
|
Observing anemones and symbionts |
45 min |
||
|
Observing anemone feeding behavior |
20 min |
||
|
Observing tentacles |
20 min |
||
|
Homogenizing anemones |
20 min |
||
|
Quantifying symbiont density |
40 min |
||
|
Discussion |
20 min |
||
|
Authentic Research Experience #3 (Part c): How do body size and symbiotic status affect the metabolic needs of the host? Note: this activity utilizes Protocols A, C/D, F/G, and H |
|||
|
Session #2 |
Pre-laboratory lesson |
15 min |
Teacher preparation:
During laboratory:
Outside of laboratory time:
|
|
Discussion of experimental design |
30 min |
||
|
Observing anemone feeding behavior |
60 min |
||
|
Weighing anemones |
15 min |
||
|
Quantifying symbiont density |
60 min |
||
|
Session #3 |
Pre-laboratory lesson |
10 min |
Teacher preparation:
During laboratory:
|
|
Homogenizing anemones |
30 min |
||
|
Quantifying anemone protein content |
120 min |
||
|
Session #4 |
Generating standard curve |
45 min |
During laboratory:
|
|
Analyzing data |
60 min |
||
|
Generating figures |
30 min |
||
|
Discussion |
20 min |
||
|
Writing workshop |
20 min |
||
|
Session(s) #5+ |
Scientific communication and reflection |
As needed |
During laboratory:
|
Table 4. Lesson timeline for implementing Authentic Research Experience #4 (part a). This example lesson spans five or more laboratory sessions plus additional instructor preparation time.
| Laboratory Session # | Description | Estimated Time | Notes and Relevant Protocols |
|---|---|---|---|
|
Preliminary Laboratory Activity Note: this activity utilizes Protocols A, B, C/D, and F/G |
|||
|
Session #1 |
Pre-laboratory lesson |
15 min |
Teacher preparation:
During laboratory:
|
|
Observing anemones and symbionts |
45 min |
||
|
Observing anemone feeding behavior |
20 min |
||
|
Observing tentacles |
20 min |
||
|
Homogenizing anemones |
20 min |
||
|
Quantifying symbiont density |
40 min |
||
|
Discussion |
20 min |
||
|
Authentic Research Experience #4 (Part a, testing temperature): How do environmental stressors affect the symbiotic relationship? Note: this activity utilizes Protocols A and F/G |
|||
|
Session #2 |
Pre-laboratory lesson |
10 min |
Teacher preparation:
During laboratory:
Outside of laboratory time:
|
|
Discussion of relevant literature (optional) |
60 min |
||
|
Discussion of experimental design and selecting treatments |
30 min |
||
|
Plating anemones |
30 min |
||
|
Establishing temperature treatments |
30 min |
||
|
Outside of laboratory time throughout intervening week |
Maintaining anemones |
30 min |
Teacher preparation:
Outside of laboratory time:
|
|
Observing anemones |
15 min |
||
|
Sampling anemones (for teams that have early time points) |
optional |
||
|
Session #3 |
Maintaining anemones |
30 min |
Teacher preparation:
During laboratory:
|
|
Observing anemones |
15 min |
||
|
Sampling anemones |
optional |
||
|
Writing workshop |
90 min |
||
|
Session #4 |
Sampling anemones |
30 min |
Teacher preparation:
During laboratory:
|
|
Quantifying symbiont density |
60 min |
||
|
Analyzing data |
30 min |
||
|
Generating figures |
30 min |
||
|
Discussion |
20 min |
||
|
Session(s) #5+ |
Scientific communication and reflection |
As needed |
During laboratory:
|
Teaching Discussion
Ideas for Implementation
One of the inherent strengths of the SEAS CURE is its versatility, which allows it to be used in courses of a variety of levels and topic areas. We intentionally designed the AREs so that instructors have the ability to mix and match protocols to address questions that are relevant to their interests and course content. For example, an instructor may choose to implement just the PLA for an introductory organismal biology course, whereas an advanced animal physiology course might also include an ARE. An instructor may also wish to combine multiple AREs to teach a full semester research methods course. Furthermore, instructors can design their own AREs with the SEA System to explore other aspects of symbiosis or coral biology.
The implementation of each ARE is also very flexible. Instructors have the ability to modify which and how many treatments are used depending on their course needs, the availability of materials, and class size. While we provided four example ARE timelines in which specific independent variables are tested, instructors may alternatively select their own treatments or outcomes to measure to further tailor the AREs to their courses. Overall, our goal was to design a CURE that is easy to integrate into the framework of an existing course and therefore decrease the barriers to implementation.
Common Challenges
Instructors should be aware of potential challenges before adopting the SEA System in their courses. First, an initial time investment will be required to obtain anemone and symbiont cultures of sufficient densities. Furthermore, if ordering anemones from commercial suppliers, some variation in the size and symbiotic state of the animals obtained can be expected. In most cases, commercial suppliers will provide fully symbiotic anemones, but we have also experienced times when the anemones arrived mostly bleached. In these cases, instructors will need time to repopulate anemones with symbionts prior to use. Lastly, instructors will need to allow for time to generate aposymbiotic anemones, a process that can take up to six weeks depending on the protocol used. Thus, we recommend that anemone and dinoflagellate stocks are acquired at least 8-10 weeks prior to use.
Another common challenge is the time required for care of this system, particularly if instructors intend to maintain anemone and symbiont clone lines indefinitely. Anemone maintenance is non-trivial and, depending on animal numbers, can require several hours each week, including feeding and water changes. Additionally, other tasks such as culturing dinoflagellates, making sea water, and preparing materials for each laboratory session can be time-consuming. If possible, we recommend having a single point person to care for this system, as doing so will require less training of students or staff and will ensure consistency with care over time.
Instructors can also use several strategies to make the initial setup of the SEA System less daunting. First, instructors can start small by just incorporating the PLA or the PLA plus a modified part of a single ARE in their curriculum. For example, using ARE Question 1a to test the effect of different light levels on symbiont growth requires minimal equipment, as students could set up their experiments using one light source, with some samples either shaded or farther away from the light source. For ARE Question 2b, examining the impact of salinity on the onset of symbiosis would also simplify setup. Additionally, to prepare aposymbiotic anemones, instructors could utilize the quicker menthol bleaching protocol (S3. SEAS CURE - Protocol C). If instructors only want to use symbiotic anemones, they can complete ARE Question 3a, eliminating the need to prepare aposymbiotic anemones or maintain long term stocks of animals. For example, instructors could order symbiotic anemones from a biological company and have these animals arrive just prior to the laboratory session in which they will be used. For this ARE, the instructor could also use aposymbiotic animals that are not fully bleached to reduce the required preparation time. Lastly, for ARE Question 4b, selecting a treatment such as added nutrients in conjunction with using cell counts to quantify symbionts would reduce equipment needs. This flexibility highlights the versatility of the SEA System so that instructors can alter various components to best meet the needs of their class.
Lesson Effectiveness
For instructors, the SEA System and the SEAS CURE facilitate both the teaching of fundamental biological concepts and the implementation of authentic research experiences in undergraduate courses. As students progress through the PLA and one or more AREs, they learn about not only the structural and functional features of anemone hosts and their algal symbionts but also the connections among multiple levels of biological organization. For example, cell signaling pathways dictate the uptake, maintenance, and expulsion of algal symbionts by anemones, thereby also determining the metabolic needs and performance of the hosts, which play critical roles in reef ecosystems. While learning concepts from multiple subdisciplines of biology and across multiple scales, students also refine important skills including using common laboratory equipment, designing experiments, analyzing and presenting data, communicating scientific findings, and working collaboratively. Learning gains associated with these concepts and skills can be assessed using paired pre- and post-ARE assessments that are available as supporting files (Supporting Files S16, S17).
We have found that students who complete the PLA and one or more AREs are uniformly enthusiastic about working with the SEA System, as it is a charismatic model with which to teach important concepts and skills. Showing students one or more of the movies (e.g., Chasing Coral) and requiring them to read one or more of the articles provided in a supporting file (S14. SEAS CURE - Foundational Resources About Coral-Algal Symbiosis) early in a course helps build excitement about the real world relevance of studying cnidarians and their symbionts, even among students who do not live near the ocean. Many of these foundational resources highlight some of biology’s most pressing “grand challenges,” including climate change and extinction, so leveraging these resources early in a course helps students feel personally invested in the projects they will subsequently undertake. Moreover, because the SEA System comprises tractable and readily observable organisms living in a captivating symbiotic relationship, students who study biology using this system can truly see science happening in front of them.
In post-course surveys, students reflected on how the SEAS CURE affected their biological knowledge. For example:
“the effects that climate change can have on an ecosystem, and how small molecular changes can have cascading impacts within and outside of that ecosystem”;
“how genetics techniques can be used to address questions about ecological and environmental issues”;
“coral symbiosis and how environmental stressors can lead to the bleaching response”;
“the lab project was exciting and a good illustration of conceptual topics”;
“I appreciated the continuity of the Aiptasia project, as it was helping in building my understanding more with each week”;
“the ecological significance of researching coral symbiosis.”
Students also reflected on how the experience affected their understanding of the scientific process:
“...I found the activity to be very beneficial in allowing us to practice making educated observations and connecting this with a hypothesis. The experiment was simple and great for our level of understanding”;
“...working with sea anemones made it easier to understand experimental design because it was tangible. For example, coming up with hypotheses based on the color or size of the anemones was effortless. For me, anemones allowed for an easy transition from studying to developing my experimental design for my semester project”;
“I really liked applying science to real situations and this was not too abstract, so I really knew what I was doing.”
Scientific Teaching
Instructors who use scientific teaching employ evidence-based, active learning strategies to teach students the process, not just the outcomes, of science as a discipline (19). A common hallmark of scientific teaching is the intentional integration of research into both the classroom and the teaching laboratory, often through the use of CUREs. Our SEAS CURE facilitates sustained yet logistically simple integration of research into biology courses across scales and at all levels of biology. SEAS CURE is flexible enough that instructors can implement activities that range in duration from a single lesson to an entire course. As students complete their SEAS CURE activities, they design experiments, collect and analyze data, and communicate their findings. Because student research projects are authentic, data generated by the students can be published, thereby contributing to the scholarly productivity of their institutions while simultaneously bolstering the students’ academic progress. Furthermore, the experiments that students design as part of their SEAS CURE activities can be readily expanded into capstone senior or honors thesis projects. The SEAS CURE provides institutions an opportunity to adopt a novel and charismatic system and thus expand the research scope of their science department(s) at little cost. Lastly, students and instructors who engage with the SEA System become part of a growing international research community that is leveraging E. diaphana to study coral biology.
Conclusion
Understanding the connection between the cellular events associated with coral symbiosis and coral reef decline requires students to take an interdisciplinary approach, integrating information from multiple levels of biological organization. This task is challenging for students, particularly those at the introductory level. Additionally, corals are difficult to rear and manipulate in the laboratory setting, making coral symbiosis and coral reef decline difficult topics for students to explore at most institutions. To address these challenges, we developed an experimental platform called the SEA System, which utilizes the model anemone Exaiptasia diaphana, its dinoflagellate symbionts, and a set of eight protocols. This system represents a cost-effective, low maintenance platform for students to explore symbiosis in the laboratory setting. Additionally, we presented a series of authentic research experiences, collectively called the SEAS CURE, consisting of a preliminary laboratory activity designed to introduce students to the basic biology of the symbiosis along with four authentic research experiences. We designed the SEAS CURE to be highly versatile, so that instructors can mix and match protocols in a way that best suits their class level and subject area, making it easy to integrate into the framework of existing courses.
Supporting Materials
-
S1. SEAS CURE - Protocol A (Culture Anemones)
-
S2. SEAS CURE - Protocol B (Culture Symbionts)
-
S3. SEAS CURE - Protocol C (Generate Aposymbiotic Anemones - Menthol)
-
S4. SEAS CURE - Protocol D (Generate Aposymbiotic Anemones - Cold Shock)
-
S5. SEAS CURE - Protocol E (Reestablish Symbiosis)
-
S6. SEAS CURE - Protocol F (Quantify Density of Symbionts Living Within an Anemone Host)
-
S7. SEAS CURE - Protocol G (Detect and Quantify Symbionts in Situ)
-
S8. SEAS CURE - Protocol H (Quantify Anemone Size)
-
S9. SEAS CURE - Brightfield Image
-
S10. SEAS CURE - Fluorescence Image
-
S11. SEAS CURE - Preliminary Laboratory Activity Handout
-
S12. SEAS CURE - Preliminary Laboratory Activity Supplies
-
S13. SEAS CURE - Teaching Script Outline
-
S14. SEAS CURE - Foundational Resources About Coral-Algal Symbiosis
-
S15. SEAS CURE - Readings About Scientific Communication
-
S16. SEAS CURE - Pre-ARE Assessment
-
S17. SEAS CURE - Post-ARE Assessment
-
S18. SEAS CURE - Scientific Paper Rubric
-
S19. SEAS CURE - Research Presentation Rubric
-
S20. SEAS CURE - Peer Evaluation Form
Acknowledgments
This project originated at a workshop (Developing Aiptasia as a model system for coral symbiosis) in July of 2018 at Oregon State University in Corvallis, Oregon. We give special thanks to Virginia Weis, Cawa Tran, Michael Morgan, Elisha Wood-Charlson, and countless students and laboratory technicians for their assistance in developing and testing our lesson materials. We thank Rick Jones for creating the SEA System illustration shown in Figure 2. Travel funds were provided by Vassar College. The collection and inclusion of student feedback about lesson materials were approved by the Furman University and Vassar College Institutional Review Boards (FU 092019 and exempt approval, respectively). We also thank two anonymous reviewers and the CourseSource editorial staff for constructive feedback on the manuscript.
References
- Weis VM, Davy SK, Hoegh-Guldberg O, Rodriguez-Lanetty M, Pringle JR. 2008. Cell biology in model systems as the key to understanding corals. Trends Ecol Evol 23:369–376. doi:10.1016/j.tree.2008.03.004
- Davy SK, Allemand D, Weis VM. 2012. Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol Mol Biol Rev 76:229–261. doi:10.1128/MMBR.05014-11
- Yellowlees D, Rees TAV, Leggat W. 2008. Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ 31:679–694. doi:10.1111/j.1365-3040.2008.01802.x
- Weis VM. 2019. Cell biology of coral symbiosis: Foundational study can inform solutions to the coral reef crisis. Integr Comp Biol 59:845–855. doi:10.1093/icb/icz067
- Hoegh-Guldberg O, Poloczanska ES, Skirving W, Dove S. 2017. Coral reef ecosystems under climate change and ocean acidification. Front Mar Sci 4. doi:10.3389/fmars.2017.00158
- Weis VM. 2008. Cellular mechanisms of cnidarian bleaching: Stress causes the collapse of symbiosis. J Exp Biol 211:3059–3066. doi:10.1242/jeb.009597
- American Association for the Advancement of Science (AAAS). 2011. Vision and change in undergraduate biology education: A call to action. AAAS, Washington, DC. http://visionandchange.org/finalreport
- Lopatto D. 2010. Science in solution: The impact of undergraduate research on student learning. Research Corporation for Science Advancement, Tucson, AZ.
- Brownell SE, Hekmat-Scafe DS, Singla V, Chandler Seawell P, Conklin Imam JF, Eddy SL, Stearns T, Cyert MS. 2015. A high-enrollment course-based undergraduate research experience improves student conceptions of scientific thinking and ability to interpret data. CBE Life Sci Educ 14:ar21. doi:10.1187/cbe.14-05-0092
- Olimpo JT, Fisher GR, DeChenne-Peters SE. 2016. Development and evaluation of the Tigriopus course-based undergraduate research experience: Impacts on students’ content knowledge, attitudes, and motivation in a majors introductory biology course. CBE Life Sci Educ 15:ar72. doi:10.1187/cbe.15-11-0228
- Brownell SE, Kloser MJ, Fukami T, Shavelson R. 2012. Undergraduate biology lab courses: Comparing the impact of traditionally based “cookbook” and authentic research-based courses on student lab experiences. J Coll Sci Teach 41:36-45.
- Esparza D, Wagler AE, Olimpo JT. 2020. Characterization of instructor and student behaviors in CURE and non-CURE learning environments: Impacts on student motivation, science identity development, and perceptions of the laboratory experience. CBE Life Sci Educ 19:ar10. doi:10.1187/cbe.19-04-0082
- Shortlidge EE, Bangera G, Brownell SE. 2016. Faculty perspectives on developing and teaching course-based undergraduate research experiences. BioScience 66:54–62. doi:10.1093/biosci/biv167
- Bangera G, Brownell SE. 2014. Course-based undergraduate research experiences can make scientific research more inclusive. CBE Life Sci Educ 13:602–606. doi:10.1187/cbe.14-06-0099
- Cheruvelil KS, Soranno PA, Weathers KC, Hanson PC, Goring SJ, Filstrup CT, Read EK. 2014. Creating and maintaining high-performing collaborative research teams: The importance of diversity and interpersonal skills. Front Ecol Environ 12:31–38. doi:10.1890/130001
- Cheruvelil KS, Palma-Dow A de, Smith KA. 2020. Strategies to promote effective student research teams in undergraduate biology labs. Am Biol Teach 82:18–27. doi:10.1525/abt.2020.82.1.18
- Reiser BJ. 2004. Scaffolding complex learning: The mechanisms of structuring and problematizing student work. J Learn Sci 13:273–304. doi:10.1207/s15327809jls1303_2
- Howitt S, Wilson A. 2016. Scaffolded reflection as a tool for surfacing complex learning in undergraduate research projects. Counc Undergrad Res Q 36:33–38. doi:10.18833/CURQ/36/4/8
- Handelsman J, Ebert-May D, Beichner R, Bruns P, Chang A, DeHaan R, Gentile J, Lauffer S, Stewart J, Tilghman SM, Wood WB. 2004. Scientific teaching. Science 304:521–522. doi:10.1126/science.1096022
Article Files
Login to access supporting documents
Poole-Mitchell-Roark-Schwarz-SEAS CURE Exploring Coral Biology Across Scales.pdf(PDF | 1 MB)
S1. SEAS CURE - Protocol A.docx(DOCX | 35 KB)
S10. SEAS CURE - Fluorescence Image.JPG(JPG | 1 MB)
S11. SEAS CURE - Preliminary Laboratory Activity Handout.docx(DOCX | 17 MB)
S12. SEAS CURE - Preliminary Laboratory Activity Supplies.docx(DOCX | 22 KB)
S13. SEAS CURE - Teaching Script Outline.docx(DOCX | 24 KB)
S14. SEAS CURE - Foundational Resources About Coral-Algal Symbiosis.docx(DOCX | 27 KB)
S15. SEAS CURE - Readings About Scientific Communication.docx(DOCX | 18 KB)
S16. SEAS CURE - Pre-ARE Assessment.docx(DOCX | 18 KB)
S17. SEAS CURE - Post-ARE Assessment.docx(DOCX | 18 KB)
S18. SEAS CURE - Scientific Paper Rubric.docx(DOCX | 22 KB)
S19. SEAS CURE - Research Presentation Rubric.docx(DOCX | 21 KB)
S2. SEAS CURE - Protocol B.docx(DOCX | 26 KB)
S20. SEAS CURE - Peer Evaluation Form.docx(DOCX | 17 KB)
S3. SEAS CURE - Protocol C.docx(DOCX | 26 KB)
S4. SEAS CURE - Protocol D.docx(DOCX | 26 KB)
S5. SEAS CURE - Protocol E.docx(DOCX | 28 KB)
S6. SEAS CURE - Protocol F.docx(DOCX | 66 KB)
S7. SEAS CURE - Protocol G.docx(DOCX | 2 MB)
S8. SEAS CURE - Protocol H.docx(DOCX | 35 KB)
S9. SEAS CURE - Brightfield Image.JPG(JPG | 944 KB)
- License terms

Comments
Comments
There are no comments on this resource.