Engaging Students in Pharmacogenetics: Patient Case Studies Using the PharmGKB Website
Editor: Wade Powell
Published online:
Abstract
Cytochrome P450 (CYP) enzymes are important regulators of drug efficacy and toxicity. Genetic variation in CYP isoforms can impact how well patients respond to medications or experience unwanted toxicities. PharmGKB is an online pharmacogenomics resource that collates the latest data and clinical guidelines on genetic variation and drug responses. The purpose of this lesson was to develop an interactive, case-based activity that demonstrated how pharmacogenetics can be used to influence the prescribing of medications. This lesson was provided to 71 students during a two-hour online interactive session. The lesson consisted of (a) a didactic lecture on pharmacogenetic principles, (b) an overview of the PharmGKB website by the instructor, and (c) patient cases that used the PharmGKB website to answer questions and make recommendations about drug therapy. Patient cases explored the impact of genetic variation in CYP enzymes on patients prescribed medications for different diseases including depression (citalopram, CYP2C19), pain (codeine, CYP2D6), organ transplantation (tacrolimus, CYP3A5), and viral infection (efavirenz, CYP2B6). Four additional cases are included in this lesson. Students reviewed the patient cases in small groups, used PharmGKB to answer questions and design treatment plans, and presented their recommendations to instructors and other students. Based on pre-/post-lesson assessment questions and student feedback, we conclude that an interactive, group-based activity can be used to teach basic principles of pharmacogenetics and connect students to online resources for drug dosing.
Primary Image: The metabolism of drugs by the liver can determine not only how well they work, but also the side effects a patient experiences. This lesson explores how a patient’s genetics influence the metabolism of a drug and in turn, its effectiveness and toxicity.
Citation
Mosquera AM, Aleksunes LM. 2023. Engaging Students in Pharmacogenetics: Patient Case Studies Using the PharmGKB Website. CourseSource 10. https://doi.org/10.24918/cs.2023.10Society Learning Goals
Genetics
- Transmission - Patterns of Inheritance
- How can one deduce information about genes, alleles, and gene functions from analysis of genetic crosses and patterns of inheritance?
Toxicology
- Biological information
- What effects can the environment have on gene expression?
- What differences occur in how individuals or populations are affected by exposure to different doses of a toxicant?
- Systems toxicology
- How are body systems affected by exposure to toxicants?
- Pathways and transformations of energy and matter
- How can ADME be quantified using toxicodynamics and toxicokinetics (TDTK)?
Lesson Learning Goals
Students will:- compare and contrast pharmacokinetics and pharmacodynamics.
- learn how CYP enzymes metabolize drugs to more or less active forms.
- understand how genetic variation affects a patient’s response to medications including their toxicity.
- utilize an internet database to evaluate how a patient’s genotype influences their phenotype to metabolize drugs.
- identify the four CYP metabolizer types (poor, intermediate, extensive/normal, and ultrarapid) based on a patient’s genotype.
- recommend medication therapy based upon the CYP genotype of a patient.
Lesson Learning Objectives
Students will be able to:- explain the pharmacokinetics and pharmacodynamics of a drug.
- demonstrate their understanding of basic pharmacogenetic concepts.
- derive possible genotype combinations of offspring when provided parental genotypes.
- describe how different CYP phenotypes impact clinical responses to drugs (both effectiveness and toxicity).
- utilize the PharmGKB website as a source of a genotypic and phenotypic data.
- work collaboratively to optimize drug therapy for a hypothetical patient based on genetics.
- define toxicokinetics, pharmacokinetics, toxicodynamics, and pharmacodynamics.
- describe how genetic factors (pharmacogenetics) can influence xenobiotic metabolism in the body.
- describe genetic polymorphisms that affect toxicokinetics and risk.
- explain how differences in individuals result in differences in susceptibility of a population to toxicants.
- design genetic crosses to provide information about genes, alleles, and gene functions.
- evaluate how genes and the environment can interact to produce a phenotype.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
Introduction
Personalized or precision medicine aims to improve treatment outcomes by replacing the traditional model in medicine that ‘one size fits all’ with an individualized patient-specific approach. One source of interpatient variation results from genetic differences in the enzymes that metabolize medications. Cytochrome P450 (CYP) enzymes are responsible for metabolizing many drugs currently on the market. CYPs are expressed at high levels in the liver and their activity can convert an inactive prodrug to an active form. Alternatively, CYPs can aid in eliminating drugs from the body thereby reducing their activity. Genetic variation and polymorphisms in CYP enzymes can determine whether a patient’s response to a particular drug yields a beneficial (considered ‘pharmacologic’) or a harmful (considered ‘toxic’) response. In clinical practice, how drugs interact with one or more CYP enzymes is an important determinant in designing treatment plans. Although the pharmacogenetics of CYP-mediated metabolism is increasingly being used to support the practice of personalized medicine, students of all education levels often have limited understanding of (a) the basic concepts of pharmacogenetics, (b) where to obtain useful information, and (c) how to use pharmacogenetics to inform clinical drug recommendations. Despite these challenges, there is interest and enthusiasm amongst students to implement pharmacogenetic content into health science and genetics curricula (1).
The Pharmacogenomics Knowledge Base (PharmGKB) is a publicly available internet resource that collates data on gene summaries, signaling pathways, and annotated literature related to drug metabolism, pharmacokinetics, pharmacodynamics, and toxicity. PharmGKB attracts a wide range of users, including students and postdoctoral fellows, clinical scientists, and researchers (2). This database connects students and healthcare professionals instantly to pharmacogenetic data by generating information on gene-drug associations and dosing guidelines. Of the 141 medications with clinical guideline annotations on PharmGKB in 2022, 103 are metabolized by a CYP enzyme. The drug dosing guidelines provide a basis for personalized medicine by showcasing how variations of the CYP alleles determine what an optimal treatment would be for a patient. However, there are few educational resources that teach users how to utilize PharmGKB to guide pharmacotherapy recommendations and apply basic principles of pharmacogenetics.
In this lesson, students were presented with an interactive lecture on pharmacogenetics and tasked with applying genetic concepts to review a patient case, answer pharmacology questions, and develop a clinical recommendation about drug therapy. Learners were comprised of rising high school juniors and seniors with an interest in STEM careers who were completing a 1-week toxicology-focused intensive summer program at the university. This activity was held in the second half of the week after students had learned the basics of dose-response toxicant relationships, forensics, pharmacokinetics, and cell death pathways. Each patient case required students to work in small groups and consider possible genotype-phenotype relationships for their patient based on the diplotype of each parent. Students used PharmGKB to evaluate patient genotypes and extract key information and recommendations for drug therapy when answering case questions. The primary aims of this lesson were to provide students with the opportunity to learn and apply aspects of pharmacogenetics through a group-based learning activity using PharmGKB. This lesson has broad applicability to undergraduate courses in biology, biochemistry, genetics, pharmacology, toxicology, and molecular biology.
Intended Audience
This lesson is intended for undergraduate biology-related majors at a university or in a professional program including entry level pharmacy, nursing, pre-medicine, and pre-health science.
Required Learning Time
The didactic lesson lasts for 45 minutes (either live or flipped classroom) followed by interactive case studies for 50–60 minutes and optional case presentations (5 minutes/case).
Prerequisite Student Knowledge
Key knowledge that learners should have include the basics of genetics (i.e., DNA structure, Punnett squares), inheritance of alleles (i.e., dominant-recessive versus additive/co-dominance), side effects of medications (i.e., therapeutic index), and cytochrome 450-mediated metabolism of drugs (i.e., bioactivation versus detoxification).
Prerequisite Teacher Knowledge
Instructors should practice using the PharmGKB website including the dropdown boxes for genotypes/phenotypes, as well as understand the cytochrome P450-mediated metabolism of all drugs used in the case studies and basic principles of pharmacogenetics/genomics.
Scientific Teaching Themes
Active Learning
This interactive session includes polling questions, open ended questions, case based learning, use of an online database, and small group learning. Polling questions provide students the ability to anonymously participate and reflect on the instructional material (3). Open-ended questions about internal and external factors that influence how drugs work in different individuals allow students to share different perspectives that may include social determinants of health. Use of clinical cases enables the translation of basic knowledge about genetics and drug metabolism to hypothetical patients. This approach provides scenarios that reinforce the ‘real world’ nature of pharmacogenetics. Since its inception, the potential for using PharmGKB as an educational tool has been proposed (4, 5), though few structured activities have been disseminated for use in the classroom. PharmGKB continues to be a premier resource for collating pharmacogenetics knowledge and can serve as a great resource for future clinicians or scientists (6). Case studies follow a standardized approach and enable students to showcase their knowledge about disease, drug therapies, use of web resources, and genetics.
Assessment
Student understanding of concepts was assessed using pre- and post-lesson polling with three questions (Table 1). The first question focused on basic pharmacology and the other two questions focused on pharmacogenetics. As this activity was run during a summer program, students were asked to rate the likelihood that they would recommend this activity to their colleagues (Table 1). A five-point Likert scale was applied to these responses to assess the collective evaluation of the students. Lastly, students were also asked to provide a word to describe what they learned during this session (Table 1)—these words were used to assemble a word cloud in real-time.
Table 1. Assessment questions. Asterisks (*) denote correct answers.
| Where Question Is Included | Type of Question | Questions |
|---|---|---|
| Pre- and Post-Lesson | Multiple Choice Question 1 |
What does the term ‘pharmacodynamics’ mean?
|
| Pre- and Post-Lesson | Multiple Choice Question 2 |
Which are more common: mutations or polymorphisms?
|
| Pre- and Post-Lesson | Multiple Choice Question 3 |
Gene duplication leads to which of the following phenotypes?
|
| Post-Lesson | Overall Activity Assessment |
How likely would you recommend this session to your colleague?
|
| Post-Lesson | Word Cloud | Use one word to describe what you learned today. |
Inclusive Teaching
During the interactive session, students work as a group to investigate a clinical case and formulate therapeutic recommendations for a hypothetical patient. This format allows students to assume different roles (reading case, looking up information, populating data, etc.) based on their comfort level. As part of the summer program, instructors and participants are asked to identify and use preferred personal pronouns while working in small groups (7). While the race and ethnicity of hypothetical patients were not included in the case histories, the genetic variants used reflect a wide array of backgrounds including those that may be marginalized in health care systems. Likewise consider more inclusive terms such as ‘variant’ rather than ‘mutant’ when describing alleles (8).
Lesson Plan
Pre-Lesson Preparation and Assessment
Before introducing this activity, the instructor should familiarize themself with PharmGKB. As they navigate to the PharmGKB homepage, two primary sections should be noted as they will be used in this activity: (a) Curated Pathways and (b) Clinical Guideline Annotations. PharmGKB is routinely updated as new CPIC guidelines are received and more information about pharmacokinetic and pharmacodynamic pathways are elucidated.
In 2021, this lesson was taught to 71 students who participated in the pre-college Toxicology, Health, and Environmental Disease High School Program at our university. Due to the ongoing pandemic, this activity was run entirely over Zoom however it is entirely adaptable to in-person instruction. The students in this summer program were largely enrolled in advanced placement science courses and/or attended a STEM or biomedical-focused high school. We encourage instructors to use the case questions and polling questions at a level appropriate to their learner population. This lesson can be divided into a didactic lecture that delivers information on pharmacogenetic concepts in a synchronous or asynchronous flipped classroom setting, and an activity portion that will both demonstrate an example case study and test the students’ knowledge of these concepts through group-based case studies. If students or instructors need assistance pronouncing drug names, there are online resources with pronunciation guidance.
Students will begin the lesson by answering pre-lesson questions through live polling conducted via a response service. The assessment questions target basic concepts of pharmacology and pharmacogenetics to establish the baseline comprehension of the students.
Didactic Instruction
Students are presented with a 45-minute didactic lecture on introductory pharmacology and pharmacogenetics (Table 2, Supporting File S1). Topics include a comparison of pharmacodynamics and pharmacokinetics, CYP-mediated metabolism, personalized/precision medicine, genetic polymorphisms and mutations, and the implications of genotypic and phenotypic differences. The instructor will also ask questions about the material pertaining to the slides to encourage active discussion, reflection, and critical thinking. The final portion of lecture includes instructions on how to use the PharmGKB database to search dosing guidelines for different drugs.
Table 2. Timeline of didactic instruction. The lesson provides an overview of pharmacogenetic and genetic concepts while actively asking students questions relating to the material. The didactic portion of this activity can be delivered synchronously or as an asynchronous flipped classroom video using Supporting File S1. This portion of the lesson is estimated to last ~45 minutes.
| Slides | Description | Time | Details of Instruction |
|---|---|---|---|
| 1–3 | Explain the differences between pharmacology and toxicology | ~2 min | A brief overview on the similarities and differences between pharmacology and toxicology should be introduced. |
| 4–6 | Explain pharmacodynamics and pharmacokinetics | ~8 min | On slide 6, four scenarios are described. The instructor should ask whether the scenario describes pharmacokinetics (PK) or pharmacodynamics (PD). Answers are as follows: neuron PD, intestinal disease PK, grapefruit PK, and cancer cells PD. These can be converted to polling questions or done as open Q-and-A. |
| 7 | Explain the role of cytochrome P450 enzymes in metabolism | ~5 min | Explain how CYP-mediated metabolism can 1) take an inactive parent drug (or prodrug) and convert it to an active drug or 2) participate in the clearance of an active drug from the body which will reduce its availability to alter pharmacodynamics. |
| 8–9 | Discuss personalized/ precision medicine | ~3 min | Ask students to describe some factors (either internal or external) that influence how an individual responds to a medication. Answers can include genetics/race/ethnicity, age, diet, smoking, co-morbidities, environment, socioeconomic status, etc. This question can be done as an open Q-and-A, word cloud, up/down voting, etc. |
| 10–12 | Explain basics of genetics and pharmacogenetics | ~5 min | Another example of comparing genotype and phenotype is eye color. The instructor can discuss the genetics of how two brown-eyed parents can have a blue-eyed child. In that case, both parents would be have 1 copy of a blue gene and 1 copy of a brown gene. Since blue is recessive, the parent's eye color would be brown. The child could inherit two copies of blue genes. |
| 13–17 | Review genetic variation including differences between mutations and polymorphisms | ~5 min | There are a number of key terms that students should understand in this section including mutations, polymorphisms, SNP, and gene duplication. For advanced learners, the calculation of activity scores such as for CYP2D6 can be added here. |
| 18–20 | Define genotype and phenotype | ~5 min | This section provides terminology for describing combinations of genes from both parents (alleles) to determine their genotype. Key definitions for homozygous, heterozygous and phenotype are provided. A brief explanation of how alleles are named is provided. |
| 21 | Compare inheritance according to the dominant-recessive model and the additive/co-dominance model | ~3 min | The example of eye color from before can be repeated for explaining the dominant-recessive model and a person's height can be used to explain the additive/co-dominance model. |
| 22–27 | Provide an overview of the PharmGKB website | ~10 min | Encourage students to go to the website and follow along as you review how to access clinical guideline annotations. Explain that you will provide direct links for important guidelines they need to access in the case study activity. Provide an example of using the dropdown options to assign a patient's alleles in order to determine their genotype, phenotype, and the clinical recommendations for drug therapy. |
Demonstration Case
Prior to completing their own patient case, students will be shown an example case study by the instructor (Table 3, Supporting File S2). The instructor will provide a brief background of the case study which is a cancer patient prescribed the antiemetic drug ondansetron to prevent the nausea and vomiting associated with chemotherapy. The key pharmacogene in this case is CYP2D6, one of the most polymorphic CYPs. CYP2D6 is responsible for the hydroxylation of ondansetron to a form that can be readily excreted from the body.
Table 3. Case studies. This portion of the lesson includes an example case study narrated by the instructor as well as a timeline for students to work in groups on case studies. This portion of this activity should be delivered synchronously using slides in Supporting Files S2 and S3. The instructor-led case study is estimated to last ~20 minutes. The group-based student case studies are estimated to last ~30 minutes. If time is available, each group of students can present their completed patient cases.
| Slides | Description | Time | Details of Instruction |
|---|---|---|---|
| Instructor-Led Example Case Study | |||
| 2–3 | Background information and case study | ~5 min | Clicker question on side effects of chemotherapy (answers: nausea, vomiting, hair loss, organ damage, infections, ulcers, etc.). |
| 4–10 | Use of PharmGKB website to identify pharmacodynamics information | ~3–4 min | This demonstration be done with embedded screen grab slides (as provided) or live using web browser on PharmGKB. |
| 11 | Review the CYP-mediated metabolism of the drug being prescribed and identify CYP pharmacogene | ~2 min | For any case studies, it is important to review the role of the CYP isoform in bioactivation to an active metabolite or its role in drug clearance and excretion. |
| 12 | Provide the genotypes for the CYP pharmacogene for the patient's parents | ~3–4 min | Ask students for the 4 possible combinations of genotypes that the patient may inherit (based on parent's diplotype). |
| 13–16 | Determine the phenotypes associated with each genotype and drug therapy recommendations | ~5 min | This demonstration be done with embedded screen grab slides (as provided) or live using web browser on PharmGKB. |
| 17 | Reveal patient's genotype and discuss therapeutic recommendations based on their phenotype | ~2 min | Beyond providing the patient's ultimate genotype, this information is extracted directly from the prior slide. |
| Group-Based Case Studies | |||
| 1–2 | Case scenario | ~2–3 min | One person should start by reading the case. Students should look up any medical terms they are unfamiliar with. Where possible, each group should collaborate on the same document (via web upload). |
| 3 | Drug therapy | ~5 min | The drug of interest is introduced to the group. The group should use the PharmGKB link to look up the pharmacodynamics (mechanism of action) for the drug and add the answer to Question 1. |
| 4 | Pharmacogene | ~2 min | The CYP pharmacogene is introduced and its role in altering drug pharmacokinetics is explained. In some cases, the CYP enzyme is involved in the inactivation and clearance of the drug; in other cases, the CYP enzyme is responsible for generating the active metabolite. |
| 5–6 | Possible genotype and phenotype scenarios | ~15 min | The genetics of the parents are provided. All students should identify the 4 possible combinations of alleles that could be inherited as unique genotypes. Then, all students should use the PharmGKB link to look up the associated phenotypes (intermediate, poor metabolizer, etc.) and the recommendations for drug therapy. This information should be added to the table to answer Question 2. |
| 7 | Final recommendations for drug therapy | ~3–5 min | The students should be provided the genotyping results at this point and asked to answer Questions 3 and 4 based on the information they collected in the table in Question 2. |
| Final | Case presentation | ~5 min/ case | If time is available, each group should present their case to the class. Each member of the group should present 1–2 slides. |
As the instructor progresses through the case, four questions are presented that will mirror those assigned in the student group cases. The questions are to (a) describe the pharmacodynamic mechanism of their assigned drug (using PharmGKB), (b) derive the possible genotype combinations of the patient using parental genotypes, (c) identify clinical recommendations associated with each genotype combination (using PharmGKB), and (d) determine the best course of treatment for the patient when their genotype is revealed by the instructor. Screen captures of where to look for the answers on PharmGKB are provided in Supporting File S2. Alternatively, the instructor can go directly to the PharmGKB website and walk students through the key webpages to identify the answers to the questions in the case.
Student Group-Based Case Studies
Students will be divided into small groups and separated into Zoom breakout rooms. Table 4 provides an overview of the eight patient cases (Supporting File S3). Cases were designed to cover a wide range of diseases and medications. In each case, a CYP isoform is polymorphic and variation in its activity can impact clinical outcomes. Cases 1, 3, 5, and 7 include CYP enzymes that are responsible for the clearance or removal of the drug from the body (Table 4). For these cases, ultrarapid CYP metabolizers will generally reduce therapeutic concentrations of these drugs and impair how well the drugs improve the patient’s condition. By comparison, poor metabolizers in these cases have the potential for slow removal and in some situations, this can increase drug toxicity to the patient. Cases 2, 4, 6, and 8 include CYP enzymes that are required to bioactive the parent drug to an active form (or metabolite) (Table 4). Without this metabolism, there will be no therapeutic benefit for the patient.
Table 4. Patient cases, drugs, pharmacogenes, and clinical guidelines. The table lists the relevant pharmacokinetic information associated with the pharmacogene for each patient case.
| Case | Clinical Indication | Drug | Pharmaco-gene | Role of CYP in Drug Therapy1 | Annotated Clinical Guidelines2 | Clinical Guidelines3 |
|---|---|---|---|---|---|---|
| 1 | Depression | Citalopram | CYP2C19 |
Clearance/ Excretion |
Citalopram Annotated Link | Citalopram Link |
| 2 | Analgesia | Codeine | CYP2D6 | Bioactivation | Codeine Annotated Link | Codeine Link |
| 3 | Transplantation | Tacrolimus | CYP3A5 |
Clearance/ Excretion |
Tacrolimus Annotated Link | Tacrolimus Link |
| 4 | Anticoagulation | Clopidogrel | CYP2C19 | Bioactivation | Clopidogrel Annotated Link | Clopidogrel Link |
| 5 | Viral infection | Efavirenz | CYP2B6 |
Clearance/ Excretion |
Efavirenz Annotated Link | Efavirenz Link |
| 6 | Cancer | Tamoxifen | CYP2D6 | Bioactivation | Tamoxifen Annotated Link | Tamoxifen Link |
| 7 | Fungal infection | Voriconazole | CYP2C19 |
Clearance/ Excretion |
Voriconazole Annotated Link | Voriconazole Link |
| 8 | Attention deficit hyperactivity disorder | Atomextine | CYP2D6 | Bioactivation | Atomextine Annotated Link | Atomextine Link |
1For some drugs, the CYP-mediated metabolism causes clearance or removal of the drug from the body thereby decreasing its activity. In other cases, the CYP enzyme is needed to bioactivate or convert the drug to an active form. Notably for atomoxetine, both the parent drug and its metabolite are active in the body.
2Annotated clinical guidelines should be used by students for completion of cases.
3The full clinical guidelines are provided for reference as well.
If moderators or teaching assistants are available, they can oversee each of the breakout rooms. Instructors or moderators may take the opportunity to assign students within the group a team role while completing each case question. Two students should act as the readers of the case questions, one student will share their zoom screen while also being assigned the role to write the group’s answers for their respective case question, and everyone else in the group will proceed to the PharmGKB website to locate answers to case questions.
The first assigned reader will read the hypothetical patient scenario (2nd slide of each case). The moderator can then ask one or two questions about the case that may be in reference to the patient’s symptoms or the doctor’s diagnosis. This discussion allows students to ask questions about the clinical case. At groups progress to the 3rd slide of each case, the moderator should remind students that ‘pharmacodynamics’ is what the drug does to the body (mechanism of action) in preparation for the use of PharmGKB. In the first question of the case, students will identify how the drug prescribed works to treat the patient’s condition. Answers to this question are derived from the pharmacodynamics section of PharmGKB.
The students should then review the basic pathway of the drug and its associated CYP-mediated metabolism on the 4th slide of the case. In each case, the CYP isoform is polymorphic and serves as the key pharmacogene. As student groups progress to the 5th slide of the case, they are provided the genotypes of the parents of the patient for the key pharmacogene. Recognizing that the patient inherits one allele from their mother and one allele from their dad, four combinations of inheritance are possible. On the 6th slide of each case, students should start by listing the four possible genotype combinations in the initial column. The student group should then return to PharmGKB where they will follow the clinical guidelines. The annotated guidelines on PharmGKB contain drop down boxes that make it easy for students to look up genotypes, phenotypes, and clinical recommendations. These links are provided in the case study slides questions (Supporting File S3) and in Table 4. Students should click the dropdown box and input the four possible combinations the patient could potentially inherit. For each possible genotype combination in the dropdown box, the students should record the phenotype (poor, extensive/normal, intermediate, or ultrarapid) and clinical implications associated with each possible genotype in the table on the 6th slide. On the final (7th) slide, the instructor should inform each group of the actual genotype of the patient. Using the table they have assembled, the students should answer the final questions regarding the patient’s phenotype based on their genotype and make treatment recommendations based on their phenotype.
After the case study has been completed by the group, one or two slides can be assigned to each group member for presentation to the class. The group will be expected to present their slides at the end of the session. Groups should practice their presentation if time permits. The student previously assigned to share their screen to complete the activity will be responsible for sharing their screen during their group’s presentation.
Post-Lesson Assessment
After students have completed the patient case in their learning groups, everyone is brought back together in the main Zoom room. All groups or a select number of groups can present their cases. While the general format of each case is similar, students may find it interesting to hear the various clinical scenarios. After case presentations, students are asked to answer the same three multiple choice questions from the beginning of the lesson (Table 1). Students are then asked to describe how likely they are to recommend this activity to a friend (Table 1). The activity is completed with an open response question that asks students for a single word that would describe what they had learned from the lesson. These responses are used to form a word cloud.
Teaching Discussion
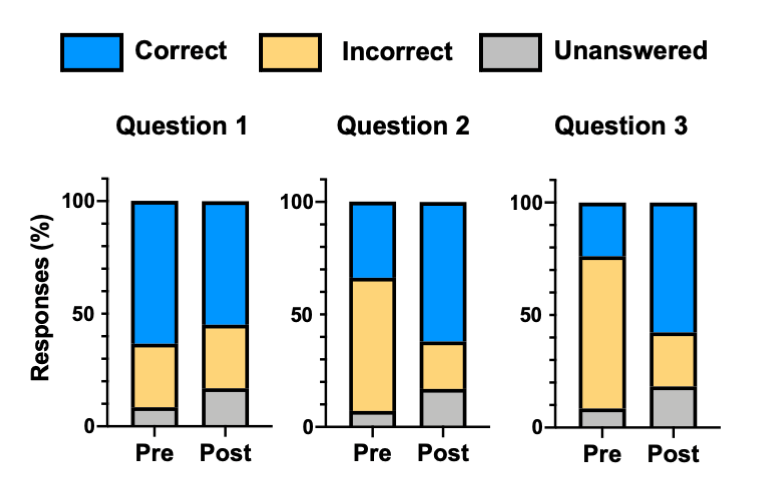
We assessed student comprehension using three pre-/post-lesson multiple-choice questions administered using PollEverywhere (Table 1). While the patient case activity was performed as a group, all pre-post assessments were completed individually. Student answers were classified as either correct, incorrect, or unanswered. Normalized gain calculations [
The first question was designed to evaluate overall pharmacology knowledge whereas the other two questions were based largely on the pharmacogenetics content (Figure 1). Interestingly, for question 1, there was a negative gain (-23%). Notably, more than 60% of students answered this question correctly during the pre-lesson assessment. This likely was due to the concept being previously covered in an overview pharmacology earlier in the day during the summer program. Likewise, the number of students that did not answer this question on the post-lesson assessment doubled, which may have accounted for this negative gain. For the two pharmacogenetics-related questions, there were positive learning gains of 43% for question 2 and 44% for question 3. These results suggest that the content within this didactic lesson and interactive training activity improved students understanding of pharmacogenetics, CYP metabolism of drugs, and personalized prescribing of medications.
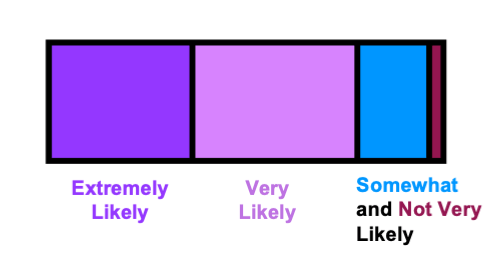
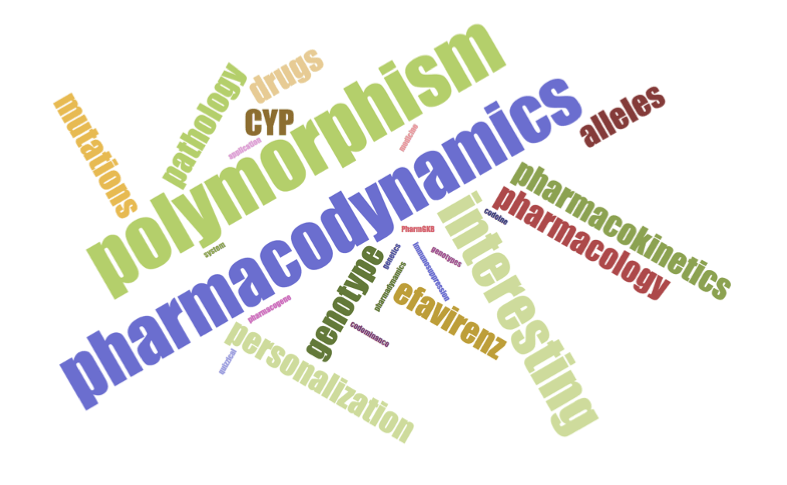
Following completion of the activity, students were asked their likelihood to recommend this activity to their colleagues (Figure 2). Over 75% were either ‘very likely’ or ‘extremely likely’ to recommend this activity. In addition, a word cloud was generated to summarize the students’ reflection of the lesson and activity with one-word answers (Figure 3). According to the word cloud, the most frequent answer was ‘pharmacodynamics’. Other common words that students reported included ‘genotype’ and ‘alleles’. Through the results of pre-/post-lesson assessments and the word cloud, students demonstrated an ability to use pharmacogenetic principles to evaluate treatment options for hypothetical patient scenarios.
This activity can be modified in many ways for use in a summer experience, workshop, or classroom setting. If there is limited time for the synchronous meetings, the didactic content can be delivered as a video using a ‘flipped’ format. Or within a course, the didactic session can be given in one lecture period and the interactive activity performed in subsequent lecture period. Likewise, the cases can be assigned to the teams to work on outside of class and presented at the next course meeting. In addition to the initial cohort described in the lesson plan, we have run this activity with two additional student groups: (a) undergraduate students at the annual meeting of the Society of Toxicology and (b) additional pre-college students during an in-person version of the toxicology summer program. In the first example, over 100 undergraduate students were divided into 14 groups with mentors (established toxicologists and toxicology graduate students). These students ranged from sophomore to seniors from undergraduate programs across diverse majors (biochemistry, molecular biology, biomedical science, chemistry, forensic science, public health, microbiology and more). Over half of these students had experience working in a toxicology research laboratory. The didactic content was provided as an asynchronous video that participants watched before the in-person session. Over 90% of participants who answered the post-survey (N=27) agreed or strongly agreed that this activity stimulated their interest in toxicology and research and provided useful new information. The second cohort were 54 high school students participating in our long-standing toxicology summer program. Due to relaxation of pandemic restrictions, students were able to return to on campus participation. A key change that was made for in-person learning was the provision of QR codes that allowed students to readily access the various PharmGKB web links on their phones and devices. Similarly, condensing the didactic material into a pre-video that the students watched the day before the activity enabled more time for group work and completion of additional cases.
At the close of the activity students are encouraged to ask questions. The most frequent questions are related to the extent of implementation of pharmacogenetic-based drug dosing currently in clinical practice. Students have typically not observed this level of precision in medicine for themselves, family members, or caregivers. To address this question, it is best to give key examples where the benefit of genetic-based selection and optimization of drug therapy has typically outweighed the costs and delays associated with genetic testing. Examples include warfarin anticoagulation clinics (notably, considering more than just genetics) as well as psychiatric clinics and comprehensive cancer care. The lesson can then conclude with a general discussion on the direction of precision medicine to optimize healthcare on an individual level.
Supporting Materials
-
S1. Engaging in Pharmacogenetics – Didactic Lecture Slides. These PowerPoint slides are used by the instructor to provide an overview of pharmacogenetic and genetic concepts. Interactive questions are included. The didactic portion of the lesson can be delivered synchronous or as an asynchronous flipped classroom video. A recorded version of the lecture is available at this Kaltura webpage. More details about the timeline for these slides are provided in Table 2.
-
S2. Engaging in Pharmacogenetics – Example Case Study. These PowerPoint slides are used by the instructor to provide an overview of how to complete a patient case using PharmGKB. More details about the timeline for these slides are provided in Table 3.
-
S3. Engaging in Pharmacogenetics – Student Case Studies. These powerpoint slides are used by student groups to review patient questions, answer questions, and develop pharmacotherapy recommendations. More details about the timeline for these slides are provided in Table 3. An overview of the patient cases is also provided in Table 4. Answers are shown in red and should be deleted before distributing to students.
Acknowledgments
Assessment of this activity was reviewed and approved by the Rutgers Institutional Review Board (Pro2021001900). This work was supported by the National Center for Advancing Translational Sciences [Grant ULTR003017] and the National Institute of Environmental Health Sciences [Grant ES005022], components of the National Institutes of Health.
References
- Coriolan S, Arikawe N, Moscati A, Zhou L, Dym S, Donmez S, Garba A, Falbaum S, Loewy Z, Lull M, Saad M, Shtaynberg J, Obeng AO. 2019. Pharmacy students' attitudes and perceptions toward pharmacogenomics education. Am J Health Syst Pharm 76:836–845. doi:10.1093/ajhp/zxz060.
- Owen RP, Klein TE, Altman RB. 2007. The education potential of the pharmacogenetics and pharmacogenomics knowledge base (PharmGKB). Clin Pharmacol Ther 82:472–475. doi:10.1038/sj.clpt.6100332.
- Sarvary MA, Gifford KM. 2017. The benefits of a real-time web-based response system for enhancing engaged learning in classrooms and public science events. J Undergrad Neurosci Educ 15:E13–E16.
- Gong L, Owen R, Gor W, Altman R, Klein T. 2008. PharmGKB: An integrated resource of pharmacogenomic data and knowledge. Curr Protoc Bioinforma 23:14.7.1–14.7.17. doi:10.1002/0471250953.bi1407s23.
- Rubin DL, Carrillo M, Woon M, Conroy J, Klein TE, Altman RB. 2004. A resource to acquire and summarize pharmacogenetics knowledge in the literature. Stud Health Technol Inform 107:793–797. doi:10.3233/978-1-60750-949-3-793.
- Caudle KE, Klein TE, Hoffman JM, Muller DJ, Whirl-Carrillo M, Gong L, McDonagh EM, Sangkuhl K, Thorn CF, Schwab M, Agundez JA, Freimuth RR, Huser V, Lee MT, Iwuchukwu OF, Crews KR, Scott SA, Wadelius M, Swen JJ, Tyndale RF, Stein CM, Roden D, Relling MV, Williams MS, Johnson SG. 2014. Incorporation of pharmacogenomics into routine clinical practice: The Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab 15:209–217. doi:10.2174/1389200215666140130124910.
- Weiler T, Caton E. 2021. Evidence-based practices for culturally responsive medical education. Med Sci Educ 31:2199–2207.
- Hales KG. 2020. Signaling inclusivity in undergraduate biology courses through deliberate framing of genetics topics relevant to gender identity, disability, and race. CBE Life Sci Educ 19:es2.
Article Files
Login to access supporting documents
Mosquera-Aleksunes-Engaging Students in Pharmacogenetics Patient Case Studies Using the PharmGKB Website.pdf(PDF | 357 KB)
S1. Engaging in Pharmacogenetics - Didactic Lecture Slides.pptx(PPTX | 2 MB)
S2. Engaging in Pharmacogenetics - Example Case Study.pptx(PPTX | 6 MB)
S3. Engaging in Pharmacogenetics - Student Case Studies.pptx(PPTX | 465 KB)
- License terms

Comments
Comments
There are no comments on this resource.