Teaching the Photoelectric Effect Using an Open-Source Interactive Simulation
Editor: Jennifier Knight
Published online:
Abstract
The photoelectric effect is one of the fundamental experiments that established the basis of quantum mechanics. Students studying this experiment in modern physics courses struggle to understand what it actually measured and why it is significant. This lesson is built around the PhET interactive simulation of this experiment. The activity involves the use of this open-source online simulation to carry out simulated experiments exploring the emission of electrons from metal surfaces when light is shone on them, and to compare those results with the predictions of the classical theory of light and its interactions with matter. This shows why Einstein’s quantum interpretation is needed to explain the observed behavior. Students complete a worksheet that guides them through the simulated experiment and comparison of observations with predictions of the classical theory of light and matter interactions. Then they are given the quantum interpretation, including exploring analogies to help develop their understanding. This presentation is supported by having students answer and discuss a set of questions in small groups. This lesson has achieved greatly improved student mastery of this fundamental experiment and how it shaped physics.
Primary Image: Image of PhET photoelectric effect simulation. This is a screenshot of the simulation which represents the apparatus used to study the photoelectric effect and is the heart of this lesson.
Citation
Wieman CE. 2023. Teaching the Photoelectric Effect Using an Open-Source Interactive Simulation. CourseSource 10. https://doi.org/10.24918/cs.2023.34Lesson Learning Goals
Students will:- understand how the photoelectric effect experiment was carried out and what results it found.
- recognize how those results are in conflict with the classical theory of light and matter interactions and why the quantum theory is required to explain them.
- recognize the significance of the photoelectric effect experiment in establishing the foundation of quantum mechanics.
Lesson Learning Objectives
Students will be able to:- qualitatively describe what is measured in the photoelectric effect experiment and explain how this implies a quantum picture of light, including explaining what results the classical theory of light would predict for this experiment.
- quantitatively analyze photoelectric data to deduce the relationship between energy of photons and frequency of light and predict how the emission of electrons will change if color of light, type of metal, or voltage of the metal is changed.
- design an experiment for determining the composition of an unknown pure metal using the photoelectric effect.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
Introduction
The photoelectric effect is one of the most important experiments in physics. It looked at the rate and energy of electrons emitted from metal surfaces due to light shone on the surface. This experiment studied the dependence on the frequency of light, the type of metal, and the time between the initiation of the light and the first emission of electrons. All of these observations gave results that were in conflict with the predictions of the classical theory of light and its interactions with matter that prevailed at the time, the start of the 20th century. These results were one of the earliest and clearest indications of the failure of classical physics, and the explanation of the results, notably by Einstein in 1905, was a foundational step in the development of quantum physics. As such, the photoelectric effect is studied in every introductory course in modern physics or quantum mechanics. However, studies have shown that standard instruction on this material leaves most students with a very weak understanding (1–3). They do not understand the experimental setup, the results obtained, or the implications of those results. References 1 and 2 provide a detailed list of specific student difficulties with this topic. To deal with this instructional challenge, we worked with the PhET team to develop an interactive simulation that simulated this experiment, allowing students to change all the relevant variables and see the results. The simulation also showed simulated electrons and their behavior, something not visible in the real world.
The lesson is built around this simulation. It first introduces students to the classical predictions for light and its interactions with matter. For some but not all students this is a review. The heart of the lesson is a worksheet that students then complete, in which they must carry out a series of simulated experiments with the simulations, exploring all the important dependencies and recording what they observe. They are also called upon to say if their results are consistent or not with the predictions of the classical theory of light. Then we give them the quantum interpretation, including exploring analogies to help develop their understanding. This presentation is supported by having students answer and discuss questions in small groups.
Intended Audience
The intended audience for this lesson is students in an introductory modern physics or quantum mechanics course. Such courses are typically taken by physics and many engineering majors in their second year at universities but are also offered at community colleges. As this lesson involves little mathematics, it would also be suitable for use in an algebra-based introduction to modern physics course that might be offered as a science distribution requirement, or as a modern physics unit in a general introductory physics course.
Required Learning Time
This lesson would typically be carried out in a 1.5-hour class, or in two 1-hour classes.
Prerequisite Student Knowledge
Students should know the basic concepts of what light is, what an electron is, and the composition of matter in terms of atoms and their component electrons. They should know that light is made up of waves that are characterized by their frequency, and equivalently by their wavelength, and that different colors represent light of different frequencies. It is desirable for students to be familiar with the classical theory of light and how it interacts with matter. In my experience, most students are familiar with light, electrons, and the composition of matter, and many but not all students in the target audience have had experience with the classical theory of light, but few have a solid understanding.
Prerequisite Teacher Knowledge
The teacher needs to be familiar with the experimental setup used in photoelectric effect experiments, the results of these experiments, what the classical theory would predict, and how observations are in conflict with classical predictions. They need to have a good understanding of Einstein’s theory of the photoelectric effect, particularly the assumptions that go into it regarding the quantum nature of light and how this is manifested in the interaction with matter. They need to have mastery of all of the different controls and display options in the photoelectric effect simulation and the results it will produce. A rewarding feature of this lesson is that it triggers many deep and subtle questions from the students about the photoelectric effect and its interpretation. An instructor needs to be prepared for getting questions they cannot answer, and to be willing to tell students they don’t know the answer but will find out, and then consult the literature for the answers.
Scientific Teaching Themes
Active Learning
There are several forms of active learning in this lesson. First, and most important, is a worksheet each student must complete while working in a small group. This worksheet calls on them to explore many different features with the simulation and record and interpret their results. In the follow-up discussion and elaboration on that worksheet activity, there are several clicker questions and small-group discussion activities. This lesson would typically be followed with a homework assignment in which students have to do further exploration with the simulation and further explain their results in terms of how the results show the failure of the classical theory and the need for quantum theory.
Assessment
The impact of this lesson has been assessed in several ways. In the typical class we look at how students complete the worksheet and how they perform on the clicker questions. There are also homework and exam questions closely aligned with the learning objectives of the lesson. For three years we asked the same two extensive questions on exams that had been used in previous research on student learning of the photoelectric effect. The numbers and materials were changed between years.
Q1. Suppose you were to perform the photoelectric effect experiment using light with a wavelength of 400 nm and a target made of cadmium. You find that when the voltage measured across the electrodes V is equal to zero volts, the ammeter reads zero current. Would the ammeter read zero current or a non-zero current if you were to:a. Double the intensity of the light source on the cadmium target? Explain your reasoning.
b. Increase the voltage V of the battery from 0 volts to +5.0 volts (using the cadmium target)? Explain your reasoning.
c. Replace the cadmium target with one made of sodium but with the original intensity and zero voltage applied? Explain your reasoning.
Q2. You perform the photoelectric effect experiment using sodium as the target metal. You find that at your present light intensity with 300 nm light, you have about 1000 electrons being ejected per second. You are making observations of both the number of electrons being ejected per second and the kinetic energy of these ejected electrons.a. Describe what you observe when you turn the intensity down and down until it is 1/1000th of its current value. (Include qualitative graphs of the # of electrons ejected per second vs intensity, and max KE vs intensity, to support your words. Label any important points on your graphs.)
b. Describe what you would observe as you vary the color of light over a broad range (from far IR to far UV). (Include qualitative graphs of # of electrons ejected per second vs frequency, and max KE vs frequency, to support your words. Label any important points on your graphs.)
c. From the observations in parts a and b, what inferences or conclusions can you make about the nature of light? List at least 2 inferences for part a and 2 for part b. Include the reasoning that leads you to these inferences.
Our students performed very well on these questions, much better than students with traditional instruction of this material, and significantly better than students who had another form of innovative instruction using a different, simpler photoelectric effect simulation (4). The class follow-up discussion of the worksheet and clicker questions, as well as the associated small-group discussion, also provide opportunities for students to self-evaluate their understanding.
Inclusive Teaching
The lesson has high structure, with very clear structured activities and objectives and extensive active learning and small-group work following best inclusive teaching practices as described in (5). There is also an ongoing theme of growth mindset (6) conveyed in the course. This is done by repeatedly telling the students that everyone initially struggles to make sense of this material, but with study and effort eventually everyone can be successful at mastering it. As they successfully complete the various activities, they are also reminded of the progress they are making and how much they have learned. The groups are formed so that there is not a single isolated woman or member of a historically marginalized group in any group. Group tasks are randomly assigned and rotated to ensure equitable distribution.
Lesson Plan
Activity Description
The different components and times of the activity are given in Table 1. The activity begins with a review of the classical theory of the interaction of light with matter. It is expected that most students will have seen this, but many will have only a weak understanding. The content and illustrations used for this review are on slide 1 of Supporting File S1, which gives the PowerPoint slides used in this lesson. It describes the wave nature of light and emphasizes that in the classical theory there is no dependence of the interaction of light with matter on the frequency of the light, and that any response to the light would build up gradually over time.
Table 1. Teaching timeline table. Provides a timeline for the lesson.
| Activity | Description | Estimated Time | Notes |
|---|---|---|---|
| Presentation | Summary review of classical description of light interacting with matter. | 5 minutes | |
| Presentation | Presentation of learning objectives. | 2 minutes | |
| Work with Simulation | |||
| Introduce simulation | Display simulation and show various control and display options. | 3–5 minutes | |
| Simulation worksheet | Working in groups, complete worksheet activities looking at different dependencies, giving classical predictions. | 40 minutes | Timing variable. Need to monitor student progress and adjust as needed. |
| Worksheet follow up | Whole class discussion, going over results observed and classical predictions. Answer questions. | 10 minutes | Usually have to decide when to limit questions. |
| Clicker question | Clicker question with peer discussion on electron energy vs light frequency graph. | 5 minutes | |
| Presentation | Present Einstein’s theory. | 5 minutes | |
| Analogy Development | |||
| Clicker question | Groups work to make analogy for electrons in metal based on balls in a pit. | 5 minutes | |
| Presentation | Analogy of balls in pit being kicked out by kickers of different strength. How is similar to electrons in a metal. | 5 minutes | |
| Presentation | Summary of equation and units for energy of emitted electrons vs light frequency. | 2 minutes | |
Students are then instructed to open the PhET photoelectric effect simulation at phet.colorado.edu shown in the primary image. The simulation is in Java, but many systems no longer support Java. The simulation can also be run using the CheerpJ browser-compatible version, which will run on nearly all systems. I briefly show how the simulation works, demonstrating the various controls and display options, particularly how to use the battery to measure stopping potential.
They complete a worksheet with the following prompts to turn in at the end of class (Supporting File S2). Although they work in their 3 to 4-person groups while completing the activity, each student has to fill in the worksheet themselves.
Describe dependence of electron emission as function of:
- Frequency of light;
- Intensity of light (don’t get too hung up on intensity dependence near the frequency threshold).
- Describe the time delay between light turn-on and when electrons are first produced.
- Describe dependence of the current on small positive and small negative battery voltages.
- What changes when you change the metal?
Describe what happens in answering all these questions and be quantitative. Use graphs and equations as useful.
Say what the classical model would predict for all your observations.
What matches classical model predictions and what does not?
(Be careful to distinguish between your observations and your inferences.)
The worksheet is followed by a whole-class discussion in which the students are called upon to produce the following summary of their observations and how well they agree with classical predictions. With only a little prompting they should be able to produce all the following observations and, to a lesser extent, the classical predictions. The instructor will likely need to provide some of the explanation of classical predictions beyond what the students offer.
Summary of photoelectric experiment results obtained by and summarized for students:1. Current linearly proportional to intensity.
Maybe agrees with classical theory. Not clear.
2. Current appears with no delay after light begins.
Classical would say there should be a delay, so does not agree.
3. Electrons only emitted if frequency of light exceeds a threshold. (Same as “if wavelength short enough.”)
Classical theory says light frequency irrelevant, so does not agree.
4. Maximum energy that electrons come off with increases linearly with light frequency (= 1/wavelength).
(Maximum energy = -stopping potential)
Classical theory says light frequency irrelevant, so does not agree.
5. Threshold frequency depends on the type of metal.
Classical theory says there should not be a threshold frequency, so does not agree.
This discussion stimulates a large number of questions from the students, many of which are quite deep, resulting in an extensive discussion about the phenomena and the accuracy of the simulation. Instructor judgment is needed to decide how much depth to go into and the amount of time spent answering various questions.
Some frequent questions from students include:
-
Does increasing the speed of the electrons lead to an increase in the current? (This is a topic of particular difficulty for students.)
-
Wouldn’t there be less current at low voltages because the electrons would fly off in different directions and not hit the other plate?
-
Can two photons give energy to a single electron?
-
How does the photon decide where it’s going to hit the metal?
-
Why does the light rip off electrons but not protons?
After that material is covered, the students are given a multiple-choice question covering the dependence of the energy of the emitted electrons on the frequency of the light. This is done because this is a critical feature which we have seen students often overlook. The question is posed in the form of the slides shown in Figure 1 and in Supporting File S1, slides 6 and 7. In answering which graph is correct, students follow the Peer Instruction format of first answering individually with clickers or phones using Poll Everywhere, then discussing with their neighbors in the classroom and then revoting. This question both tests and reinforces their understanding.
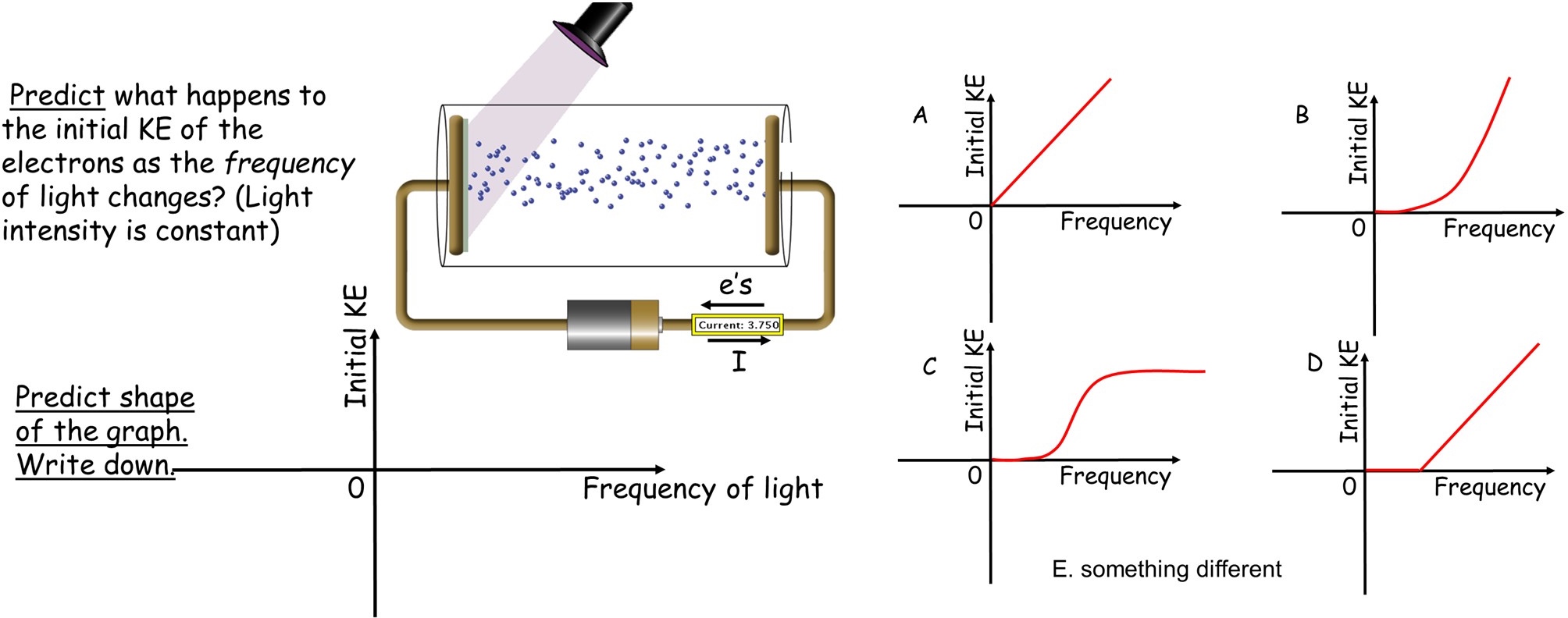
This question is followed by a brief explanation using the simulation to show the correct answer is D and noting the graph’s distinctive features of a threshold and then linear dependence.
Students are then told Einstein’s 1905 theory explaining the phenomena, summarized as follows.
Light comes in discrete chunks (“quanta”) with energy E = hf where f is the frequency of the light and where h is Planck’s constant. It is very tiny, 10-34. In interaction with matter only a full quanta of light is absorbed or emitted, so it is an all-or-nothing process. No half quanta allowed. When a light quanta is absorbed by the metal, all the energy goes into a SINGLE electron. This is true even though many electrons are covered by the classical waves. It is just a random lottery in which just one out of all the electrons exposed gets the full quantum of energy.
These are quite novel nonintuitive ideas. To help students grasp the implications of this theory, they are then guided through an analogy for the behavior of the electrons. The analogy is based on treating the electrons in a metal like soccer balls piled up in a pit, and the light is like soccer players kicking the balls, with different strength kickers analogous to light of different colors. Only the strongest kickers are capable of kicking the balls out of the pit.
The students are first asked to work in small groups with the challenge to develop the analogy themselves following the instructions and illustration of balls in a pit given in slide 10 of Supporting File S1. They are first asked to answer why or why not this is a good analogy to electrons in metal and then are asked “Can you fill out the analogy? Something to represent light and how it interacts with balls/electrons and give suitable dependencies?”
Students typically make some progress but are unable to come up with a complete accurate analogy. They may need help in thinking about the electrons in metal occupying different energy levels, like sitting on shelves at different depths in a potential well, similar to balls piled up in a pit. The following two slides shown in Figure 2 (as well as Supporting File S1, slides 11 and 12) are then used to present the full analogy to them and relate it back to the observations with the simulation. This shows how kickers of different strength are analogous to light of different colors and how metals with different work functions are analogous to balls being in pits of different depths.
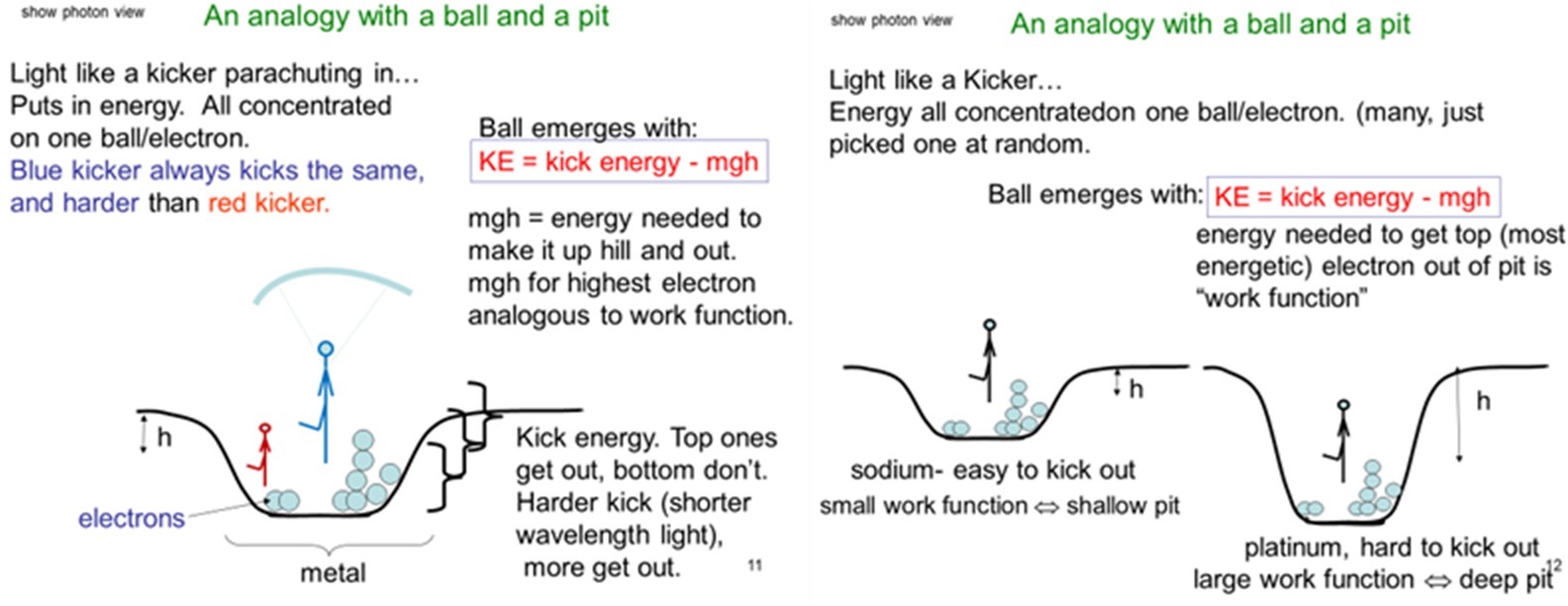
This is followed by presenting the model of a metal as a potential well with the electrons filling up many levels in the well, like soccer balls all piled on top of each other, and the light ejecting those electrons, as illustrated on slides 13–14 in Supporting File S1. The equation for the energy of the emitted electrons in terms of the metal work function, the frequency of the light, and Planck’s constant is also given along with all the proper units for the quantities.
In the class following this one, students are taught how the photoelectric effect is important for all sensitive light detectors, including the eye. Students are given homework in which they have to review and apply the ideas covered in this lesson by predicting and explaining behavior as different experimental variables are changed for a variety of conditions. They also have to work through how to determine the composition of an unknown piece of metal by using the photoelectric effect to determine its work function, and from that identifying the type of metal.
Teaching Discussion
The students become very engaged in working with the simulation and making sense of the results. As noted in the assessment section, multiple tests have shown this activity to be effective at achieving the learning objectives. The combination of simulation plus analogy development are likely quite complementary in developing understanding. The primary adjustment needed for different populations is the amount of time needed to be spent on the classical theory of light. It varies with their level of background with the subject. When there is a little additional time, I have the students work through the analogy of light hitting a metal being like ocean waves hitting the beach knocking the sand around. So what is seen in a normal beach stirring up the sand matches the classical picture of light heating up many electrons, but if the interaction of wave with sand was like the photoelectric effect observed, this would be like the waves causing just a few grains of sand to fly away with tremendous energy.
Supporting Materials
-
S1. Teaching photoelectric effect – Presentation slides
-
S2. Teaching photoelectric effect – Worksheet and instructor notes
Acknowledgments
Sarah McKagan and Katherine Perkins collaborated on the development of the simulation and some aspects of the lesson development.
References
- Steinberg RN, Oberem GE, McDermott LC. 1996. Development of a computer-based tutorial on the photoelectric effect. Am J Phys 64:1370–1379. https://doi.org/10.1119/1.18360.
- Steinberg RN, Oberem GE. 2000. Research-based instructional software in modern physics. J Comp Math Sci Teach 19:115–136.
- De Leone CJ, Oberem GE. 2004. Toward understanding student conceptions of the photoelectric effect, p 85–88. In Marx J, Franklin S, Cummings K (ed), 2003 Physics Education Research Conference Proceedings. AIP, Madison, WI. https://doi.org/10.1063/1.1807260.
- McKagan SB, Handley W, Perkins KK, Wieman CE. 2009. A research-based curriculum for teaching the photoelectric effect. Am J Phys 77:87–94. https://doi.org/10.1119/1.2978181.
- Hogan KA, Sathy V. 2022. Inclusive teaching: Strategies for promoting equity in the college classroom. West Virginia University Press, Morgantown, WV.
- Dweck CS, 2006. Mindset: The new psychology of success. Random House Publishing Group, New York, NY.
Article Files
Login to access supporting documents
Wieman-Teaching the Photoelectric Effect Using an Open-Source Interactive Simulation.pdf(PDF | 752 KB)
S1. Teaching photoelectric effect - Presentation slides.pptx(PPTX | 380 KB)
S2. Teaching photoelectric effect - Worksheet and instructor notes.docx(DOCX | 14 KB)
- License terms

Comments
Comments
There are no comments on this resource.