Keep It Shrimple: An Adaptable Student-Driven Research Project for the Introductory Biology Laboratory
Editor: Jim Burnette
Published online:
Abstract
A challenge in introductory biology laboratory courses is to provide students with authentic, engaging research opportunities that allow them to take ownership of their experiments. We present a nine-week introductory biology module that allows students to engage with the process of science, gain experience with various laboratory techniques, and communicate their results to a peer audience. These modules use the inexpensive and accessible invertebrate model of the brine shrimp Artemia, which has many applications from aquaculture to ecology to behavior. Students explore known taxis behaviors in the larval (or “naupliar/nauplii”) stages of these brine shrimp before designing their own experiments, collecting and analyzing data, presenting their results orally, and redesigning their experiments based on peer and instructor feedback. This LessonPlus article highlights the exploration of known taxis behaviors and the scaffolding for having students design their own experiments. We originally designed this module to be highly flexible and used it to teach students both remotely and in-person during the early years of the pandemic. We have since found it to be easily adaptable in terms of timing, materials used, and learning modality. Most importantly, we have observed a number of positive outcomes related to student engagement and proficiency, including increased quality of summative assessments.
Primary Image: Artemia nauplius. A scientific illustration of the nauplius stage of Artemia sp. used in these experiments to study taxis behaviors. ©2024 Liesl V. McCormick.
Citation
Karson MA, McCormick LV. 2024. Keep It Shrimple: An Adaptable Student-Driven Research Project for the Introductory Biology Laboratory. CourseSource 11. https://doi.org/10.24918/cs.2024.12Society Learning Goals
Science Process Skills
- Process of Science
- Locate, interpret, and evaluate scientific information and primary literature
- Pose testable questions and hypotheses to address gaps in knowledge
- Plan, evaluate, and implement scientific investigations
- Interpret, evaluate, and draw conclusions from data
- Address novel questions through authentic research experiences
- Modeling/ Developing and Using Models
- Build and evaluate models of biological systems
- Quantitative Reasoning/ Using Mathematics and Computational Thinking
- Use basic mathematics (e.g., algebra, probability, unit conversion) in biological contexts
- Apply the tools of graphing, statistics, and data science to analyze biological data
- Communication and Collaboration
- Share ideas, data, and findings with others clearly and accurately
- Work productively in teams with people who have diverse backgrounds, skill sets, and perspectives
- Provide and respond to constructive feedback in order to improve individual and team work
- Reflect on your own learning, performance, and achievements
Learning Goals for the Course
Students will:- use basic laboratory science skills to make observations, collect data, and keep accurate and careful records.
- work successfully with their peers in small groups.
- find and use primary literature to identify and learn about scientific research related to behavioral taxis of interest.
- develop and state a testable hypothesis, and predict results that will support the hypothesis.
- use a graphing program to represent data in a visual format.
- perform basic calculations and use statistical tests to interpret data, and explain the results of these statistical tests.
- analyze and communicate data by generating figures and expanded figure captions, and write formal portions of a lab report.
- communicate results by giving short oral presentations that describe results.
- reflect and evaluate own understanding and skill level.
Selected Learning Objectives
Justification
Exemplar 1
Near the beginning of the semester, students are led through a pre-designed experiment to test chemotaxis of the larval (or “naupliar/nauplii”) stage of brine shrimp Artemia in a variety of salt solutions based on a hypothesis derived from in-class reading. This introduces students to the experimental paradigm. Students set up the experiment and collect data for analysis and interpretation in the following week.Exemplar 2
In the middle of the semester, students work through hypothesis generation and experimental design to develop an experiment to test Artemia nauplii taxis to an environmental cue of their own choosing. They use previously-identified primary literature to ground their hypothesis, and work with the instructor and teaching assistant to design a feasible experiment to carry out in the following week.Exemplar Learning Objectives
Exemplar 1
Students will be able to:- use basic laboratory science skills to make observations, collect data, and keep accurate and careful records.
- pour a salinity column.
- use serological pipets.
- use a dissecting microscope.
- carefully observe animals and develop accurate methods for quantifying Artemia behaviors.
- practice scientific note-taking skills and laboratory notebook entry skills.
- use a spreadsheet program to enter and organize data into a table.
- work successfully with peers in small groups.
Exemplar 2
Students will be able to:- propose a plan for a testable behavioral experiment based on previous experimental findings.
- correctly format a citation of a piece of scientific literature, both in the body of a piece of text and in the literature cited portion of a lab report.
- implement the scientific method to formulate, develop, and state testable hypotheses, and predict results that will support these hypotheses.
- describe the difference between a prediction and hypothesis. Given a hypothesis and prediction related to chemotaxis and symbiosis in Artemia, develop a second related prediction.
- develop hypotheses and design experiments examining the relationship between environmental cues and Artemia taxic behaviors.
Article Context
Course
Article Type
Course Level
Bloom's Cognitive Level
Vision and Change Core Competencies
Vision and Change Core Concepts
Class Type
Class Size
Audience
Lesson Length
Pedagogical Approaches
Principles of How People Learn
Assessment Type
Introduction
Centering laboratory courses around student inquiry and authentic research experience is desirable, yet difficult to implement in an introductory biology setting. In our previous introductory biology laboratory curriculum, students participated in instructive, research-like three-week modules. Each module was intended to illustrate a topic related to the “flow of biological information,” a Vision and Change core concept (1), but these modules ended up relatively unrelated to one another and lacked obvious connections (both between topics and between skills). Three weeks was also often not enough time to refine skills and fully engage in the entire process of science, from hypothesis generation to data collection and interpretation. Students did not feel that the topics connected to lecture material in any way, and some students struggled with the transitions from one module to another. We also found that although students were exposed to the scientific process and a wide variety of biological techniques and topics, their learning remained at a superficial level.
We thus revised the laboratory curriculum under the premise that students would gain more by attaining a deeper understanding of fewer concepts than from a superficial survey of many. We took one of the short modules and expanded it into a single longer module, containing a series of intentionally related lessons. This allows students plenty of time to fully engage with the process of science, often spending an entire lab period on developing a hypothesis, or doing statistical analyses. Although the laboratory content is still not directly tied to lecture examples, both are used to explore concepts related to the flow of biological information. Additionally, we have developed case studies in lecture that explore primary literature, which we can now use to reference their new lab skills in data literacy, statistical analysis, and the process of science. This paper describes in depth portions of this nine-week module where students explore the connections between the behavior of the brine shrimp Artemia and its ability to respond to salient environmental cues.
Another goal of our redesign was to engage students throughout the nine-week module. We increase student engagement and interest by allowing them to self-select topics of interest, providing them with a scaffold for both techniques and concepts, giving them ample time to explore and complete activities, and assessing them in a variety of frequent, low-stakes ways. These are described further in the Inclusive Teaching section.
To introduce open-inquiry experimentation in an introductory biology course, we provide a framework to use a relatively inexpensive, broadly available model organism: Artemia franciscana, a species of brine shrimp. Artemia behavior can be related back to a number of interesting topics, as they are relevant to both a variety of important food webs and the global aquaculture industry. Students employ readily-available laboratory or household items as “environmental cues” to test one of several different variables that have the potential to affect Artemia behavior. Required reagents are inexpensive and can be purchased at a pet store or grocery store, or ordered easily online (Supporting File S3). Laboratory facilities and equipment are not technically required, so the module can be adapted for asynchronous/online use if necessary. Students are assessed with both an in-lab group oral presentation and with figures and accompanying captions presented in a publication style. The goal of this module is to relieve both students and instructors from the burden of technical complexities, instead giving them an opportunity to focus on quality experimental design and the process of “doing science” as a group endeavor.
Model Organism
Native to the Great Salt Lake and salt flats surrounding the San Francisco Bay, Artemia brine shrimp are commonly used in aquaculture as inexpensive live food sources for small or juvenile fish. Brine shrimp are economically important to the Great Salt Lake and aquaculture industry (see current articles at Great Salt Lake Artemia). Artemia are biologically interesting in that they are able to thrive in a wide variety of conditions (2).
These invertebrates have high utility in educational settings, as they are inexpensive to procure and maintain, are easy to raise, have a quick life cycle, and are enjoyable to watch. They are sold as dormant cysts, which can be induced to hatch quickly (within 24–36 hours). In this module we use their first larval stage (termed the “naupliar” stage), which allows for nearly immediate use of nauplii after hatching. Artemia will grow to adult stages within one week if older life stages are desired. During the naupliar stage, the nauplii subsist on yolk until they molt and do not need to be fed. Adults can be fed baker’s yeast.
Pedagogical Framework
Over the course of the module, students examine taxis behaviors of Artemia nauplii, defined as movements in response to an external stimulus. The students work in small groups to select an “environmentally meaningful cue” for the nauplii, based on their group’s interest and a literature search. They then devise a specific, self-directed research question, formulate a hypothesis, and design an accompanying experiment to test the hypothesis that the Artemia nauplii will swim toward or away from their cue of interest when presented with the choice in a test chamber.
Although there is a commonly-cited related lab using Artemia (or other small invertebrate) to examine taxis (3), the lab experience presented here is a significant revision. Our suggested techniques allow for more varieties of student inquiry as well as the ability to count significantly more Artemia due to larger experimental setups, the use of microscopes, and more time in lab (leading to a larger n and more powerful statistical analyses). Our module also has students expend more effort developing sound hypotheses and predictions, which fosters a stronger connection to their overall research goal and allows a more meaningful experience. Results from research indicate that allowing students to develop and test their own hypotheses encourages them to think critically and analyze data (4).
Intended Audience
This laboratory module is used in a large-enrollment introductory biology lab course at a small liberal arts university, serving both biology and non-biology majors. There are often 80 students enrolled per semester. These students are split into two lecture sections of 40 students each and four lab sections of 20 students each. Labs are technically capped at 24 students, although we try to maintain sections of no more than 20 when possible. Lab meets for two hours a week for nine weeks of the semester.
Within each lab section, students work in research teams of four students, with whom they will work for the entire semester. A full-time instructor runs each lab section with the help of one undergraduate teaching assistant, but the lab instructor is not always the same instructor for the related lecture course.
With more instructional time, this laboratory module could readily be adapted for an intermediate course or more advanced students by adding more iteration of experimental technique, having the students manipulate hatching conditions and observe subsequent taxis behavior, incorporating further aspects of literature reading and science writing, etc. It could alternately be adapted for a younger audience (such as high school or even middle school students) as either a multi-week project or a one-day outreach event.
Term and Context Description
This module is designed for nine two-hour laboratory class periods. It is taught in a basic laboratory classroom and is meant to accompany a full-semester lecture course in introductory biology.
Prerequisite Student Knowledge
This laboratory module is designed for students with minimal background in the subject matter. The course has no prerequisites. Students are required to use word processing, data analysis/graphing software, and presentation software to analyze their data and assemble a final presentation and lab report. We provide very basic background information about Artemia brine shrimp in the student lab workbook. One good simple resource is found at the Utah Division of Wildlife Resources’s website.
Prerequisite Teacher Knowledge
If the instructor is not familiar with Artemia as a model organism, numerous guides are available that explain basic biology, hatching protocols, and care methods (5–7). We have provided instructions about hatching and housing in the first exemplar lesson below.
Scientific Teaching Themes
Course Structure
The module we present here employs a four-step pedagogical framework (8) based on a Course-based Undergraduate Research Experience (CURE) adapted for introductory biology laboratory setting by Goudzousian and colleagues (9), where the biological inquiry process is scaffolded and students progressively gain comfort and familiarity with the process as they move through the semester. Students first learn the experimental technique, design an experiment based on the technique, carry out the experiment, interpret the data, and communicate their results (8). CURE-like experiences can be difficult to implement in introductory lab settings for myriad reasons: differential student preparation, limited seat time, limited resources, and the fact that multiple instructors (with the potential for very different backgrounds) may teach the lab course. We have found this module is flexible enough to overcome these challenges.
Assessment
The themes of intentional feedback and reiteration are key components throughout this lab module. During lab, students work on writing hypotheses and predictions, generating figures and writing figure captions, and preparing an oral presentation. Each of these are submitted in stages as graded assignments, which provides the instructor the opportunity to comment on initial drafts via rubric before they are submitted in the final lab report. Several of the assignments are re-submitted, allowing students to address and incorporate feedback. Other feedback is offered by undergraduate teaching assistants and peers. We believe that all students can succeed given adequate support and deliberate opportunities for practice, so we guide students in revising their written hypotheses, repeating their data analysis, and extending their experiments. Some sample assessments are provided in Supporting Files S2, S5, and S6.
Inclusive Teaching
CUREs have traditionally been difficult to implement at the introductory biology level, due to challenges such as the level of student preparedness, the involvement of a variety of faculty with various content expertise, time commitments outside of scheduled laboratory sections, and facilitating student engagement with a topic that may lie outside their interest.
To address some of these challenges, this laboratory module incorporates a number of inclusive learning strategies, as discussed by Tanner (10), including:
-
Providing options for promoting interest. After grounding students in the basic techniques, this module allows students to select and pursue a research question that is of interest to them, allowing them to express their individuality and investing them in their project (11).
-
Working in small (self-selecting) groups. We typically allow students to self-select their lab groups, which provides the opportunity for them to be comfortable with the people they are working with (12, 13).
-
Facilitating learning such that students become their own “content experts.” Often, the students themselves must find the answers to their queries and challenges instead of relying on the instructor to provide answers (14–16).
-
Allowing students ample time to perform lab activities. In our design, we have consciously reduced the number of activities for each lab meeting time, so that all students have enough time to complete everything that is asked of them.
-
Scaffolding and guiding written assignments. The instructions for each written assignment (such as the hypothesis, figure captions, and lab report) are presented both orally by the instructor as well as in writing. Models of appropriately-formatted assignments are also provided. Worksheets help students “walk through” the steps they should complete and questions they should answer in generating their piece of writing (17).
-
Annotated primary literature. Reading primary literature and emphasizing real science and data is a powerful tool for teaching. However, learning to read journal articles can be unwieldy for introductory students. Early in the semester, we provide an annotated paper related to phototaxis in Artemia to increase the accessibility of primary literature.
-
Transparency in assessment. In addition to providing students with guidance for how to approach and what to include in their graded assignments, we also provide students with a rubric before they submit the assignment. This allows them to see exactly how they will be graded (18).
-
Frequent low-stakes assessments. This ensures that there are very few “make-or-break” assignments during the lab module, meaning that the pressure to perform well on any one assignment is reduced.
-
Variety in assessment. We provide multiple modalities for assessment, including multiple choice quizzes, short writing assignments, figure generation, and oral presentation. In this way, diverse populations of students all have the ability to complete assignments that may cater to their background or preparedness.
Course Schedule
This lab module follows the general progression of the scientific method (Table 1). Students are first introduced to the model system and carry out a pre-designed experiment to gain familiarity with the paradigm and to practice data analysis and interpretation. They then work in small groups to re-design the initial experiment, carry it out, and analyze the data. Students then learn how to search the scientific primary literature to identify a research question that interests them, generate a hypothesis, and design an experiment. They carry out their student-designed experiment, analyze, interpret, present their results orally, and revise their experimental design based on peer and instructor feedback.
Table 1. Course schedule table.
| Week | Learning Goals/Concepts | Activities | Notes |
|---|---|---|---|
| Overview of Lab Course and Artemia Model System | |||
| 1 | Introduction to Artemia model system | • Short intro to Artemia life history and use as a model system in lab | |
| Reviewing prior research | • Read secondary source (19) related to Artemia behavior | • We use this source as the basis for the hypothesis the students test in Week 2 (Exemplar 1) | |
| Reading primary literature/reviewing prior research | • Read annotated primary source related to Artemia behavior/taxis |
• Supporting File S1 • Article is annotated to aid in vocabulary and graph interpretation |
|
| Information literacy/biological literacy | • Using secondary (19) and primary (24) sources, group discussion about primary versus secondary literature to highlight differences | ||
| Critically evaluating scientific information | • Complete worksheet on evaluating science in the news | • Based on CRAAP test (25) | |
| Reflection/metacognition | • Debrief with instructor and/or teaching assistant about the two articles and whether they expect Artemia to show preference for salinity levels and/or saline color based on the readings | ||
| Perform Salinity Experiment (Exemplar 1) | |||
| 2 | Formulating hypotheses | • Discuss instructor-generated hypothesis based on prior week’s in-class reading (19) |
• See Exemplar 1 • Students take pre-lab quiz before coming to lab, see example in Supporting File S2 |
| Predicting outcomes | • Predict outcome of salinity choice experiment | ||
|
Designing/conducting experiments Gathering data/making observations |
• Conduct prescribed salinity experiment, including set up, observation, data collection |
• Supplies needed in Supporting File S3 • Protocol in Supporting File S4 |
|
| Hands-on lab skills | • Pour salinity column, use serological pipette, use dissecting microscope | • Pouring the salinity column can be tricky, and some groups may need to attempt it 2–3 times before succeeding | |
| Reflection |
• Debrief with instructor and/or teaching assistant about their experimental trials and any technical issues they may have encountered • Double-check they have recorded their counts/data in their lab notebooks |
• Instructor checks in with groups about whether they encountered any methodological issues, if preliminary results (raw data) matched their expectations, and that they have data in usable format for entry into class spreadsheet the following week | |
| Analyze Data From Salinity Experiment | |||
| 3 |
Collaboration Organize and annotate simple data sets |
• Compile Week 2 results from each group into usable table on spreadsheet to allow for analysis of class data | • Requires access to computers equipped with spreadsheet programs |
|
Displaying/modeling results/data Numeracy |
• Calculate percentage of Artemia in each salinity • Perform chi-square test |
• Many students need help in determining the “expected” counts when calculating a chi-square statistic | |
| Interpreting results/data | • Determine whether class data support the provided hypothesis | ||
| Communicating results |
• Generate figure (bar graph) and write expanded figure caption • Write appropriate statistical results sentence |
• Much of the assistance in generating a bar graph comes with the peculiarities of using a spreadsheet program | |
| Reflection |
• Check in with instructor to ensure all criteria of graph and caption are met • Debrief with instructor and/or teaching assistant about how class results relate to the original hypothesis, whether group results matched class results/importance of replicating experiments, potential alternative hypotheses, and what factors the group/s may wish to control for in the following week |
||
| Design and Perform Controlled Salinity Experiment | |||
| 4 | Designing/conducting experiment | • Each lab group develops their own control experiment for Week 2 prescribed class experiment | |
| Predicting outcomes Testing hypotheses | • Each lab group predicts outcome of their control experiment | ||
| Gathering data/making observations | • Conduct designed salinity experiment, including set up, observation, data collection |
• Supplies and protocol will be somewhat similar to Week 2, depending on student design • Supplies needed in Supporting File S3 • Potential to be similar to protocol in Supporting File S4 |
|
|
Interpreting results/data Numeracy |
• Calculate percentage of Artemia in each experimental condition • Perform chi-square test |
• Requires access to computers equipped with spreadsheet programs • Students are more familiar with data analysis this second time through and often require less assistance |
|
| Communicating results |
• Generate bar graph and write expanded figure caption • Write appropriate statistical results sentence |
||
| Reflection |
• Check in with instructor to ensure all criteria of graph and caption are met • Debrief with instructor and/or teaching assistant about whether the results of their control experiment support the original hypothesis or suggest an alternative hypothesis |
||
| Literature Search and Identification of Research Question | |||
| 5 | Literature searching skills | • Work through web search tutorial to identify primary literature related to small group’s behavior of interest | • Requires access to computers with internet access (browser) |
| Reading research papers/reviewing prior research/understanding anatomy of a research paper |
• Complete worksheet with basic questions about the article of lab group’s choosing • Interpret one figure from identified primary source |
• The hope is that the groups find a paper that will inform their own experimental design. Thus, it is important that they select a paper that they can understand on some level | |
| Reflection | • Debrief with instructor to make sure that primary literature is usable (students generally understand abstract, can interpret one figure from the paper, and may be applicable to some aspect of experimental design) | • Much previous research on Artemia focuses on conditions for hatching and growing, rather than taxis behaviors | |
| Hypothesis Generation and Experimental Design (Exemplar 2) | |||
| 6 | Reviewing prior research/formulating hypothesis | • Work in groups and with instructor to generate biological hypothesis based on Week 5 paper |
• See Exemplar 2 • Throughout lab period, instructor helps guide groups to develop biologically-sound hypotheses based on primary literature • Prompts in Supporting File S5 |
|
Designing experiments Predicting outcomes Testing hypotheses |
• Groups design experiment to test biological hypothesis | • Prompts in Supporting File S5 | |
| Reflection | • Debrief with instructor about intended experimental design and feasibility of experiment | • Rubric for grading hypothesis in Supporting File S6 | |
| Perform Group-Designed Experiment | |||
| 7 |
Collaboration Designing/conducting experiments Gathering data/making observations |
• Conduct group-designed experiment, including set up, observation, data collection |
• Groups are not provided with protocol, instead they are free to base it loosely on salinity experiment protocol (Supporting File S4) • Each group is asked to perform at least two trials of their experiment and aim for at least 100 nauplii in each trial |
| Displaying/modeling results/data |
• Compile results within group into usable table on spreadsheet • Calculate percentage of Artemia in each condition • Perform chi-square test |
• Requires access to computers equipped with spreadsheet programs | |
| Interpreting results/data | • Determine whether data support student-generated hypothesis | ||
| Communicating results |
• Generate figure and write expanded figure caption • Write appropriate statistical results sentence • Put together results slides to be used in presentation Week 8 |
||
| Reflection | • Debrief with instructor to discuss experimental outcome and ensure all criteria of graph and caption are met | ||
| Group Oral Presentation and Peer Feedback | |||
| 8 | Communicating results | • Each group gives oral presentation with accompanying slides and receives feedback from peers and instructor |
• Requires access to audiovisual equipment • Each group is given 10 minutes to present and 5 minutes for questions • Worksheets to guide peer feedback in Supporting File S7 |
| Interpreting results/data | • Work in groups to brainstorm modifications to experimental design based on peer and instructor feedback | • Many groups with unexpected results have ideas for how to redesign their experiment. Groups with expected results are often instead the ones that need assistance envisioning next steps | |
|
Reviewing prior research/formulating hypotheses Predicting outcomes |
• Revise original experimental hypothesis, based on results and presentation feedback | • Prompts for revising and rewriting hypothesis in Supporting File S8 | |
| Reflection | • Debrief with instructor to answer questions and guide groups to design feasible follow-up experiment | ||
| Perform Revised Group-Designed Experiment | |||
| 9 |
Designing/conducting experiments Gathering data/making observations |
• Conduct group-designed experiment, including set up, observation, data collection |
• Each group is asked to perform at least two trials of their experiment and aim for at least 100 nauplii in each trial • By this point students are reasonably self-sufficient and often do not require any assistance |
| Displaying/modeling results/data |
• Compile results within group into usable table on spreadsheet • Calculate percentage of Artemia in each condition • Perform chi-square test |
• Requires access to computers equipped with spreadsheet programs | |
| Interpreting results/data | • Determine whether data support revised student-generated hypothesis | ||
| Communicating results |
• Generate figure and write expanded figure caption • Write appropriate statistical results sentence • Compile this figure with Week 7 results and compose draft of a formal written lab report |
||
| Reflection | • Check in with instructor to discuss outcome and how it relates to/extends understanding of Artemia behavior from their Week 7 result, and review content of lab report | ||
Exemplar Lesson Plan #1
Lab Week 2: Perform Salinity Experiment (Table 2)
Instructor Prep
At least 36 hours before lab begins, the instructor hatches and rears Artemia to the naupliar stage. In our facility, we use a simple brine shrimp hatchery kit made from an inverted 2 L bottle on a base, outfitted with an oxygenating aquarium pump, tubing, and hose clamp (Figure 1A, Supporting File S3). This hatchery can hold about 1.5 L of 25 ppt (parts per thousand) Instant Ocean®, which when combined with 1 teaspoon of cysts, provides more than enough Artemia nauplii for 3–4 sections of students over the course of two days. We place the hatchery near a light bulb to keep the water around 27 °C and use the pump to oxygenate the water and circulate the cysts. Cysts typically hatch within 24–36 hours after setup. We set up at least two of these hatcheries to have back-up cultures (Figure 1A, Supporting File S2).
To set up for each lab period, we transfer hatched Artemia nauplii to a small three-gallon aquarium with a large opening on top, filled with fresh 25 ppt Instant Ocean® and outfitted with a bubbling airstone to keep the water oxygenated (Figure 1B). From this communal aquarium, students can take up nauplii using a pipette, turkey baster, or other tool into a small beaker and carry the beaker back to their work stations. Unused Artemia can be added back to the home aquarium at the end of the lab. Collection of Artemia from the aquarium can be aided by temporary removal of the airstone (to cease water movement), and a small light source held near the side can be used to attract the nauplii to one area of the aquarium.

Pre-Lab Activity
Students read background information about animal sensory systems and Artemia, read through the protocol for the day, and complete a flowchart of the protocol (Supporting File S4). They then use our learning management system to take a pre-lab quiz on the background information and protocol (Supporting File S2).
In-Lab Activities
Students are given the experimental framework to test the hypothesis “Artemia nauplii will move toward the highest salt concentration in their environment because they are adapted to seek saline levels to benefit the symbiotic bacteria living in their guts,” which is based on a reading from the first week of lab (19). We provide students with both this hypothesis and three different graphs of potential results, asking them to choose which one most closely matches the prediction based on the hypothesis.
Students construct a salinity column using three Instant Ocean® salt solutions made at 15, 25, and 35 ppt (dyed green, red, and blue respectively, to aid in visualizing the solutions; Figure 2). Once students successfully layer the saline, they inject live nauplii into the middle (25 ppt) saline layer (the same salinity in which the Artemia were hatched) and let them swim freely for 10 minutes. During this time, students observe the Artemia and take notes on any interesting behaviors (e.g., swarming), allowing them to practice their observational skills.

They collect each fraction of saline and accompanying nauplii into separate beakers, then filter each solution through a sink vacuum set-up attached to a Buchner funnel lined with filter paper (Figure 3). Alternatively, a hand vacuum pump, small plug-in vacuum pump, or even a large syringe with tubing can be used to expedite collection of water from the column and filtration onto filter paper. The filter paper from each fraction, along with accompanying nauplii, can then be placed in a Petri dish and counted using a dissecting microscope.

Post-Lab Activity
Each lab group enters the data they have recorded in their lab workbooks into a Google Sheets spreadsheet that contains all lab section data for analysis the following week.
Table 2. Teaching timeline table for Exemplar 1. Lab week 2: Perform salinity experiment.
| Activity | Description | Estimated Time | Notes |
|---|---|---|---|
| Before Lab: Instructor Preparation | |||
| Growing Artemia cultures |
• Make 1.5 liters 25 ppt saline for hatching • Set up hatchery with aerator under light bulb and and add cysts |
15 minutes (do 36 hours or more before class period) |
• Instructions in text of Exemplar 1 • Photo of hatchery in Figure 1A |
| Home tank preparation | • Set up home tank from which students will collect live Artemia | 15 minutes |
• Instructions in text of Exemplar 1 • Photo of home tank in Figure 1B |
| Saline preparation | • Make stock of 3 salinities for student use (15 ppt, 25 ppt and 35 ppt) and add food coloring | 30 minutes |
• Recipe: 25 ppt is 25 grams Instant Ocean® in 1000 mL water • Total quantity depends on lab size: each group will need 300 mL of each salinity for their column (provide at least 500 mL of each salinity per group and have backup carboys to accommodate errors). Recommend 10 L total of each. |
| Vacuum/filter flask setup | • Construct filtering station(s) at classroom sink | 5 minutes |
• Instructions in text of Exemplar 1 • Photo of filtration setup in Figure 3 |
| Before Lab: Student Preparation | |||
| Reading |
• Read background information about Artemia, animal sensory systems, and taxic behaviors • Read through experimental protocol |
30–60 minutes | • Salinity experiment protocol in Supporting File S4 |
| Pre-lab quiz | • Take quiz on readings using Learning Management System (LMS) | 10 minutes |
• Quiz is open-note but time-limited to motivate preparing readings. • Sample quiz questions in Supporting File S2 |
| During Lab: Overview of Activities | |||
| Instructor overview | • Instructor gives overview of experimental hypothesis, experimental setup | 10–15 minutes | • We use this time to begin introducing hypothesis testing |
| During Lab: Salinity Experiment | |||
| Set up experiment | • Students work in groups to pour salinity column | 15–30 minutes |
• Salinity experiment protocol in Supporting File S4 • Pouring the salinity column can be tricky, and some groups may need to attempt it 2–3 times before succeeding • There is therefore a wide distribution in how long it takes each group to complete this portion • Photo of salinity column in Figure 2 |
| Run experiment | • Students work in groups to place Artemia nauplii into salinity column and observe their behavior | 15–30 minutes |
• We choose to have students observe the Artemia for 10 minutes, due to the size of the column used • Prompts in the lab workbook guide students in observations of Artemia behavior (Supporting File S4) |
| Data collection |
• Students work in groups to remove fractions from salinity column • Filtration of fractions to separate Artemia nauplii • Microscopy to observe and count number of nauplii in each fraction |
30–45 minutes |
• Photo of filtration system in Figure 3 • Though filtration does not take long, there is a potential bottleneck of student use because we only have two sinks in our lab |
| Data entry | • Recording of number of nauplii into lab notebook (physical) and class data spreadsheet (digital) | 10–15 minutes |
• Students are provided a template table in the lab notebook to fill in provided in (Supporting File S4) • A shared Google Sheet is provided for all groups to enter data into |
Exemplar Lesson Plan #2
Lab Week 6: Hypothesis Generation and Experimental Design (Table 3)
Instructor Prep
Bring lists and/or examples of supplies available for student use (contained as part of the prompts in Supporting File S5).
Pre-Lab Activity
Students complete a worksheet guiding them in reading a piece of primary literature, using a piece of primary literature they identified in lab the previous week. They then use our learning management system to take a pre-lab quiz on the components of primary literature.
In-Lab Activities
Students work with their group to develop a novel hypothesis, prediction, and protocol for their experiment. This is informed by background information from the piece of primary literature they found and read in the previous week. In lab, they begin by writing a bulleted outline of background information to motivate their experiment, which they will use in both their oral presentation and final lab report later in the semester. Students then work closely within their lab groups to formalize a hypothesis and outline an experimental protocol (Supporting File S5).
Although this seems like a relatively simple task, we allow for a lot of time for discussion, editing, and thinking through this very important step of the scientific process. There is typically a lot of back-and-forth between each group and the instructor or undergraduate teaching assistant, as we guide them to refine their hypotheses and experimental design. We allow students to practice their own skill in backwards design by having them explain what they are trying to demonstrate, survey what tools they have available to them, and discuss appropriate controls they can build into their experiment. Typically, each group already has a basic idea of what they are interested in studying, but needs to spend time discussing the best approach. A list or display of materials that are available for student use is particularly helpful as they discuss how they might manipulate their set-ups (contained as part of the prompts within Supporting File S5).
If students wish to use a horizontal choice chamber for their experiments, additional choice chamber options we have used are shown in Figure 4 (small and large water volume options). Both setups allow users to start the experiment with Artemia in a central starting point equidistant from the cues on either side of the chamber.
The hypothesis that students write this week also serves as a rough draft of what will ultimately be included in their final lab report. Each group debriefs with the instructor and/or teaching assistant before leaving.
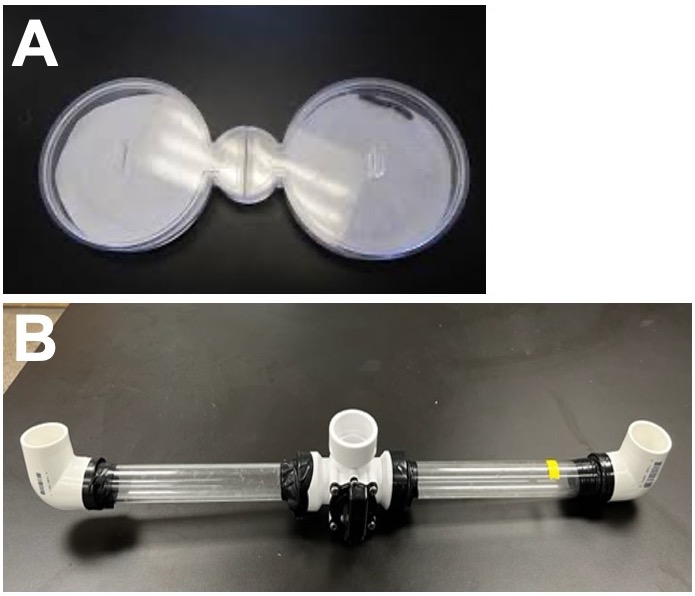
Post-Lab Activity
Each student submits a version of their experimental hypothesis (written in their own words) for grading by the lab instructor (Supporting File S6).
Table 3. Teaching timeline table for Exemplar 2. Lab week 6: Hypothesis generation and experimental design.
| Activity | Description | Estimated Time | Notes |
|---|---|---|---|
| Before Lab: Instructor Preparation | |||
| Organization | • Make a list or display of possible supplies that are available to students for use in experiments | 30 minutes |
• We put possible materials into bins sorted by type of taxis (e.g., “phototaxis”) to show students during lab and allow them the chance to think creatively about how various materials might be used • Example of supply list provided to our students in Supporting File S5 |
| During Lab: Hypothesis and Experimental Design | |||
| Hypothesis generation |
• Students work in groups and with instructor to generate biological hypothesis that will inform their experiment • Instructor is present to answer questions and help guide groups to develop biologically-sound hypothesis based on primary literature |
up to 1 hour |
• Hypothesis generation prompts in Supporting File S5 • Students often have a good first idea for their biological hypothesis, but need guidance to refine it and connect it to what is already known (from the piece of primary literature they researched) • Some students struggle with the difference between a hypothesis and a prediction • Rubric for grading the hypothesis in Supporting File S6 |
| Experimental design |
• Students work in groups and with instructor to design experiment that can test their biological hypothesis • Instructor is present to answer questions and help guide groups to design experiment that is feasible within the constraints of the lab setting |
up to 1 hour |
• Choice chamber options we offer students shown in Figure 4 • Instructors can give feedback about what has worked in the past to design similar experiments |
Teaching Discussion
The goal of redesigning this introductory biology laboratory course was to give students a more authentic research experience. This includes the development of a number of scientific skills, including literature searching, hypothesis generation, experimental design, data analysis and statistical analysis, and communication of results in multiple formats (oral presentation and written lab report). One of the major benefits we saw in expanding into a single longer module was providing context to the students. With the focus on a single experimental paradigm (rather than four over the course of the semester), students had a clearer understanding of how each of these components fits together, as demonstrated by the greater ease with which they generated biologically-relevant hypotheses as well as their more cohesive lab reports.
After the redesign to the new format, lab instructors saw increases in student evaluation scores in the following categories: “The activities/assignments for this class enhanced my learning,” “This course was clearly organized and planned,” “What I was supposed to learn in this course was clear to me,” “This course has been a valuable learning experience for me,” “This course increased my knowledge,” and “This course expanded my thinking.” This seems noteworthy, especially as the first few semesters that we taught this redesigned lab were during the 2020–2021 academic year, a period where many students were still attending class and lab remotely and this laboratory module was taught in a HyFlex (hybrid-flexible) format.
Other meaningful benchmarks that we have observed for this lab course include:
-
Increased student and group independence. When students approach lab instructors with questions now, they are more likely to be complex, rather than “What are we supposed to be doing” or “How do I calculate a p value?”
-
Better success in forming testable hypotheses that are grounded in biological rationale. Conducting the primary literature search for background information and reading some secondary literature help them articulate why their chosen stimulus might be biologically-relevant. Because we ask students to separate their hypothesis (including a biological mechanism) from their prediction (which predicts the results of the experiment if the hypothesis is supported), we have also seen an increase in the ability of students to distinguish between hypotheses and predictions.
-
Higher quality and more engaging oral presentations that generate more interest from peers, along with more meaningful feedback from their peers. Students seem generally invested in and excited about their experiments and results, and they have interesting ideas about how to revise their experiments and extend their findings.
-
Higher scores and overall improvement on the final lab report. All students seem to have a better understanding of what they have spent the semester doing and how each of the different pieces relates to each other. Having iteration in the hypothesis assignments has led to drastic improvements in the readability of their final product.
-
Enjoyment of working with a group. Although we still observe the occasional difficulties that come from complex group dynamics, students are generally pleased to be able to select their own groups and work with their peers on a project for the majority of the semester.
We are constantly redesigning this lab, and we have several suggestions for ways in which it could be adapted or modified. We have already found success using this module in a HyFlex environment, with some students physically present in the lab and some students synchronously attending the lab remotely. During the early semesters of the COVID-19 pandemic, we worked to implement this module as one that could be carried out both in the lab setting as well as at a distance. We mailed remote students all of the supplies they needed to hatch and raise their own Artemia and carry out modified versions of all of the experiments. For example, instead of a one-liter glass graduated cylinder, remote students performed the salinity experiment using graduated 16-ounce plastic cups and counted nauplii using a hand magnifying glass. Although nothing can substitute for in-person instruction and group collaboration, we found success in having students practice experimental design and revision using physical materials. This module has the flexibility to be adapted to a number of instructional situations where students may or may not be physically present in the laboratory.
We are currently trialing an updated version of this module to expand on the controls for the salinity experiment by having students create their own color salinities to test the Artemia nauplii preference for particular colors versus salinities. This allows us to teach micropipetting and dilution skills before having students practice using micropipettes to create various colors of the three salt solutions. There are a number of additional skills-based outcomes that could be incorporated into this module, including DNA extraction and sequencing, microscopy, and toxicology.
We have also considered making connections to molecular biology by including DNA barcoding to determine the species of Artemia. We previously included a short DNA barcoding module in this lab course, which could easily be altered to use Artemia nauplii as the DNA source. This could be used to address the question of whether any particular differences in taxis behavior could be due to differences in species. Although many commercial sources of Artemia sell Artemia franciscana, there does exist some genetic diversity within this species as well as among different species (20–22).
Finally, other modifications could include extensions for an upper-division course. There is an extensive literature on ideal conditions for hatching and raising Artemia, since they are a crucial component of the aquaculture industry. There is therefore the possibility that hatching and raising conditions might influence Artemia nauplii behavior (23). Students could raise Artemia under different conditions and then test their behavioral preferences. In fact, this is a somewhat common belief among our students: they posit that the Artemia in our lab will have particular preferences based on the conditions that we raise them under (red light, 25 ppt salt solution, post hatching development stage, etc.).
Supporting Materials
Peer-Reviewed Supporting Files
-
S2. Keep it shrimple – Sample LMS quiz
-
S3. Keep it shrimple – Experimental supplies
-
S4. Keep it shrimple – Salinity experiment protocol
-
S5. Keep it shrimple – Hypothesis and experimental design
-
S6. Keep it shrimple – Hypothesis rubric
Other Supporting Files
-
S0. Keep it shrimple – Complete course contents
-
S1. Keep it shrimple – Annotated primary literature
-
S7. Keep it shrimple – Peer feedback form
-
S8. Keep it shrimple – Hypothesis and experimental design revision
Acknowledgments
We would like to thank our colleagues in the Pacific University Biology Department for their collaboration and support.
References
- American Association for the Advancement of Science (AAAS). 2011. Vision and change in undergraduate biology education: A call to action. AAAS, Washington, DC.
- Gajardo GM, Beardmore JA. 2012. The brine shrimp Artemia: Adapted to critical life conditions. Front Physiol 3. doi:10.3389/fphys.2012.00185.
- Podwall KS, Talbert RR, Lener WB. 1993. Response to stimuli: The basis of behavior, p 33–48. In Goldman CA, Hauta PL, O’Donnell MA, Andrews SE, van der Heiden R (ed), Tested studies for laboratory teaching, vol 5. Proceedings of the 5th Workshop/Conference of the Association for Biology Laboratory Education (ABLE), Clemson, SC.
- Killpack TL, Fulmer SM, Roden JA, Dolce JL, Skow CD. 2020. Increased scaffolding and inquiry in an introductory biology lab enhance experimental design skills and sense of scientific ability. J Microbiol Biol Educ 21:21.2.56. doi:10.1128/jmbe.v21i2.2143.
- Bengtson DA, Léger P, Sorgeloos P. 1991. Use of Artemia as a food source for aquaculture, p 255–285. In Browne RA, Sorgeloos P, Trotman CNA (ed), Artemia biology. CRC Press, Boca Raton, FL.
- Fox R. 2004. Invertebrate zoology laboratory exercises. Invertebrate Anatomy OnLine. Retrieved from https://web.archive.org/web/20060423082302/http://www.lander.edu/rsfox/310artemiaLab.html (accessed 20 September 2023).
- Carolina Biological Supply. n.d. Care guide: Brine shrimp. Retrieved from https://www.carolina.com/teacher-resources/Interactive/care-guide-brine-shrimp/tr10481.tr (accessed 14 September 2023).
- McLaughlin JS, Favre DE, Weinstein SE, Goedhart CM. 2017. The impact of a four-step laboratory pedagogical framework on biology students’ perceptions of laboratory skills, knowledge, and interest in research. J Coll Sci Teach 47:83–91. doi:10.2505/4/jcst17_047_01_83.
- Goudsouzian LK, McLaughlin JS, Slee JB. 2017. Using yeast to make scientists: A six-week student-driven research project for the cell biology laboratory. CourseSource 4. doi:10.24918/cs.2017.4.
- Tanner KD. 2013. Structure matters: Twenty-one teaching strategies to promote student engagement and cultivate classroom equity. CBE Life Sci Educ 12:322–331. doi:10.1187/cbe.13-06-0115.
- Raffini JP. 1995. 150 ways to increase intrinsic motivation in the classroom. Allyn and Bacon, New York, NY.
- Fearon C, McLaughlin H, Yoke Eng T. 2012. Using student group work in higher education to emulate professional communities of practice. Educ Train 54:114–125. doi:10.1108/00400911211210233.
- Hodges LC. 2018. Contemporary issues in group learning in undergraduate science classrooms: A perspective from student engagement. CBE Life Sci Educ 17:es3. doi:10.1187/cbe.17-11-0239.
- Weaver GC, Russell CB, Wink DJ. 2008. Inquiry-based and research-based laboratory pedagogies in undergraduate science. Nat Chem Biol 4:577–580. doi:10.1038/nchembio1008-577.
- Holmes NG, Wieman CE, Bonn DA. 2015. Teaching critical thinking. Proc Natl Acad Sci 112:11199–11204. doi:10.1073/pnas.1505329112.
- Gouvea J, Appleby L, Fu L, Wagh A. 2022. Motivating and shaping scientific argumentation in lab reports. CBE Life Sci Educ 21:ar71. doi:10.1187/cbe.21-11-0316.
- Deiner LJ, Newsome D, Samaroo D. 2012. Directed self-inquiry: A scaffold for teaching laboratory report writing. J Chem Educ 89:1511–1514. doi:10.1021/ed300169g.
- Winkelmes M-A, Boye A, Tapp S. 2019. Transparent design in higher education teaching and leadership: A guide to implementing the transparency framework institution-wide to improve learning and retention. Stylus Publishing, Sterling, VA.
- Yong E. 2015. Why sea monkeys love salt: A fable on the cost of symbiosis. National Geographic. Retrieved from https://www.nationalgeographic.com/science/article/why-sea-monkeys-love-salt-a-fable-on-the-cost-of-symbiosis (accessed 7 June 2023).
- Curreri N, Dilbaryan A, Thomas B, Torok K. 2016. Sea monkey business: What species of Artemia are you hatching? Cold Spring Harbor Laboratory DNA Learning Center. Retrieved from https://dnabarcoding101.org/files/uploads/0000/sullivan-1222-poster-C1MR.pdf (accessed 20 September 2023).
- Van Stappen G. 1996. Introduction, biology and ecology of Artemia. Food and Agriculture Organization of the United Nations. Retrieved from https://www.fao.org/3/w3732e/w3732e0m.htm (accessed 20 September 2023).
- Utah Division of Wildlife Resources. 2023. Brine shrimp: What are they, and how do they survive in such an environment? Retrieved from https://wildlife.utah.gov/gslep/wildlife/brine-shrimp.html (accessed 20 September 2023).
- Dey P, Bradley TM, Boymelgreen A. 2023. The impact of selected abiotic factors on Artemia hatching process through real-time observation of oxygen changes in a microfluidic platform. Sci Rep 13:6370. doi:10.1038/s41598-023-32873-1.
- Aiken RB, Hailman JP. 1978. Positive phototaxis of the brine shrimp Artemia salina to monochromatic light. Can J Zool 56:708–711. doi:10.1139/z78-098.
- Blakeslee S. 2004. The CRAAP test. LOEX Quarterly 31. https://commons.emich.edu/loexquarterly/vol31/iss3/4/
Article Files
Login to access supporting documents
Karson-McCormick-Keep It Shrimple An Adaptable Student-Driven Research Project for the Introductory Biology Laboratory.pdf(PDF | 668 KB)
S0. Keep it shrimple - Complete course contents_notPR.docx(DOCX | 16 KB)
S1. Keep it shrimple - Annotated primary literature_notPR.docx(DOCX | 320 KB)
S2. Keep it shrimple - Sample LMS quiz_PR.docx(DOCX | 18 KB)
S3. Keep it shrimple - Experimental supplies_PR.docx(DOCX | 20 KB)
S4. Keep it shrimple - Salinity experiment protocol_PR.docx(DOCX | 1 MB)
S5. Keep it shrimple - Hypothesis and experimental design_PR.docx(DOCX | 30 KB)
S6. Keep it shrimple - Hypothesis rubric_PR.docx(DOCX | 17 KB)
S7. Keep it shrimple - Peer feedback form_notPR.docx(DOCX | 15 KB)
S8. Keep it shrimple - Hypothesis and experimental design revision_notPR.docx(DOCX | 26 KB)
- License terms

Comments
Comments
There are no comments on this resource.